Abstract
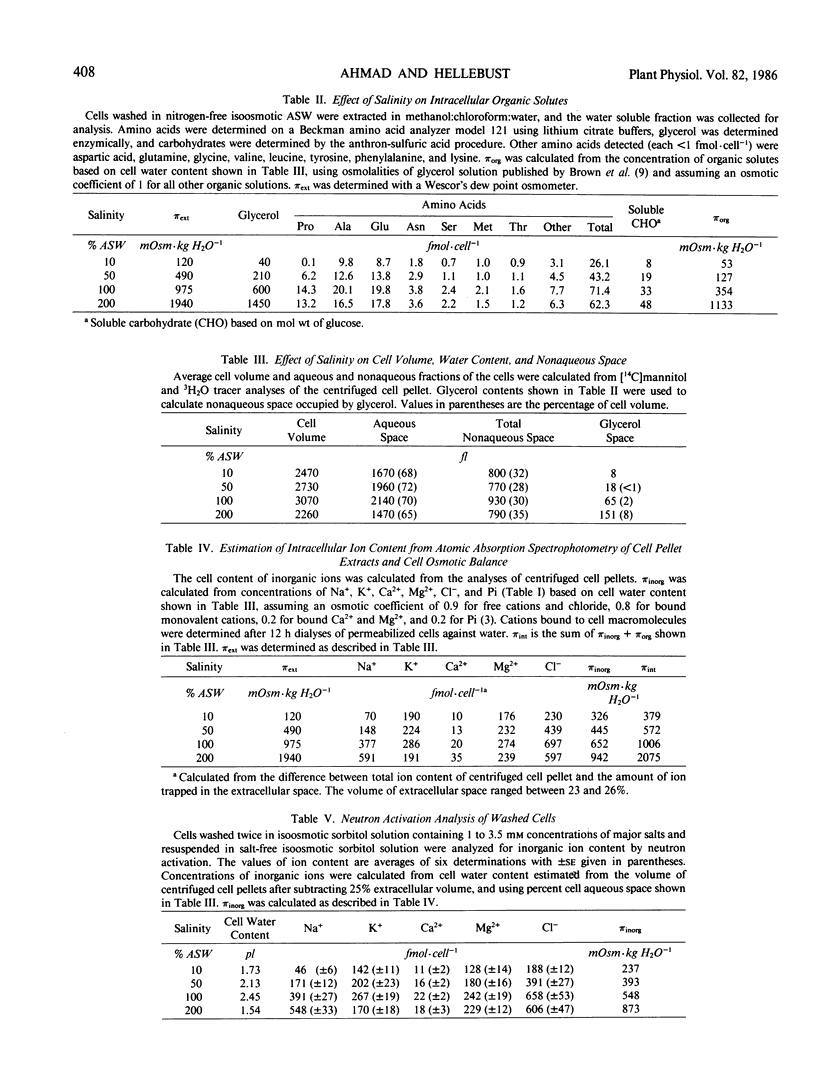
The green euryhaline flagellate Chlamydomonas pulsatilla Wollenweber, isolated from a coastal marine environment, was grown exponentially over the salinity range of 10 to 200% artificial seawater (ASW). The cellular volume and aqueous space of the alga, measured by [14C] mannitol and 3H2O tracer analyses of centrifuged cell pellets, ranged between 2.3 and 3.1 picoliters and between 1.5 and 2.1 picoliters, respectively. The nonaqueous space determined in those analyses (28-35%) was consistent with the cell composition of the alga. The glycerol content of the alga increased almost linearly with increasing salinity; its contribution to intracellular osmolality at 200% ASW was about 57%. The contribution of amino acids and soluble carbohydrates to the cell osmotic balance was small. Intracellular ion concentrations determined by analyzing centrifuged cell pellets of known [14C]mannitol space by atomic absorption spectrophotometry, and by neutron activation analyses of washed cells were similar. At 10% ASW, potassium and magnesium were the major cations, and chloride and phosphate were the major anions. The sodium and chloride content of the alga increased with increasing salinity; at 200% ASW the intracellular concentration of both sodium and chloride was about 400 millimolar. The intracellular osmolality (πint) matched closely the external osmolality (πext) over the entire salinity range except at 10% ASW where πint exceeded πext by 120 to 270 milliosmoles per kilogram H2O.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Hellebust J. A. Nitrogen Metabolism of the Marine Microalga Chlorella autotrophica. Plant Physiol. 1984 Nov;76(3):658–663. doi: 10.1104/pp.76.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I., Hellebust J. A. Osmoregulation in the Extremely Euryhaline Marine Micro-Alga Chlorella autotrophica. Plant Physiol. 1984 Apr;74(4):1010–1015. doi: 10.1104/pp.74.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Brown A. D. The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol. 1974 Mar 1;96(1):37–52. doi: 10.1007/BF00590161. [DOI] [PubMed] [Google Scholar]
- Katz A., Avron M. Determination of intracellular osmotic volume and sodium concentration in dunaliella. Plant Physiol. 1985 Aug;78(4):817–820. doi: 10.1104/pp.78.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M., Thompson G. A., Jr On the mechanism of rapid plasma membrane and chloroplast envelope expansion in Dunaliella salina exposed to hypoosmotic shock. J Cell Biol. 1986 Jan;102(1):289–297. doi: 10.1083/jcb.102.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]


