Abstract
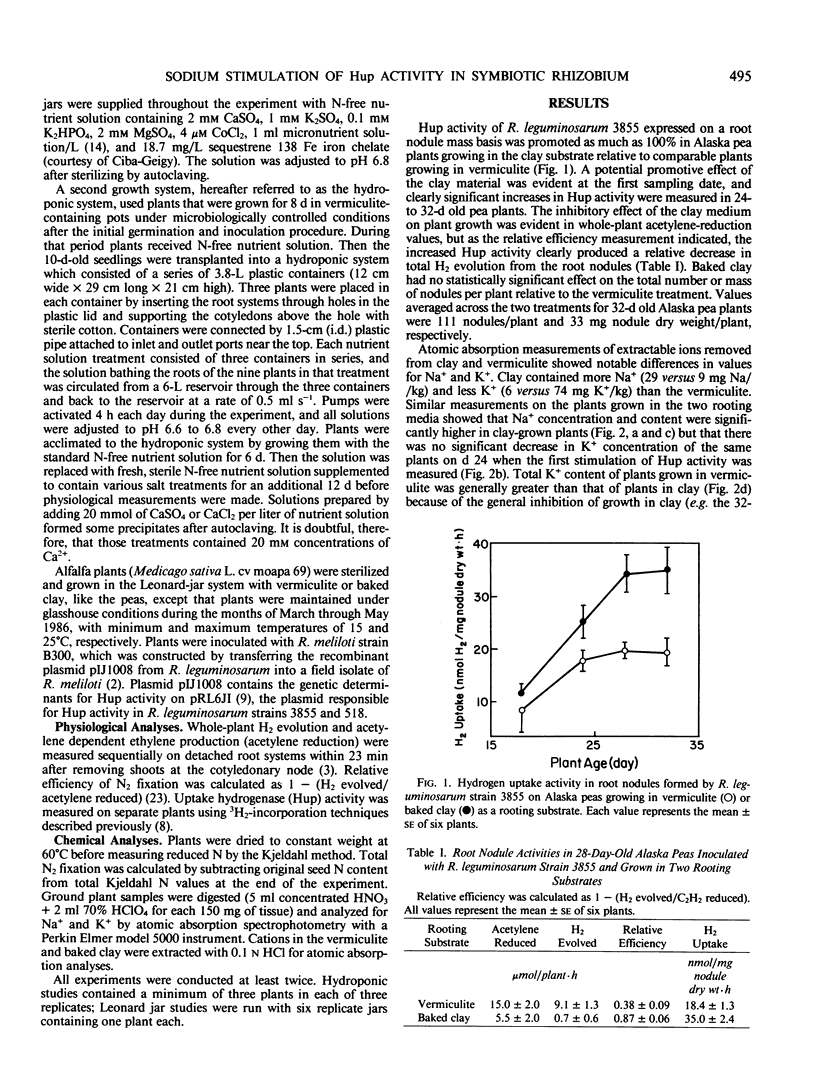
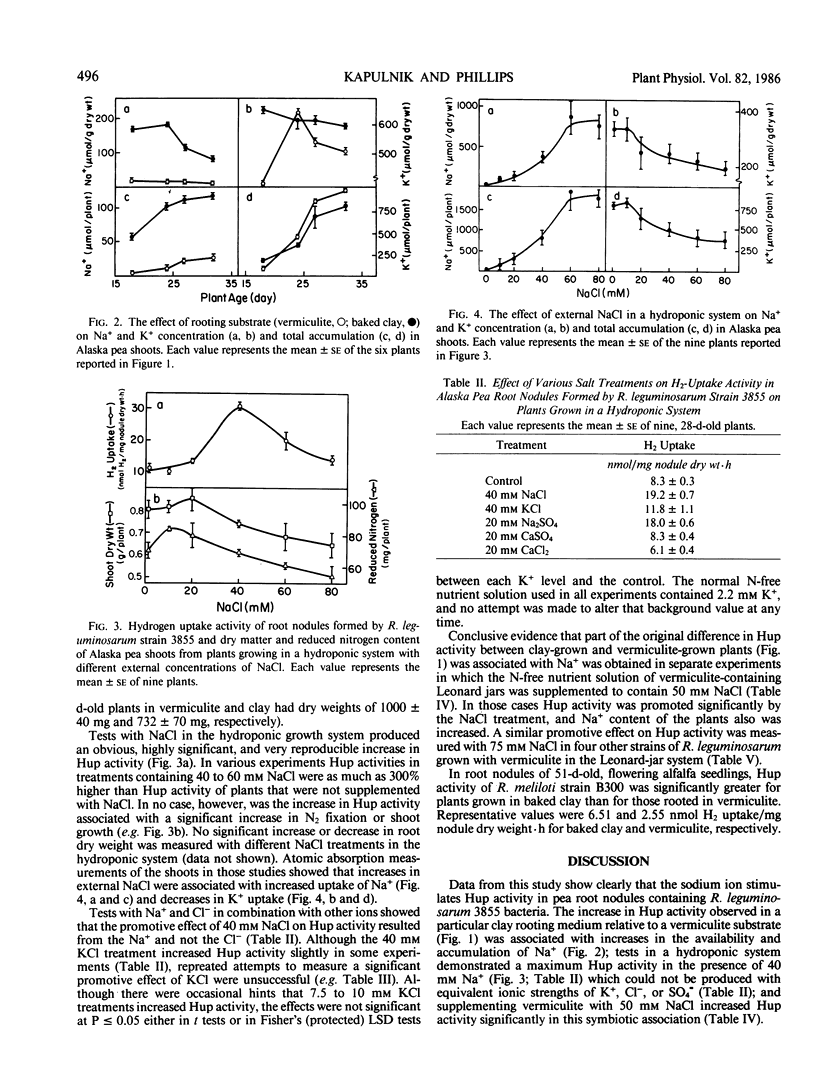
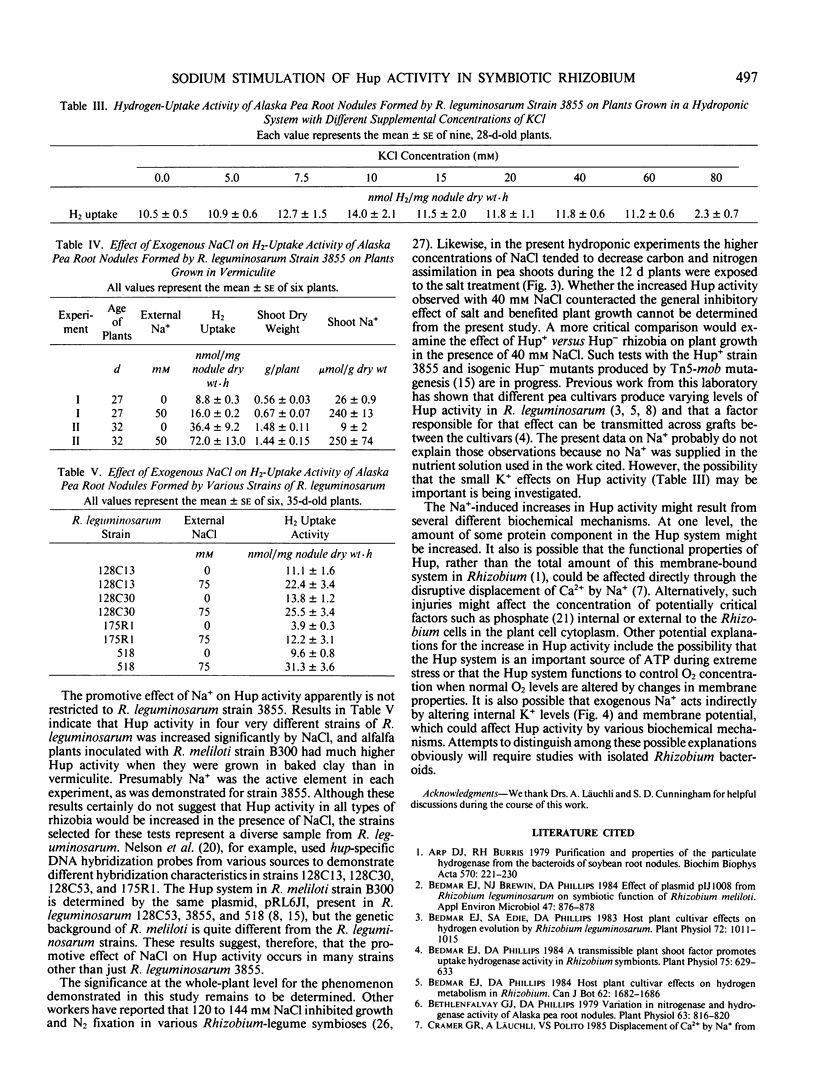
Initial observations showed a 100% increase in H2-uptake (Hup) activity of Rhizobium leguminosarum strain 3855 in pea root nodules (Pisum sativum L. cv Alaska) on plants growing in a baked clay substrate relative to those growing in vermiculite, and an investigation of nutrient factors responsible for the phenomenon was initiated. Significantly greater Hup activity was first measured in the clay-grown plants 24 days after germination, and higher activity was maintained relative to the vermiculite treatment until experiments were terminated at day 32. The increase in Hup activity was associated with a decrease in H2 evolution for plants with comparable rates of acetylene reduction. Analyses of the clay showed that it contained more Na+ (29 versus 9 milligrams per kilogram) and less K+ (6 versus 74 milligrams per kilogram) than the vermiculite. Analyses of plants, however, showed a large increase in Na+ concentration of clay-grown plants with a much smaller reduction in K+ concentration. In tests with the same organisms in a hydroponic system with controlled pH, 40 millimolar NaCl increased Hup activity more than 100% over plants grown in solutions lacking NaCl. Plants with increased Hup activity, however, did not have greater net carbon or total nitrogen assimilation. KCl treatments from 5 to 80 millimolar produced slight increased in Hup activity at 10 millimolar KCl, and tests with other salts in the hydroponic system indicated that only Na+ strongly promoted Hup activity. Treating vermiculite with 50 millimolar NaCl increased Na+ concentration in pea plant tissue and greatly promoted Hup activity of root nodules in a manner analogous to the original observation with the clay rooting medium. A wider generality of the phenomenon was suggested by demonstrating that exogenous Na+ increased Hup activity of other R. leguminosarum strains and promoted Hup activity of R. meliloti strain B300 in alfalfa (Medicago sativa L.).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Bedmar E. J., Brewin N. J., Phillips D. A. Effect of Plasmid pIJ1008 from Rhizobium leguminosarum on Symbiotic Function of Rhizobium meliloti. Appl Environ Microbiol. 1984 Apr;47(4):876–878. doi: 10.1128/aem.47.4.876-878.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedmar E. J., Edie S. A., Phillips D. A. Host Plant Cultivar Effects on Hydrogen Evolution by Rhizobium leguminosarum. Plant Physiol. 1983 Aug;72(4):1011–1015. doi: 10.1104/pp.72.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedmar E. J., Phillips D. A. A transmissible plant shoot factor promotes uptake hydrogenase activity in Rhizobium symbionts. Plant Physiol. 1984 Jul;75(3):629–633. doi: 10.1104/pp.75.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Variation in nitrogenase and hydrogenase activity of alaska pea root nodules. Plant Physiol. 1979 May;63(5):816–820. doi: 10.1104/pp.63.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S. D., Kapulnik Y., Brewin N. J., Phillips D. A. Uptake Hydrogenase Activity Determined by Plasmid pRL6JI in Rhizobium leguminosarum Does Not Increase Symbiotic Nitrogen Fixation. Appl Environ Microbiol. 1985 Oct;50(4):791–794. doi: 10.1128/aem.50.4.791-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejong T. M., Phillips D. A. Nitrogen Stress and Apparent Photosynthesis in Symbiotically Grown Pisum sativum L. Plant Physiol. 1981 Aug;68(2):309–313. doi: 10.1104/pp.68.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in legume root nodule bacteroids: occurrence and properties. Arch Mikrobiol. 1972;85(3):193–201. doi: 10.1007/BF00408844. [DOI] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Linker C. S., Benoit A. G., Jardetzky O., Nieman R. H. Salt stimulation of phosphate uptake in maize root tips studied by p nuclear magnetic resonance. Plant Physiol. 1984 Aug;75(4):947–950. doi: 10.1104/pp.75.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F. B., Burris R. H. A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science. 1984 Jun 8;224(4653):1095–1097. doi: 10.1126/science.6585956. [DOI] [PubMed] [Google Scholar]


