Abstract
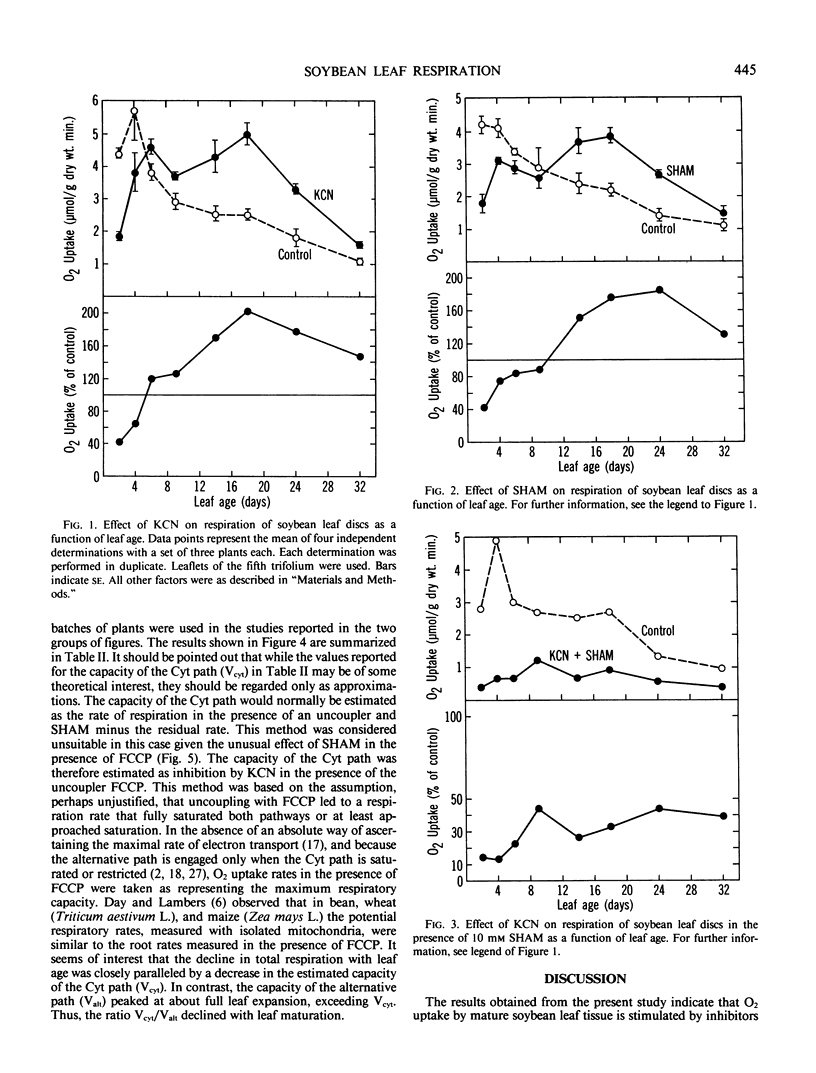
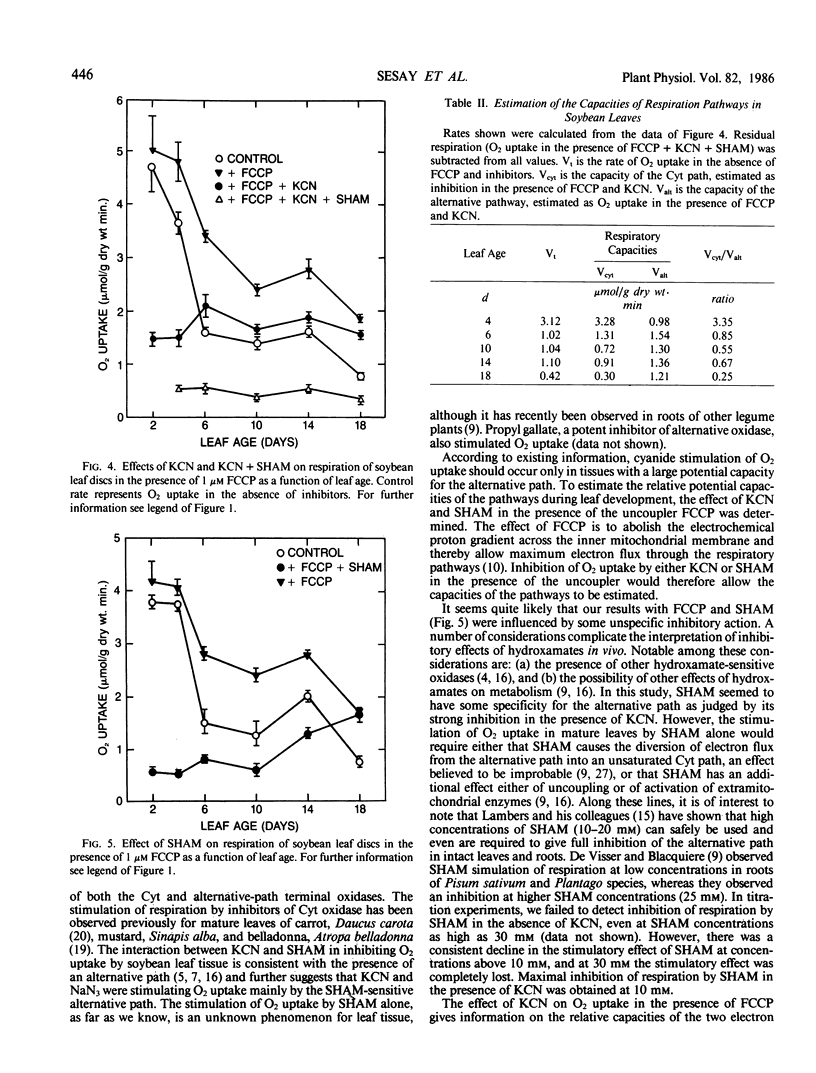
Measurements of respiration were made on leaf discs from glasshouse-grown soybean (Glycine max [L.] Merr. cv `Corsoy') plants in the presence and absence of cyanide (KCN) and salicylhydroxamic acid (SHAM). O2 uptake by mature leaves measured at 25°C was stimulated by 1 millimolar KCN (63%) and also by 5 millimolar azide (79%). SHAM, an inhibitor of the alternative oxidase and a selection of other enzymes, also stimulated O2 uptake by itself at concentration of 10 millimolar. However, in combination, KCN and SHAM were inhibitory. The rate of O2 uptake declined consistently with leaf age. The stimulation of O2 uptake by KCN and by SHAM occurred only after a certain stage of leaf development had been reached and was more pronounced in fully expanded leaves. In young leaves, O2 uptake was inhibited by both KCN and SHAM individually. The uncoupler, p-trifluoromethoxy carbonylcyanide phenylhydrazone, stimulated leaf respiration at all ages studied, the stimulation being more pronounced in fully expanded leaves. The uncoupled rate was inhibited by KCN and SHAM individually. The capacity of the cytochrome path declined with leaf age, paralleling the decline in total respiration. However, the capacity of the alternative path peaked at about full leaf expansion, exceeding the cytochrome capacity and remaining relatively constant. These results are consistent with the presence in soybean leaves of an alternative path capacity that seems to increase with age, and they suggest that the stimulation of O2 uptake by KCN and NaN3 in mature leaves was mainly by the SHAM-sensitive alternative path. The stimulation of O2 uptake by SHAM was not expected, and the reason for it is not clear.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahr J. T., Bonner W. D., Jr Cyanide-insensitive respiration. II. Control of the alternate pathway. J Biol Chem. 1973 May 25;248(10):3446–3450. [PubMed] [Google Scholar]
- Bishop P. D., Atkinson D. E. Adenine nucleotide control of the rate of oxygen uptake by rat heart mitochondria over a 15- to 20-fold range. Arch Biochem Biophys. 1984 Apr;230(1):335–344. doi: 10.1016/0003-9861(84)90116-4. [DOI] [PubMed] [Google Scholar]
- Day D. A., De Vos O. C., Wilson D., Lambers H. Regulation of Respiration in the Leaves and Roots of Two Lolium perenne Populations with Contrasting Mature Leaf Respiration Rates and Crop Yields. Plant Physiol. 1985 Aug;78(4):678–683. doi: 10.1104/pp.78.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant N. G., Hommersand M. H. The Respiratory Chain of Chlorella protothecoides: I. Inhibitor Responses and Cytochrome Components of Whole Cells. Plant Physiol. 1974 Jul;54(1):50–56. doi: 10.1104/pp.54.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubec T. C., Robinson J. M., Donaldson R. P. Isolation of mitochondria from soybean leaves on discontinuous percoll gradients. Plant Physiol. 1985 Apr;77(4):1010–1012. doi: 10.1104/pp.77.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. G., Obendorf R. L. Use of Tetraethylthiuram Disulfide to Discriminate between Alternative Respiration and Lipoxygenase. Plant Physiol. 1981 May;67(5):962–964. doi: 10.1104/pp.67.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow J. N., Girvin M. E. Alternative Respiratory Pathway: ITS ROLE IN SEED RESPIRATION AND ITS INHIBITION BY PROPYL GALLATE. Plant Physiol. 1980 Apr;65(4):669–674. doi: 10.1104/pp.65.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Kremer D. F., Lee D. R. Carbon assimilation and translocation in soybean leaves at different stages of development. Plant Physiol. 1978 Jul;62(1):54–58. doi: 10.1104/pp.62.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M. Photosynthetic & respiratory activities of growing pea leaves. Plant Physiol. 1962 Nov;37(6):716–721. doi: 10.1104/pp.37.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallihan E. F. Modification and Use of an Electric Hygrometer for Estimating Relative Stomatal Apertures. Plant Physiol. 1964 Jan;39(1):86–90. doi: 10.1104/pp.39.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Day D. A. Malate oxidation, rotenone-resistance, and alternative path activity in plant mitochondria. Plant Physiol. 1982 Oct;70(4):959–964. doi: 10.1104/pp.70.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yentur S., Leopold A. C. Respiratory Transition during Seed Germination. Plant Physiol. 1976 Feb;57(2):274–276. doi: 10.1104/pp.57.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser R., Blacquière T. Inhibition and stimulation of root respiration in pisum and plantago by hydroxamate : its consequences for the assessment of alternative path activity. Plant Physiol. 1984 Jul;75(3):813–817. doi: 10.1104/pp.75.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]


