Abstract
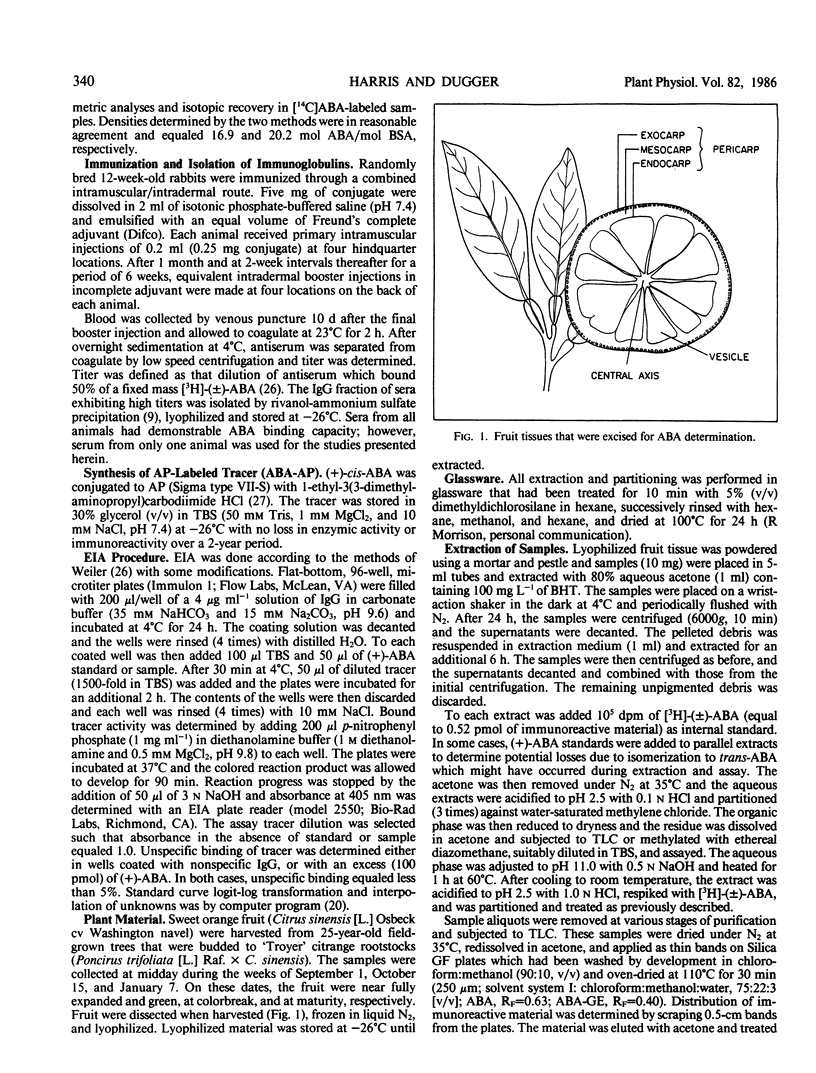
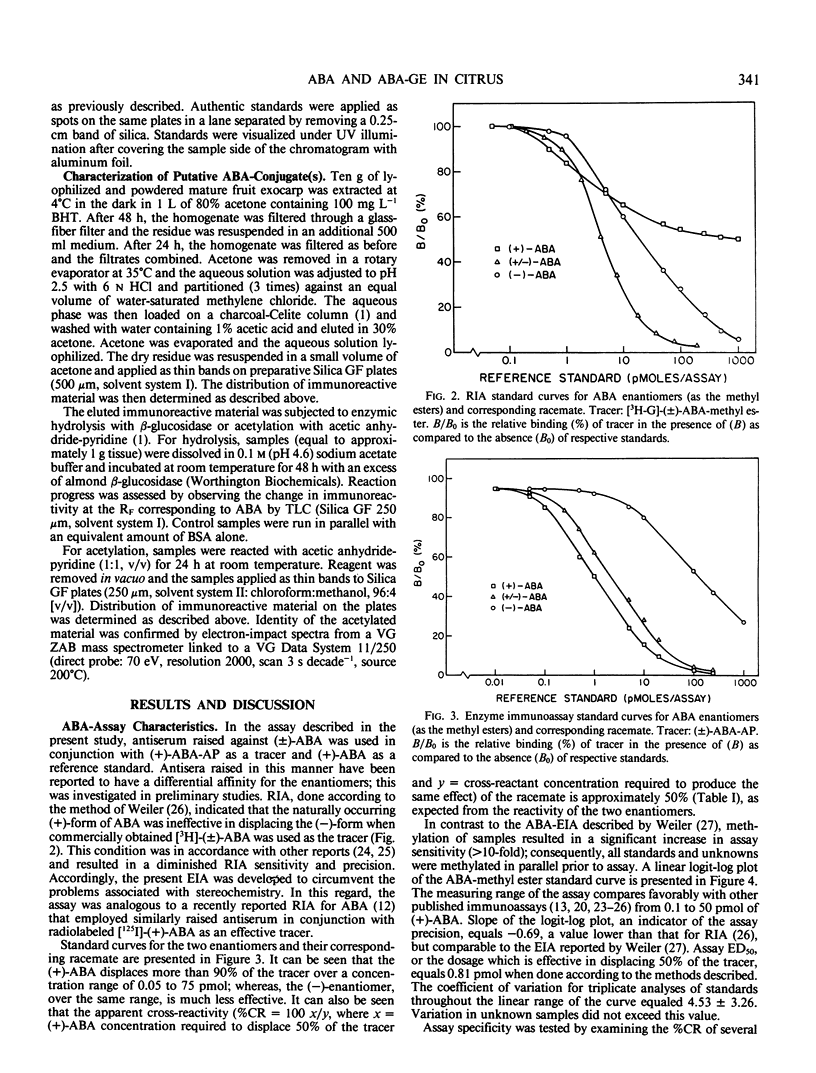
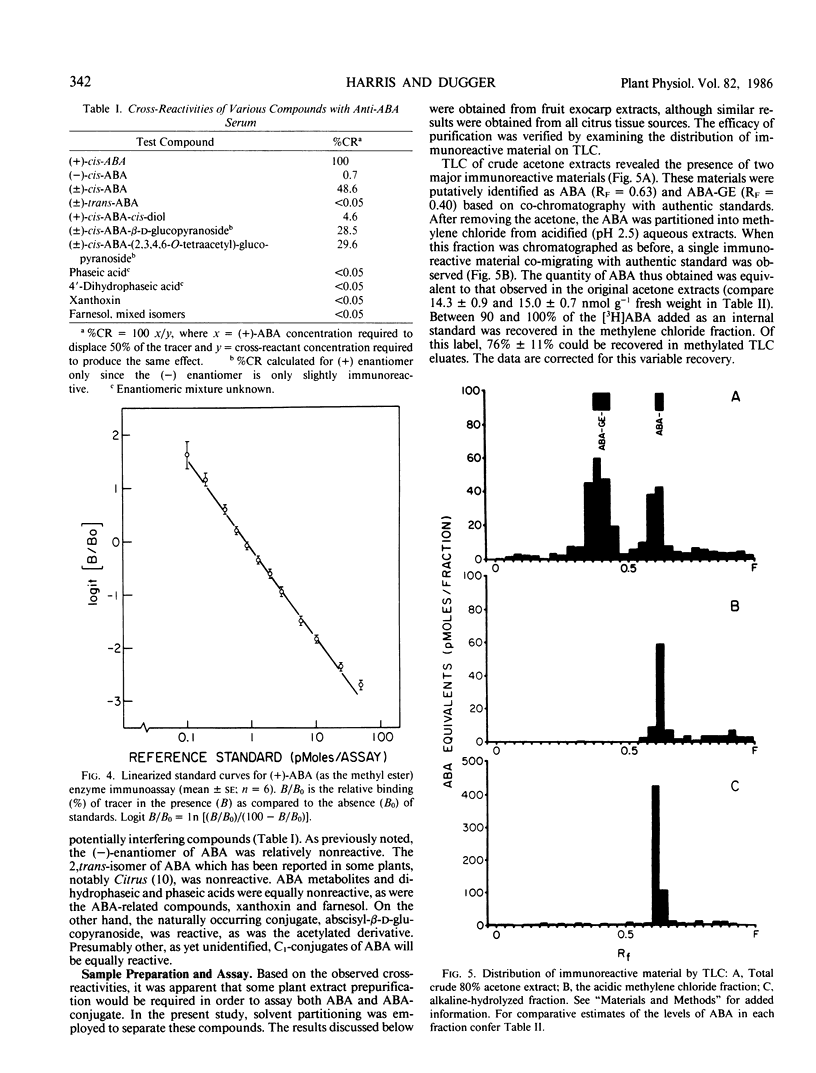
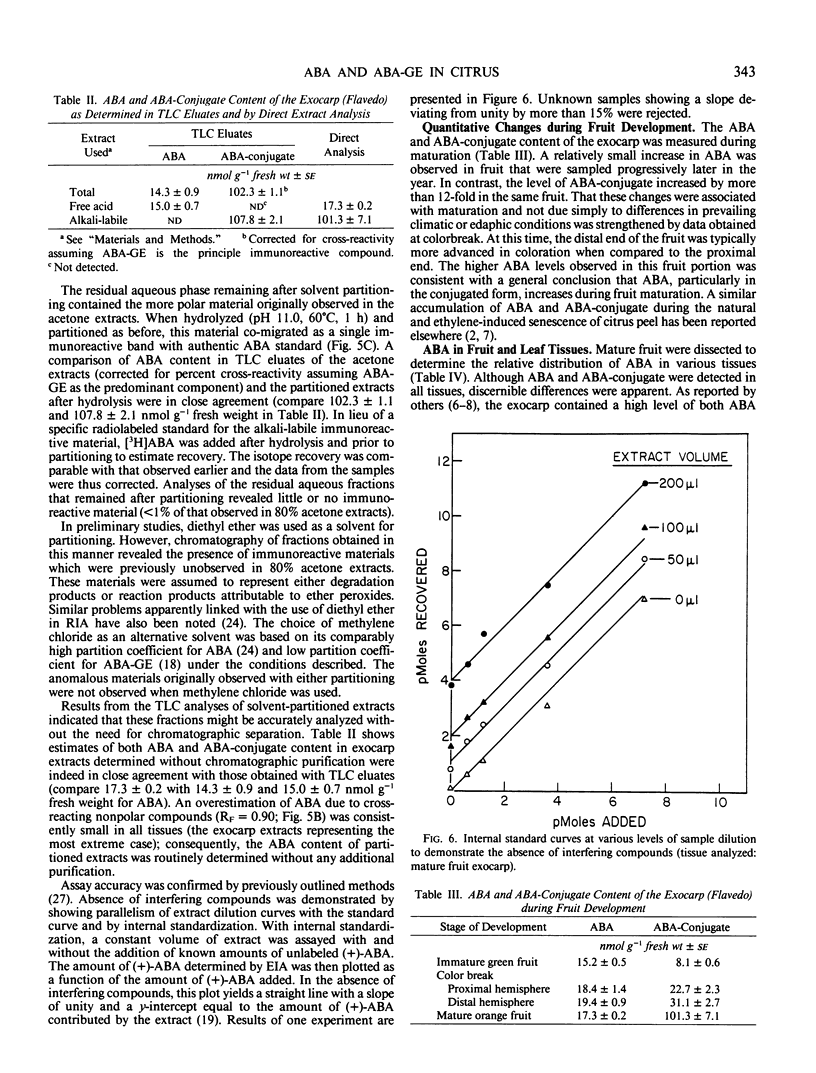
The contents of (+)-cis-abscisic acid (ABA) and alkaline-hydrolyzable ABA-conjugate(s) were analyzed by means of enzyme immunoassay in partially purified extracts of developing and mature sweet orange fruit (Citrus sinensis [L.] Osbeck cv Washington navel). A relatively small increase in ABA was observed in the fruit exocarp during the natural color transition from green to orange. At the same time, the ABA-conjugate level increased approximately 12-fold in this tissue. The contents of ABA and ABA-conjugate equaled 15.0 ± 0.7 and 107.8 ± 2.1 nanomoles per gram fresh weight, respectively, in the exocarp at harvest. Other tissues also contained considerable quantities of these compounds. Whereas the highest ABA content was observed in the exocarp, the highest ABA-conjugate content was observed in the central vascular axis of the fruit and equaled 187.0 ± 10.3 nanomoles per gram fresh weight. The only immunoreactive conjugate found in significant quantity in mature fruit was identified as abscisyl-β-d-glucopyranoside (ABA-GE) based on (a) immunological cross-reactivity, (b) thin layer chromatography co-chromatography with authentic standards in two solvent systems, (c) susceptibility to both chemical and enzymic degradation, and (d) mass spectroscopy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer G. L., Zeevaart J. A. Isolation and Quantitation of beta-d-Glucopyranosyl Abscisate from Leaves of Xanthium and Spinach. Plant Physiol. 1982 Jul;70(1):227–231. doi: 10.1104/pp.70.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisker H. E., Goldschmidt E. E., Goren R. Ethylene-induced Formation of ABA in Citrus Peel as Related to Chloroplast Transformations. Plant Physiol. 1976 Sep;58(3):377–379. doi: 10.1104/pp.58.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E. E., Goren R., Even-Chen Z., Bittner S. Increase in Free and Bound Abscisic Acid during Natural and Ethylene-induced Senescence of Citrus Fruit Peel. Plant Physiol. 1973 May;51(5):879–882. doi: 10.1104/pp.51.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Jones W. W., Coggins C. W., Embleton T. W. Endogenous abscisic Acid in relation to bud growth in alternate bearing ;valencia' orange. Plant Physiol. 1976 Nov;58(5):681–682. doi: 10.1104/pp.58.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi K. V., Sardeshpande P. D., Jalnapurkar B. V., Ajinkya S. M. A case of uterine adenocarcinoma in dog. Indian Vet J. 1967 Feb;44(2):114–116. [PubMed] [Google Scholar]
- Lewis L. N., Khalifah R. A., Coggins C. W. Seasonal Changes in Citrus Auxin and 2 Auxin Antagonists as Related to Fruit Development. Plant Physiol. 1965 May;40(3):500–505. doi: 10.1104/pp.40.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengelly W. L., Bandurski R. S., Schulze A. Validation of a radioimmunoassay for indole-3-acetic Acid using gas chromatography-selected ion monitoring-mass spectrometry. Plant Physiol. 1981 Jul;68(1):96–98. doi: 10.1104/pp.68.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


