Abstract
Inactivation of the water splitting enzyme complex in leaves or isolated chloroplasts results in increased susceptibility of photosystem II (PSII) to damage by light. Photoinhibition under this condition occurs in very weak light. The site of damage is exclusive of the water splitting complex yet still on the oxidizing side of PSII, as the QB locus is unaffected while photoreduction of silicomolybdate is inhibited. The kinetics of loss in PSII activity are more complex than apparent first-order, and the quantum efficiency is low. We observe no evidence of deletion from thylakoid membranes of any PSII polypeptide as a consequence of photoinhibition, although recovery from the photoinhibition is dependent upon both light and 70S protein synthesis. Enhanced synthesis of two proteins occurs during recovery, only one of which (D2) appears to be causally related to the recovery. We present a model which describes the relationship of weak light photoinhibition and its recovery to photoactivation of the S-state water oxidizing complex.
Full text
PDF
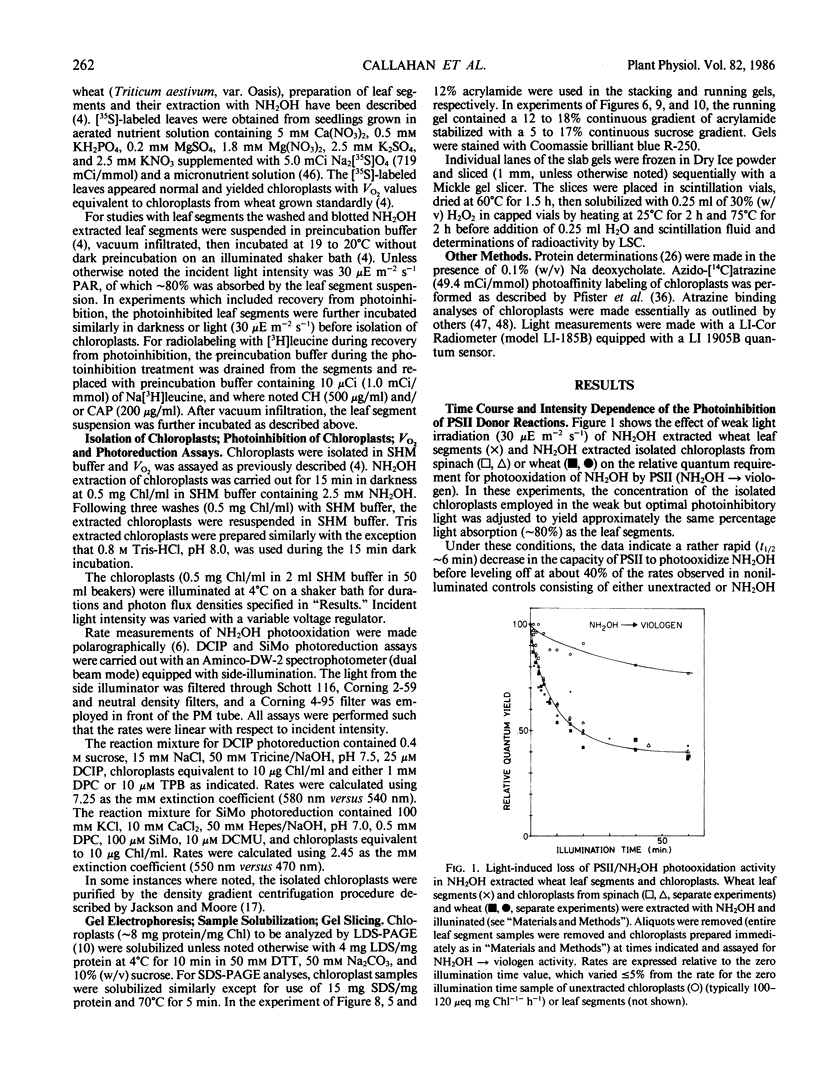
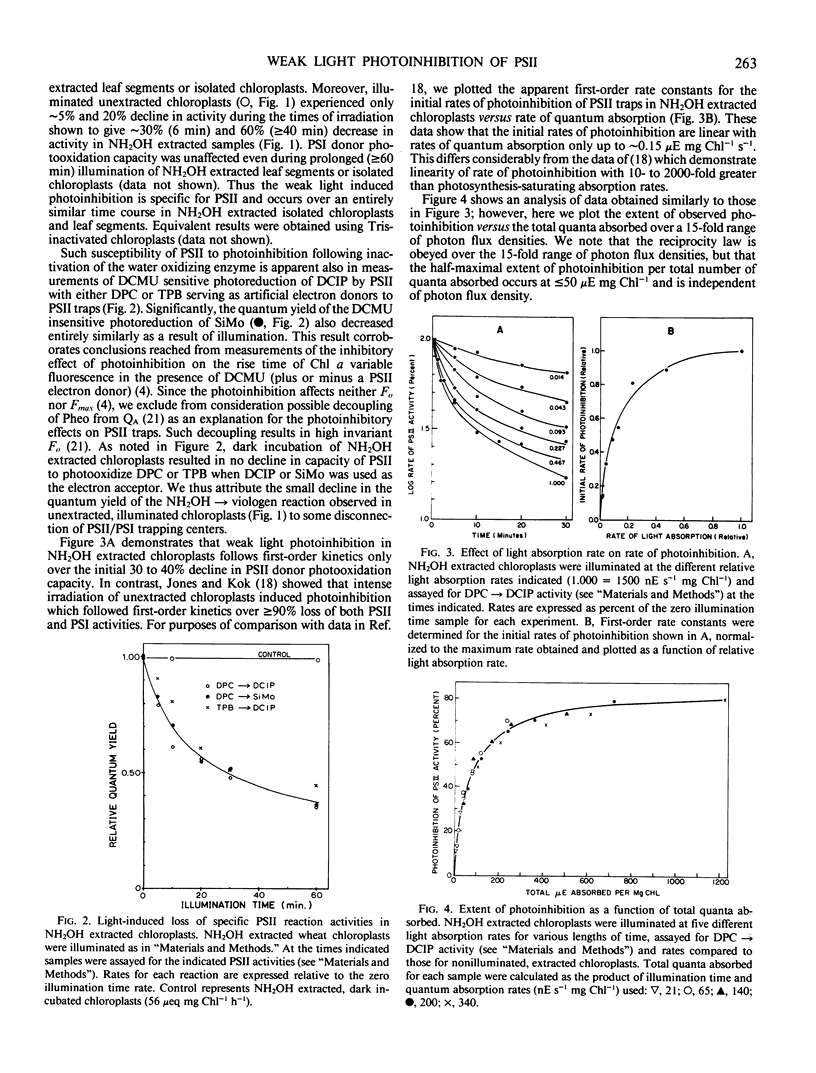
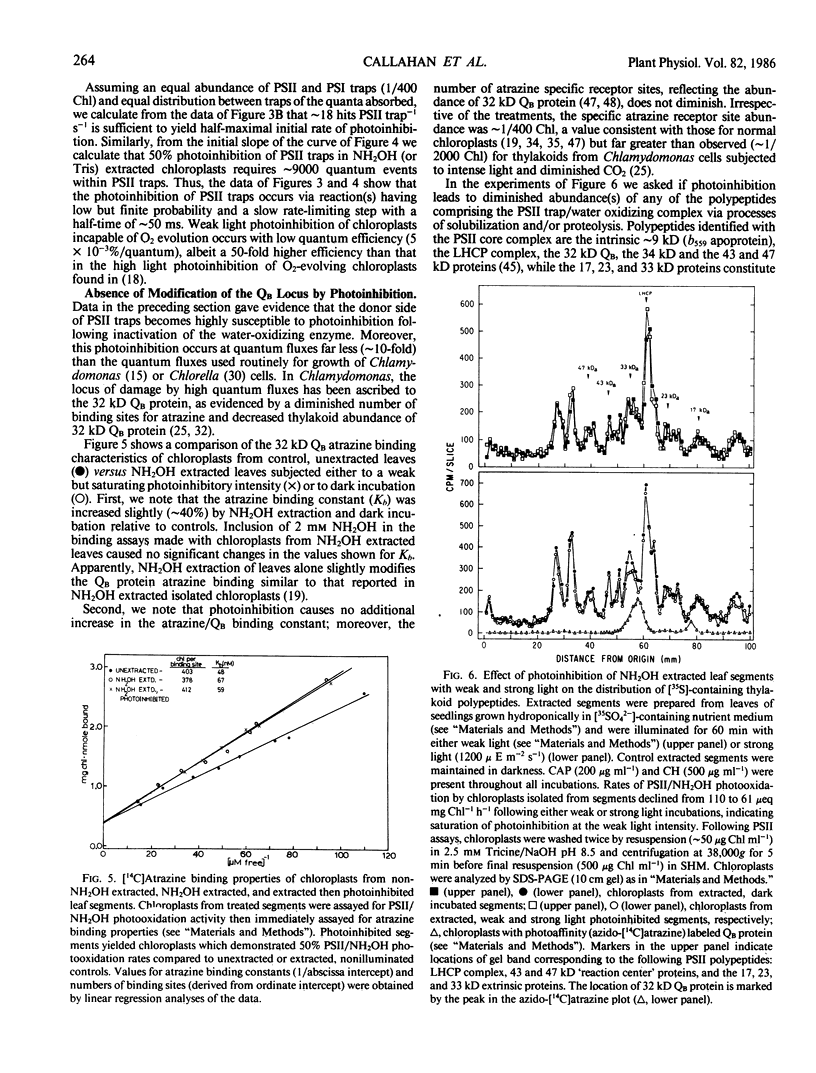

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan F. E., Cheniae G. M. Studies on the photoactivation of the water-oxidizing enzyme : I. Processes limiting photoactivation in hydroxylamine-extracted leaf segments. Plant Physiol. 1985 Nov;79(3):777–786. doi: 10.1104/pp.79.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G., Miginiac-Maslow M. Photoinhibition of Photosynthesis in Broken Chloroplasts as a Function of Electron Transfer Rates during Light Treatment. Plant Physiol. 1985 Aug;78(4):724–729. doi: 10.1104/pp.78.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley C. Studies on the Mechanism of Photoinhibition in Higher Plants: I. EFFECTS OF HIGH LIGHT INTENSITY ON CHLOROPLAST ACTIVITIES IN CUCUMBER ADAPTED TO LOW LIGHT. Plant Physiol. 1981 Jun;67(6):1161–1165. doi: 10.1104/pp.67.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos C. P., Hall D. O. Thylakoid polypeptides of light and dark aged chloroplasts. Plant Physiol. 1982 Sep;70(3):795–802. doi: 10.1104/pp.70.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Dilley R. A. A partial reaction in photosystem II: reduction of silicomolybdate prior to the site of dichlorophenyldimethylurea inhibition. Biochim Biophys Acta. 1975 May 15;387(2):288–305. doi: 10.1016/0005-2728(75)90111-5. [DOI] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jursinic P., Stemler A. Changes in [C]Atrazine Binding Associated with the Oxidation-Reduction State of the Secondary Quinone Acceptor of Photosystem II. Plant Physiol. 1983 Nov;73(3):703–708. doi: 10.1104/pp.73.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDLER O., SIRONVAL C. Photoxidation processes in normal green Chlorella cells. II. Effects on metabolism. Biochim Biophys Acta. 1959 May;33(1):207–215. doi: 10.1016/0006-3002(59)90515-3. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Kok B., Malkin S., Owens O., Forbush B. Observations on the reducing side of the O2-evolving photoact. Brookhaven Symp Biol. 1966;19:446–459. [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y. Inhibition of photosynthetic oxygen evolution by calmodulin-type inhibitors and other calcium-antagonists. Biochem Biophys Res Commun. 1984 Jun 15;121(2):626–633. doi: 10.1016/0006-291x(84)90228-6. [DOI] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Hirschberg J. Light-dependent degradation of the Q(B)-protein in isolated pea thylakoids. EMBO J. 1985 Jul;4(7):1655–1659. doi: 10.1002/j.1460-2075.1985.tb03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Radosevich S. R., Arntzen C. J. Modification of Herbicide Binding to Photosystem II in Two Biotypes of Senecio vulgaris L. Plant Physiol. 1979 Dec;64(6):995–999. doi: 10.1104/pp.64.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisfeld A., Mattoo A. K., Edelman M. Processing of a chloroplast-translated membrane protein in vivo. Analysis of the rapidly synthesized 32 000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur J Biochem. 1982 May;124(1):125–129. doi: 10.1111/j.1432-1033.1982.tb05914.x. [DOI] [PubMed] [Google Scholar]
- Samuelsson G., Lönneborg A., Rosenqvist E., Gustafsson P., Oquist G. Photoinhibition and Reactivation of Photosynthesis in the Cyanobacterium Anacystis nidulans. Plant Physiol. 1985 Dec;79(4):992–995. doi: 10.1104/pp.79.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- Zilinskas B. A., Govindjee Silicomolybdate and silicotungstate mediated dichlorophenyldimethylurea-insensitive photosystem II reaction: electron flow, chlorophyll a fluorescence and delayed light emission changes. Biochim Biophys Acta. 1975 May 15;387(2):306–319. doi: 10.1016/0005-2728(75)90112-7. [DOI] [PubMed] [Google Scholar]