Abstract
Type 2 diabetes is associated with an increased risk of herpes zoster and postherpetic neuralgia. However, the association of type 1 diabetes with herpes zoster or postherpetic neuralgia remains unclear. This retrospective cohort study using Taiwan’s Health Insurance Research Database included 199,566 patients with type 1 diabetes and 1,458,331 with type 2 diabetes, identified during the period 2000 to 2012. Patients with type 1 diabetes had a significantly higher risk of developing herpes zoster than those with type 2 diabetes (p < 0.001). Across all age groups, the impact of diabetes on herpes zoster was greater in type 1 than in type 2 diabetes. Patients with both type 1 and type 2 diabetes had a 1.45-fold higher risk of post-herpetic neuralgia than those without diabetes (hazard ratio 1.45, 95% confidence interval 1.28–1.65; hazard ratio 1.45, 95% confidence interval 1.37–1.52, respectively), and there was no difference between the 2 types of diabetes (hazard ratio 1.06; 95% confidence interval 0.93–1.21). The results recommend consideration of herpes zoster vaccination at an earlier age in patients with type 1 diabetes.
SIGNIFICANCE
This study found that people with type 1 diabetes have a significantly higher risk of developing herpes zoster than those with type 2 diabetes. Both type 1 and type 2 diabetic patients were at much higher risk of developing post-herpetic neuralgia than were non-diabetic people. Notably, people with type 1 diabetes aged 40–59 years had a 1.45-fold higher risk of developing herpes zoster than those without diabetes, while diabetic people in this age group had a 2-fold higher risk of post-herpetic neuralgia than non-diabetic people. Thus, it is recommended to consider giving herpes zoster vaccination at an earlier age in people with type 1 diabetes.
Key words: herpes zoster, post-herpetic neuralgia, type 1 diabetes mellitus, type 2 diabetes mellitus, vaccine
Herpes zoster (HZ) is a ubiquitous, highly neurotropic viral infection caused by reactivation of varicella-zoster virus (VZV), which is latent in cranial or dorsal root nerve ganglia following primary varicella infection (5). More than 90% of adults in the United States have been infected with VZV and are therefore at risk for herpes zoster (HZ) (6). In the United States, the lifetime risk for HZ infection is about 30% (7–9). The most common, debilitating complication of HZ is post-herpetic neuralgia (PHN), which may persist for months to years after resolution of the HZ rash (8–10), with a severe impact on the patient’s quality of life (11, 12). The risk of HZ and PHN increased with age due to age-related decline in memory cell-mediated immunity (CMI) to VZV (9, 13). Antiviral medications could reduce the duration and severity of HZ, but do not prevent PHN (9, 14). Thus, vaccination is recommended to prevent HZ and its sequelae.
Several studies have reported that diabetes is associated with an increased risk of HZ and PHN (15, 16). The management of HZ and PHN in patients with diabetes is challenging, as it may be hindered by diabetes-related complications, such as diabetic nephropathy or diabetic neuropathy (17, 18). In addition, PHN may further deteriorate the already poor quality of life of patients with diabetes (19, 20). To date, studies regarding the associations of diabetes with HZ or PHN have relied on data from patients with type 2 diabetes (T2DM) or unspecified diabetes. The association of type 1 diabetes (T1DM) with HZ and PHN remains unclear. To date, only 4 studies (21–24) have examined the risk of HZ or PHN in T1DM and T2DM patients separately, and none of these studies directly compared the effects of the 2 types of diabetes on developing HZ or PHN. Furthermore, most of these studies were not designed to investigate the association of diabetes with HZ or PHN, and had very small sample sizes.
The HZ vaccine was introduced in Taiwan in 2013. The current study examined patient data from the prevaccine era, in order to accurately measure the influence of diabetes on the risk of HZ and PHN. The aim of this study was to conduct a very large population-based cohort study to assess the impact of T1DM on developing HZ and PHN and to compare the effects of T1DM vs T2DM on HZ and PHN.
MATERIALS AND METHODS
Data source
This population-based, retrospective study utilized patient data from Taiwan’s Health Insurance Research Database (NHIRD). Taiwan implements a single-payer universal health insurance programme that covers more than 99.8% of Taiwan’s total population, approximately 23 million people. The NHIRD contains registration files, detailed medical data, and original claims data from reimbursement files.
Study design
A total of 1,789,610 patients with diabetes were identified among all beneficiaries in the NHIRD (data source 1) between 1 January 2000 and 31 December 2012. Diabetes was defined as 1 inpatient visit or 2 or more outpatient visits (at least 30 days apart) with an International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic code of 250 and a minimum 12-month prescription for antidiabetic medication. T1DM was identified based on the registry for catastrophic illness, which is applied only when medical history or specific laboratory data are met, including diabetic ketoacidosis, glucagon test, low C-peptide levels after glucagon stimulation test, presence of glutamate decarboxylase antibodies, insulin autoantibodies, and islet cell antibody. The index date of patients with diabetes was defined as the date of diagnosis of diabetes.
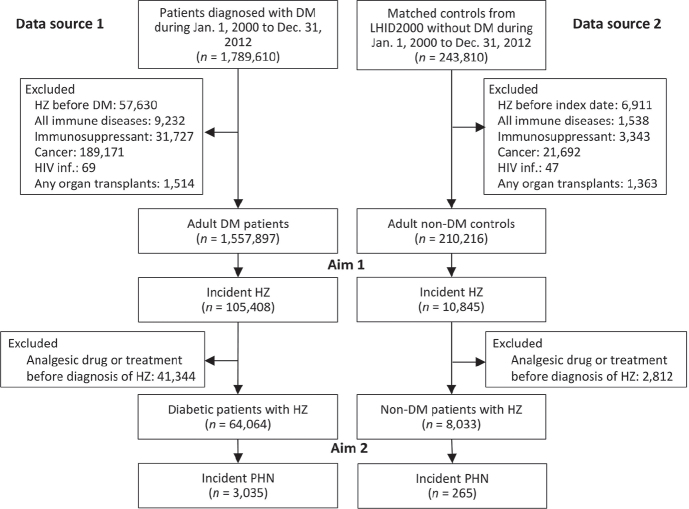
Due to the protection of personal privacy, controls without diabetes could only be obtained from Longitudinal Health Insurance Databases 2000 (LHID2000, data source 2), a representative subset of NHIRD containing longitudinal data on medical claims of 1,000,000 enrollees, rather than from all beneficiaries in the NHIRD. Subjects in data source 2 were matched by age and sex with patients in data source 1 based on the index date of diabetes diagnosis. A total of 243,810 subjects with a diagnosis of diabetes between 2000 and 2012 were identified. Exclusion criteria were: a history of HZ before the index date, any immune disease, previous use of immunosuppressants, a diagnosis of cancer, HIV infection, or any organ transplant (21). After exclusion, data from 1,557,897 patients with diabetes and 210,216 controls without diabetes were analysed. The study flowchart is shown in Fig. 1.
Fig. 1.
Flowchart for the inclusion and exclusion of the subjects. DM: diabetes; HZ: herpes zoster; PHN: post-herpetic neuralgia; HIV: human immunodeficiency virus infection; LHID2000: Longitudinal Health Insurance Databases 2000.
Main outcomes
This study included 2 main outcomes of interest. First, incident HZ after diagnosis of diabetes; and, secondly, incident PHN after diagnosis of HZ. The presence of HZ (ICD-9-CM: 053.xx) was defined as having at least 2 outpatient diagnoses or any inpatient diagnosis during the follow-up periods. The presence of PHN was defined as a HZ patient >90 days after initial diagnosis with the following: (i) ICD-9-CM code 053.12 (post-herpetic trigeminal neuralgia) or ICD-9-CM code 053.13 (post-herpetic polyneuropathy); or (ii) HZ code and a relevant prescription for analgesics, antidepressants or anticonvulsants at that visit. The current study did not follow the method proposed by Klompas et al. (25) to identify PHN, due to possible confusion with diabetic neuropathy; therefore, patients with non-specific neuralgia code but no HZ code > 90 days after initial HZ diagnosis were excluded. In the analysis of PHN, subjects who had received any medications typically used to treat HZ pain before the diagnosis of HZ were excluded. Subjects were followed from the index date until the outcome date, withdrawal from the National Health Insurance (NHI) programme or December 31, 2012.
Covariates
The covariates included were demographics and comorbidities (Table SI). Demographic characteristics were age at diagnosis of diabetes, sex, urbanization level of residence, and monthly income. The comorbidities included depression, obstructive sleep apnoea, chronic kidney disease, hyperlipidaemia, hypertension, cardio-vascular diseases, chronic obstructive pulmonary disease, and asthma. Comorbidity was defined as having at least 2 outpatient diagnoses or any single inpatient diagnosis in the previous year.
Statistical analysis
To compare the risk of HZ/PHN in patients with diabetes and controls without diabetes, the inverse probability of treatment weighting (IPTW) method was used to create an additional cohort. The propensity score represents the predicted probability of being in the group with diabetes given the specific values of covariates derived from the multivariable logistic regression model. To avoid the impact of extreme weights, stabilized weights are used. Balance between patients with diabetes and controls without diabetes was assessed using standardized differences (STD), where an absolute value < 0.1 indicates a negligible difference. The risk of HZ/PHN between subjects with diabetes and controls without diabetes was compared using the Cox proportional hazard model in the IPTW-adjusted cohort. A 2-sided p-value of less than 0.05 was considered statistically significant. Analyses were performed in SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
After applying the exclusion criteria, data from 1,557,897 patients with diabetes and 210,216 controls without diabetes were analysed. The study populations included 99,566 people with T1DM and 1,458,331 people with T2DM, respectively. The mean age of the groups with diabetes and without diabetes was 57.5 and 55.4 years, respectively. The proportions of men in the groups with diabetes and without diabetes were 52.4% and 55.9%, respectively. After IPTW adjustment, the baseline characteristics of the 2 groups were balanced with all absolute STD values ≤ 0.1 (Table SI).
The mean follow-up of groups with and without diabetes was 7.2 ± 4.2 and 7.1 ± 4.2 years, respectively. A total of 105,408 patients with diabetes and 10,845 subjects without diabetes were diagnosed with HZ during the follow-up period. In the IPTW-adjusted cohort, HZ incidence was 9.7 and 8.8 events per 1,000 PY in the groups with diabetes and without diabetes, respectively. Patients with diabetes demonstrated a significantly higher risk of HZ than those without diabetes (hazard ratio (HR) 1.10; 95% confidence interval (95% CI) 1·09–1·11). For the whole cohort with diabetes, the effect of diabetes was greater in younger populations and similar between the male and female groups.
Compared with controls without diabetes, patients with T1DM had a 1·35-fold (HR 1.35; 95% CI 1.32–1.37) higher risk of developing HZ and patients with T2DM had a 1.09-fold (HR 1.09; 95% CI 1.08–1.10) higher risk of developing HZ. Patients with T1DM had significantly increased risk of HZ than those with T2DM when adjusting for the known risk factors (HR 1.07; 95% CI 1.05–1.10, p < 0.001) (Table I). Compared with the controls without diabetes, the HRs for HZ were 1.68, 1.45 and 1.16 in subjects with T1DM aged 20–39, 40–59 and 60–79 years, respectively, and 1.49, 1.16 and 1.03 in subject with T2DM aged 20–39, 40–59 and 60–79 years, respectively. The impact of diabetes on HZ was higher in T1DM than in T2DM in all age groups. The HRs for HZ decreased with age in both T1DM and T2DM.
Table I.
Subgroup analysis of the risk of herpes zoster (HZ) according to the diabetes status
| DM | Non-DM | HR of HZa (95% CI) | |||
|---|---|---|---|---|---|
| HZ cases | ID (95% CI)a | HZ cases | ID (95% CI)a | ||
| Total incident HZ cases | 105,408 | 9.7 (9.7–9.8) | 10,845 | 7.5 (7.4–7.7) | 1.10 (1.09–1.11) |
| Age group | |||||
| < 20 years | 205 | 2.68 (2.31–3.04) | 25 | 1.73 (1.05–2.41) | 1.67 (1.36–2.05) |
| 20–39 years | 3,484 | 3.82 (3.69–3.94) | 390 | 2.51 (2.26–2.76) | 1.52 (1.45–1.60) |
| 40–59 years | 47,528 | 8.86 (8.78–8.94) | 5,386 | 6.69 (6.51–6.87) | 1.17 (1.16–1.19) |
| 60–79 years | 50,746 | 12.28 (12.18–12.39) | 4,655 | 10.81 (10.50–11.12) | 1.04 (1.02–1.05) |
| ≥80 years | 3,445 | 13.39 (12.94–13.84) | 389 | 10.23 (9.22–11.25) | 1.08 (1.03–1.13) |
| Sex | |||||
| Female | 55,946 | 10.61 (10.52–10.69) | 5,631 | 8.54 (8.32–8.77) | 1.07 (1.06–1.09) |
| Male | 49,462 | 9.05 (8.97–9.13) | 5,214 | 6.65 (6.47–6.83) | 1.12 (1.11–1.13) |
| Type of diabetes mellitus | |||||
| Type 1 diabetes mellitus | 8,433 | 10.01 (9.88–10.14) | 10,845 | 8.84 (8.78–8.89) | 1.35 (1.32–1.37) |
| Type 2 diabetes mellitus | 96,975 | 9.71 (9.66–9.77) | 1.09 (1.08–1.10) | ||
| Type 1 diabetes mellitus | |||||
| < 20 years | 145 | 2.74 (2.33–3.15) | 25 | 1.60 (1.35–1.85) | 1.73 (1.39–2.15) |
| 20–39 years | 516 | 4.12 (3.87–4.36) | 390 | 2.47 (2.28–2.66) | 1.68 (1.52–1.85) |
| 40–59 years | 3,718 | 9.83 (9.65–10.01) | 5,386 | 6.84 (6.69–7.00) | 1.45 (1.41–1.49) |
| 60–79 years | 3,855 | 12.75 (12.49–13.01) | 4,655 | 11.08 (10.83–11.33) | 1.16 (1.13–1.20) |
| ≥ 80 years | 199 | 13.91 (12.95–14.86) | 389 | 10.40 (9.51–11.30) | 1.35 (1.21–1.51) |
| Type 2 diabetes mellitus | |||||
| < 20 years | 60 | 2.39 (1.88–2.89) | 25 | 1.61 (1.24–1.99) | 1.50 (1.10–2.05) |
| 20–39 years | 2,968 | 3.73 (3.61–3.86) | 390 | 2.51 (2.41–2.62) | 1.49 (1.41–1.57) |
| 40–59 years | 43,810 | 8.72 (8.65–8.80) | 5,386 | 7.50 (7.43–7.58) | 1.16 (1.14–1.17) |
| 60–79 years | 46,891 | 12.21 (12.10–12.31) | 4,655 | 11.81 (11.71–11.91) | 1.03 (1.02–1.04) |
| ≥ 80 years | 3,246 | 13.27 (12.84–13.69) | 389 | 12.43 (12.02–12.84) | 1.07 (1.02–1.12) |
| Type 1 vs Type 2 diabetes mellitus | HR (95% CI) | p - value | |||
| Type 1 | 1.07 (1.05–1.10) | < 0.001 | |||
| Type 2 | Reference | ||||
Adjusted for inverse probability of treatment weighting by propensity score.
IPTW: inverse probability treatment weighting; ID: incidence density, number of events per 1,000 person-years; 95% CI: 95% confidence interval; HR: hazard ratio.
After applying the exclusion criteria to patients with HZ, the study identified 64,064 and 8,033 subjects in the groups with diabetes and without diabetes, respectively, and followed up for PHN outcomes. The mean follow-up of groups with diabetes and without diabetes was 4.5 ± 3.2 and 4.3 ± 3.2 years, respectively. The baseline characteristics of the 2 groups were balanced with all absolute STD values ≤ 0.1 after IPTW adjustment (Table SII).
Patients with both T1DM and T2DM had a significantly (45%) increased risk of developing PHN compared with subjects without diabetes (HR = 1.45; 95% CI 1.28–1.65, p < 0.001 and HR = 1.45; 95% CI 1.37–1.52, respectively; both p < 0.001) (Table II). There was no significant difference in the risk of developing PHN between T1DM and T2DM (HR = 1.06; 95% CI 0.93–1.21). The aforementioned results were adjusted for the known risk factors. The HRs for PHN was particularly increased in the younger populations and decreased with age, with no increase observed after age 80 years. Compared with patients without diabetes, patients with diabetes had a 3.17-fold (95% CI 1.43–7.01), 2.00-fold (95% CI 1.77–2.27), and 1.54-fold (95% CI 1.45–1.65) increased risk of developing PHN in the respective age groups 20–39, 40–59 and 60–79 years.
Table II.
Subgroup analysis of the risk of poste-herpetic neuralgia among the zoster patients according to diabetes status
| DM | Non-DM | ||||
|---|---|---|---|---|---|
| PHN cases (n = 3,035) | PHN (%)a | PHN cases (n=265) | PHN (%)a | HR of PHNa (95% CI) | |
| Type of DM | |||||
| Type 1 | 233 | 5.0 | 265 | 3.3 | 1.45 (1.28–1.65) |
| Type 2 | 2,802 | 4.7 | 1.45 (1.37–1.52) | ||
| Age group | |||||
| < 20 years | 1 | 1.1 | 1 | 8.8 | 0.16 (0.02–1.17) |
| 20–39 years | 23 | 2.0 | 1 | 0.6 | 3.17 (1.43–7.01) |
| 40–59 years | 666 | 3.0 | 48 | 1.5 | 2.00 (1.77–2.27) |
| 60–79 years | 1,913 | 5.5 | 152 | 3.6 | 1.54 (1.45–1.65) |
| ≥ 80 years | 432 | 7.1 | 63 | 7.5 | 0.94 (0.83–1.06) |
| Sex | |||||
| Female | 1,399 | 4.4 | 124 | 3.0 | 1.51 (1.40–1.64) |
| Male | 1,636 | 5.0 | 141 | 3.6 | 1.41 (1.31–1.51) |
| Type 1 vs Type 2 DM | HR (95% CI) | P value | |||
| Type 1 | 1.06 (0.93–1.21) | 0.410 | |||
| Type 2 | Reference | ||||
Adjusted for inverse probability of treatment weighting by propensity score.
DM: diabetes mellitus; PHN: post-herpetic neuralgia; IPTW: inverse probability treatment weighting; 95% CI: 95% confidence interval; HR: hazard ratio.
DISCUSSION
This large population-based study, including 99,566 patients with T1DM, is the largest study to assess the effect of T1DM on HZ and PHN to date, and the only study to directly compare the impact of T1DM vs T2DM on the development of HZ and PHN. The results showed that patients with T1DM had a significantly higher risk of HZ than those with T2DM. The effect of diabetes on HZ was higher in patients with T1DM than in those with T2DM in all age groups, while the effect of both types of diabetes on the development of HZ decreased with age. Both T1DM and T2DM patients had a significantly (45%) higher risk of developing PHN than non-diabetic individuals, and there was no difference in the risk of PHN between the 2 types of diabetes.
The impact of diabetes on PHN was much greater in younger than older populations, and the impact attenuated with age. Furthermore, diabetes had a much greater impact on the risk of PHN than on the risk of HZ. Notably, patients with T1DM in the 40–59-year age group had a 1.45-fold higher risk of developing HZ than non-diabetic individuals, while diabetic patients in this age group had a 2-fold higher risk of PHN than non-diabetic patients.
To date, only a few studies have reported the association between T1DM and HZ, and their results are inconsistent. A Taiwanese study with a much smaller sample size of 4,736 patients with T1DM reported a 2.37-fold higher risk of HZ in patients with T1DM compared with those without T1DM (26), which was consistent with the current findings. A UK study quantifying the risk factors for HZ also showed an association between T1DM and HZ (23). However, in contrast to the results of the current study, this UK study showed no association between T2DM and HZ. In addition, in contrast to the current results, a study by Guignard et al. (21) using a US claims database showed no evidence of an effect of T1DM (n = 20.397) on the development of HZ. In addition to ethnic factors and sample size, the age distribution of HZ events in Guignard et al.’s study differed considerably from the results from the current study. Patients with HZ over the age of 40 years accounted for 40% of event cases in the T1DM cohort in Guignard et al.’s study, while these patients accounted for 80% in the current study. Since HZ is more common among older adults, the current study comprehensively assessed the effects of T1DM on HZ in middle-aged and older adults. In the study conducted in Belgium, Ogunjimi et al. (22) similarly reported no association between T1DM (cases and controls, n = 77) and HZ. As for T1DM and PHN, another UK study examined risk factors for PHN in patients with HZ, of whom 282 patients with T1DM had 10 PHN events, and suggested no significant association between T1DM and PHN (24), which was different from the observation in the current study. The results of these studies differed from the current study mainly because they were not specifically designed to assess the association between diabetes and HZ or PHN, and because they had very small sample sizes.
The effects of diabetes at different ages on the development of HZ or PHN are sparsely studied. Ke et al. (27) examined newly diagnosed patients with HZ in Taiwan, and found that, compared with patients without diabetes, those with diabetes aged < 30 years had the highest odds ratios and those aged > 70 years had the lowest odds ratios. Another Taiwan study of 4,736 patients with T1DM reported that patients aged 20–39 years had a higher HR of HZ than those > 40 years compared with non-T1DM patients (26). The observations of the current study were consistent with these 2 reports. However, the US study by Guignard et al. (21) proposed that age was an effect modifier for HZ in patients with T2DM, with a particularly high risk in patients ≥ 65 years and a moderately high risk in those < 65 years. However, another US study by Suaya et al. (28) had different findings, showing no age-dependent trend in the risk of HZ in subjects with diabetes. The study by Suaya et al. also found an increased risk of PHN among people with diabetes under the age of 60 years and no difference over the age of 65 years, which was compatible with the current study results that diabetes had a lesser effect on PHN in elderly subjects.
Both T1DM and T2DM were reported to be associated with a disturbed T-cell balance, as manifested by expansion of CD4+ CD28null T-cells and reduction in CD4+CD25+Foxp3+ regulatory T-cells (29). Okamoto et al. (30) indicated that the CMI of VZV in individuals with diabetes was significantly lower than that in healthy controls, which explains why people with diabetes are more likely to develop HZ. Persistent inflammation in patients with DM leads to nerve damage and increased pain sensitivity, which might contribute to the increased incidence of PHN in patients with DM (31). Ma et al. (32) proposed that autoimmune pathogenesis resulted in impaired function of monocyte/macrophage in patients with T1DM. The production of interleukins IL-1 and IL-6 by monocytes of patients with T1DM was reported to be significantly lower than that of patients with T2DM and healthy subjects (33). These findings might explain the higher risk of HZ in patients with T1DM than in those with T2DM. The current study revealed that the effects of diabetes on HZ and PHN were greater in the younger population and attenuated with age. We speculate that the age-related decline in VZV-CMI is so strong that the effect of diabetes on VZV-CMI in the elderly population becomes insignificant compared with the effect of ageing on HZ and PHN.
The strengths of the current study are, first, that it used a very large population representative sample, which provided sufficient statistical power to detect the effect of diabetes on HZ and PHN; moreover, the current study included 99,566 patients with T1DM, the largest study ever to evaluate the association between T1DM and HZ. Secondly the current study is the first study designed to assess the association between T1DM and PHN, and, thirdly, the patients with T1DM in the current study were retrieved from the Catastrophic Illness Patient Database (34), and diagnosis was made based on the patient’s medical history, clinical presentation, and laboratory data, providing the correct classification of T1DM and T2DM. This differs from other studies using administrative data, which have inherent limitations in distinguishing between the 2 types of diabetes mellitus. The current study has several limitations. It was conducted based on diagnostic codes from a claims database, and verification of HZ through PCR or other diagnostic tools was not available. In the report of Klompas et al. (25) identification of HZ by the ICD-9-CM code was regarded as a valid method with a positive predictive value of 93%. However, HZ in Taiwan is primarily diagnosed by dermatologists, and some are diagnosed by internists and family physicians, rather than solely by dermatologists. Furthermore, no glycaemic data were available, which may affect the incidence or severity of HZ or PHN. Despite including known HZ risk factors as exclusion criteria, detailed information on critical confounders, such as malignancy, autoimmune disease, and immunosuppressive drug use, was still lacking due to the absence of medical record review. In the current study, there was potential selection bias due to disparities in healthcare service utilization, or people with diabetes might have a higher likelihood of receiving a HZ diagnosis because of routine medical follow-up. Nevertheless, hospitals and clinics in Taiwan are densely distributed and the Taiwanese population can easily access medical services at extremely low cost (35), hence the population has a strong incentive to use medical services regardless of the severity of their symptoms.
In conclusion, this study found that patients with T1DM have a significantly higher risk of HZ than those with T2DM, and that there is no significant difference in the risk of PHN between the 2 types of diabetes. Both T1DM and T2DM had a strong effect on the risk of developing PHN compared with non-diabetic individuals. It is noteworthy that T1DM aged 40–59 years had a 1.45-fold increased risk of developing HZ compared with those without diabetes, and diabetic patients in this age group had a 2-fold increased risk of PHN compared with non-diabetic individuals. The results recommend consideration of HZ vaccination at an earlier age in patients with T1DM.
Supplementary Material
Impact of Type 1 Versus Type 2 Diabetes on Developing Herpes Zoster and Post-herpetic Neuralgia: A Population-based Cohort Study
ACKNOWLEDGEMENTS
The study protocol was approved by the Institutional Review Board of Taipei City Hospital (TCHIRB-10805001-W).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Speight J, Holmes-Truscott E, Hendrieckx C, Skovlund S, Cooke D. Assessing the impact of diabetes on quality of life: what have the past 25 years taught us? Diabet Med 2020; 37: 483–492. [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019; 157: 107843. [DOI] [PubMed] [Google Scholar]
- 3.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab 2012; 16: S27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey IM, Critchley JA, DeWilde S, Harris T, Hosking FJ, Cook DG. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care 2018; 41: 513–521. [DOI] [PubMed] [Google Scholar]
- 5.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc 2009; 84: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta N, Breuer J. A global perspective of the epidemiology and burden of varicella-zoster virus. Curr Pediatr Rev 2009; 5: 207–228. [Google Scholar]
- 7.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007; 82: 1341–1349. [DOI] [PubMed] [Google Scholar]
- 8.Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57: 1–30. [PubMed] [Google Scholar]
- 9.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open 2014; 4: e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol 2010; 48: S2–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6: 356–363. [DOI] [PubMed] [Google Scholar]
- 12.Gater A, Uhart M, McCool R, Préaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health 2015; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alper BS, Lewis PR. Treatment of postherpetic neuralgia: a systematic review of the literature. J Fam Pract 2002; 51: 121–128. [PubMed] [Google Scholar]
- 14.Arvin AM, Moffat JF, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res 1996; 46: 263–309. [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc 2017; 92: 1806–1821. [DOI] [PubMed] [Google Scholar]
- 16.Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain 2016; 157: 30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim AKH. Diabetic nephropathy – complications and treatment. Int J Nephrol Renovasc Dis 2014; 7: 361–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckenthal P, Barkin RL. Options for treating postherpetic neuralgia in the medically complicated patient. Ther Clin Risk Manag 2013; 9: 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torcel-Pagnon L, Bricout H, Bertrand I, Perinetti E, Franco E, Gabutti G, et al. Impact of underlying conditions on zoster-related pain and on quality of life following zoster. J Gerontol A Biol Sci Med Sci 2017; 72: 1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002; 18: 350–354. [DOI] [PubMed] [Google Scholar]
- 21.Guignard AP, Greenberg M, Lu C, Rosillon D, Vannappagari V. Risk of herpes zoster among diabetics: a matched cohort study in a US insurance claim database before introduction of vaccination, 1997–2006. Infection 2014; 42: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogunjimi B, Buntinx F, Bartholomeeusen S, Terpstra I, De Haes I, Willem L, et al. Herpes zoster is associated with herpes simplex and other infections in under 60-year-olds. J Infect 2015; 70: 171–177. [DOI] [PubMed] [Google Scholar]
- 23.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population-based case-control study. BMJ 2014; 348: g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Mansfield K, et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: a cohort study. Neurology 2016; 87: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc 2011; 86: 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HH, Lin IC, Chen HJ, Yeh SY, Kao CH. Association of herpes zoster and type 1 diabetes mellitus PLoS One 2016; 11: e0155175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke CC, Lai HC, Lin CH, Hung CJ, Chen DY, Sheu WH, et al. Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: a population-based study in Taiwan. PLoS One 2016; 11: e0146750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suaya JA, Chen SY, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis 2014; 1: ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedicino D, Liuzzo G, Trotta F, Giglio AF, Giubilato S, Martini F, et al. Adaptive immunity, inflammation,and cardiovascular complications in type 1 and type 2 diabetes mellitus. J Diabetes Res 2013; 2013: 184258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto S, Hata A, Sadaoka K, Yamanishi K, Mori Y. Comparison of varicella-zoster virus-specific immunity of patients with diabetes mellitus and healthy individuals. J Infect Dis 2009; 200: 1606–1610. [DOI] [PubMed] [Google Scholar]
- 31.Galiero R, Caturano A, Vetrano E, Beccia D, Brin C, Alfano M, et al. Peripheral neuropathy in diabetes mellitus: pathogenetic mechanisms and diagnostic options. Int J Mol Sci 2023; 24: 3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma WT, Gao F, Gu K, Chen DK. The Role of Monocytes and Macrophages in Autoimmune Diseases: a Comprehensive Review. Front Immunol 2019; 10: 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohno Y, Aoki N, Nishimura A. In vitro production of interleukin-1, interleukin-6, and tumor necrosis factor-alpha in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993; 77: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol 2019; 11: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreng VB, Yang CT. The equality of resource allocation in health care under the National Health Insurance System in Taiwan. Health Policy 2011; 100: 203–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Impact of Type 1 Versus Type 2 Diabetes on Developing Herpes Zoster and Post-herpetic Neuralgia: A Population-based Cohort Study