Abstract
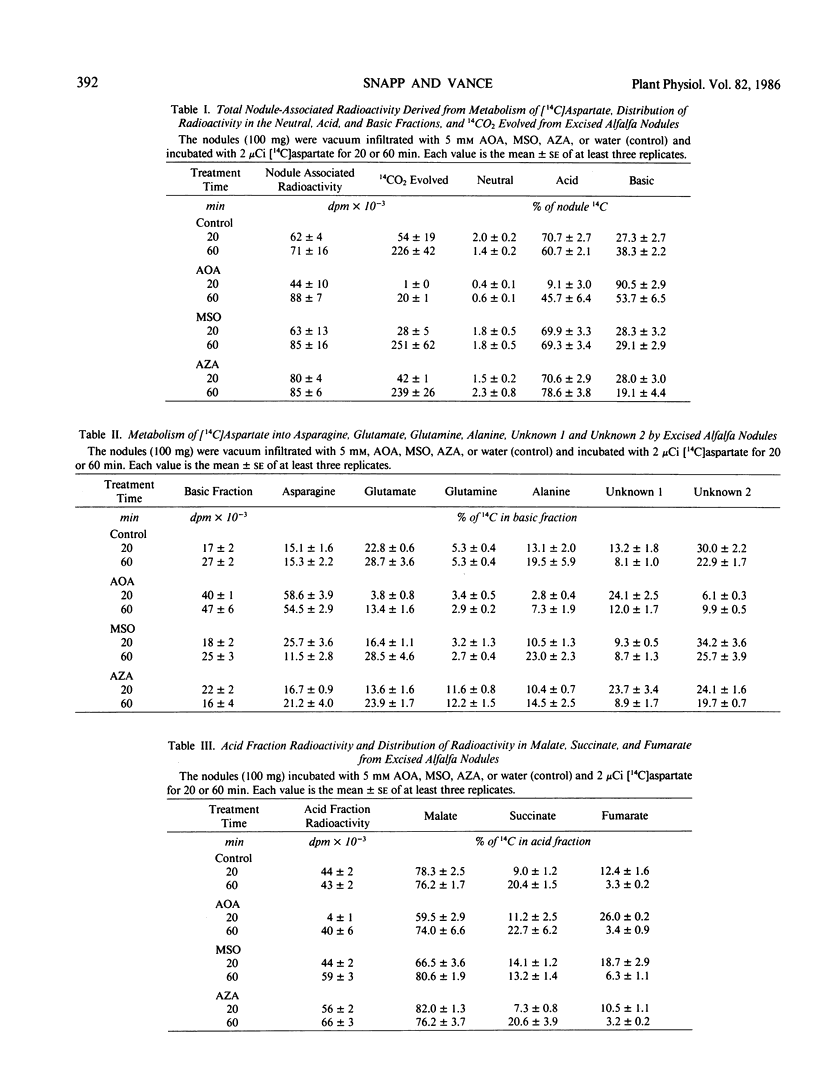
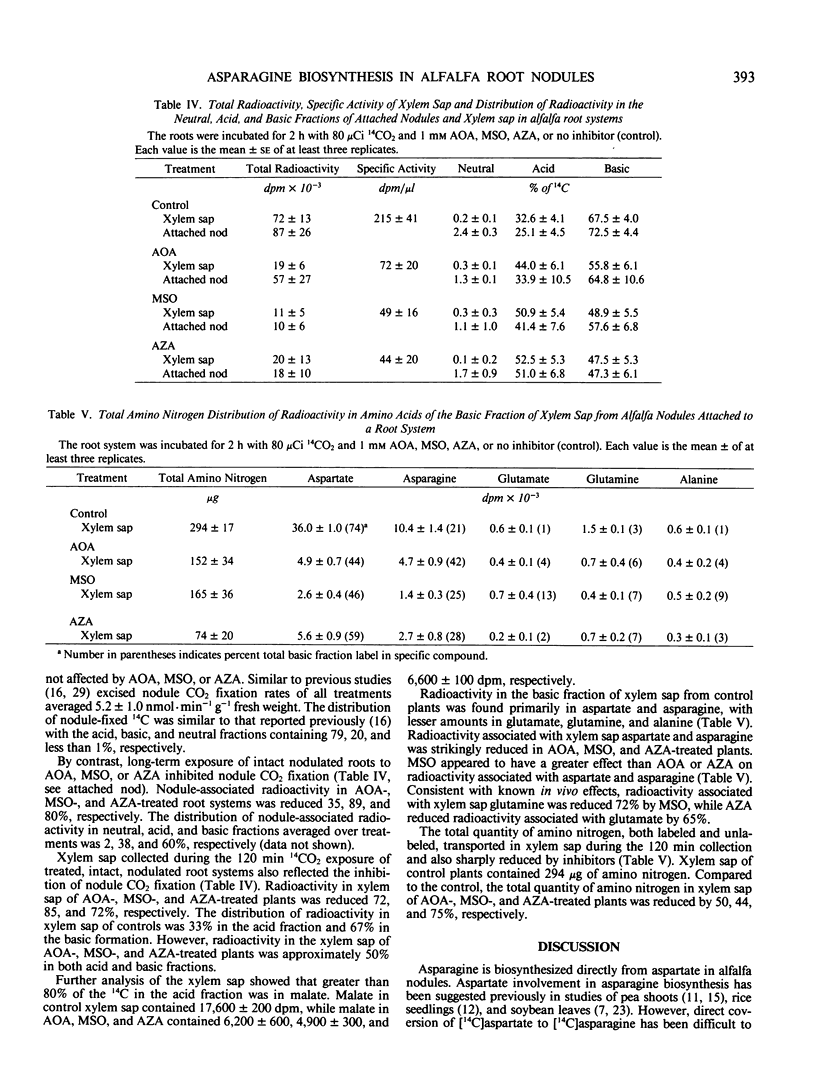
Rapid direct conversion of exogenously supplied [14C]aspartate to [14C] asparagine and to tricarboxylic cycle acids was observed in alfalfa (Medicago sativa L.) nodules. Aspartate aminotransferase activity readily converted carbon from exogenously applied [14C]aspartate into the tricarboxylic acid cycle with subsequent conversion to the organic acids malate, succinate, and fumarate. Aminooxyacetate, an inhibitor of aminotransferase activity, reduced the flow of carbon from [14C]aspartate into tricarboxylic cycle acids and decreased 14CO2 evolution by 99%. Concurrently, maximum conversion of aspartate to asparagine was observed in aminooxyacetate treated nodules (30 nanomoles asparagine per gram fresh weight per hour. Metabolism of [14C]aspartate and distribution of nodulefixed 14CO2 suggest that two pools of aspartate occur in alfalfa nodules: (a) one involved in asparagine biosynthesis, and (b) another supplying a malate/aspartate shuttle. Conversion of [14C]aspartate to [14C]asparagine was not inhibited by methionine sulfoximine, a glutamine synthetase inhibitor, or azaserine, a glutmate synthetase, inhibitor. The data did not indicate that asparagine biosynthesis in alfalfa nodules has an absolute requirement for glutamine. Radioactivity in the xylem sap, derived from nodule 14CO2 fixation, was markedly decreased by treating nodulated roots with aminooxyacetate, methionine sulfoximine, and azaserine. Inhibitors decreased the [14C]aspartate and [14]asparagine content of xylem sap by greater than 80% and reduced the total amino nitrogen content of xylem sap (including nonradiolabeled amino acids) by 50 to 80%. Asparagine biosynthesis in alfalfa nodules and transport in xylem sap are dependent upon continued aminotransferase activity and an uninterrupted assimilation of ammonia via the glutamine synthetase/glutamate synthase pathway. Continued assimilation of ammonia apparently appears crucial to continued root nodule CO2 fixation in alfalfa.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeller J. T., Laing W. A., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: I. Characterization, Association with Phosphoenolpyruvate Carboxylase, and Correlation with Nitrogen Fixation during Nodule Development. Plant Physiol. 1977 Jul;60(1):47–50. doi: 10.1104/pp.60.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schubert K. R. Carbon Dioxide Fixation in Soybean Roots and Nodules: I. CHARACTERIZATION AND COMPARISON WITH N(2) FIXATION AND COMPOSITION OF XYLEM EXUDATE DURING EARLY NODULE DEVELOPMENT. Plant Physiol. 1981 Apr;67(4):691–696. doi: 10.1104/pp.67.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. H., Schrader L. E., Miller M. G. Low Temperature Effects on Soybean (Glycine max [L.] Merr. cv. Wells) Free Amino Acid Pools during Germination. Plant Physiol. 1978 Oct;62(4):642–647. doi: 10.1104/pp.62.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentem P. A., Lea P. J., Stewart G. R. Action of Inhibitors of Ammonia Assimilation on Amino Acid Metabolism in Hordeum vulgare L. (cv Golden Promise). Plant Physiol. 1983 Mar;71(3):502–506. doi: 10.1104/pp.71.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. A., Streeter J. G. Asparagine biosynthesis in soybean nodules. Plant Physiol. 1984 Mar;74(3):605–610. doi: 10.1104/pp.74.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W., Ireland R. J., Lea P. J. Asparagine synthesis in pea leaves, and the occurrence of an asparagine synthetase inhibitor. Plant Physiol. 1983 Sep;73(1):165–168. doi: 10.1104/pp.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A., Christeller J. T., Sutton W. D. Carbon Dioxide Fixation by Lupin Root Nodules: II. Studies with C-labeled Glucose, the Pathway of Glucose Catabolism, and the Effects of Some Treatments That Inhibit Nitrogen Fixation. Plant Physiol. 1979 Mar;63(3):450–454. doi: 10.1104/pp.63.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K. Synthesis and Interconversion of Amino Acids in Developing Cotyledons of Pea (Pisum sativum L.). Plant Physiol. 1977 Sep;60(3):344–348. doi: 10.1104/pp.60.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus L. M. Glutamine binding sites. Methods Enzymol. 1977;46:414–427. doi: 10.1016/s0076-6879(77)46049-x. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Allantoin and Allantoic Acid in Tissues and Stem Exudate from Field-grown Soybean Plants. Plant Physiol. 1979 Mar;63(3):478–480. doi: 10.1104/pp.63.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. Asparaginase and asparagine transaminase in soybean leaves and root nodules. Plant Physiol. 1977 Aug;60(2):235–239. doi: 10.1104/pp.60.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Transport, metabolism, and redistribution of xylem-borne amino acids in developing pea shoots. Plant Physiol. 1982 May;69(5):1226–1232. doi: 10.1104/pp.69.5.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Boylan K. L., Maxwell C. A., Heichel G. H., Hardman L. L. Transport and Partitioning of CO(2) Fixed by Root Nodules of Ureide and Amide Producing Legumes. Plant Physiol. 1985 Aug;78(4):774–778. doi: 10.1104/pp.78.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Heichel G. H., Barnes D. K., Bryan J. W., Johnson L. E. Nitrogen Fixation, Nodule Development, and Vegetative Regrowth of Alfalfa (Medicago sativa L.) following Harvest. Plant Physiol. 1979 Jul;64(1):1–8. doi: 10.1104/pp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


