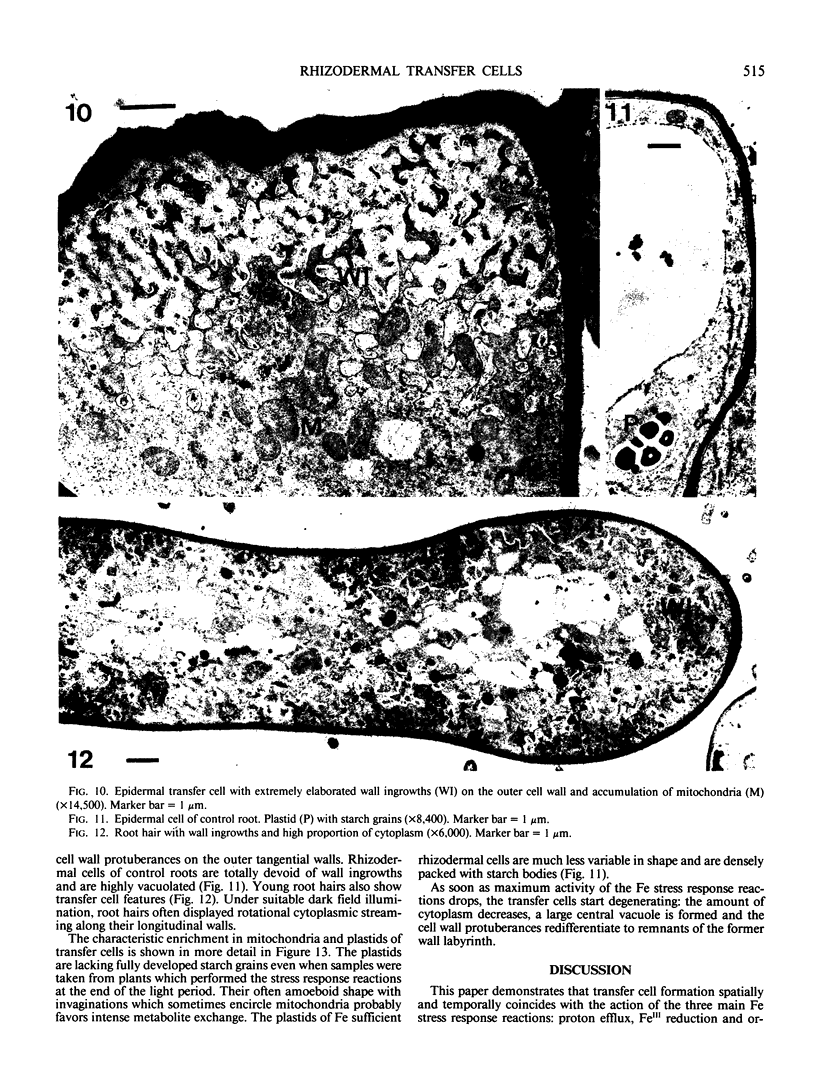
Abstract
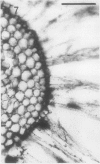
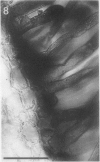
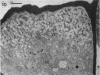
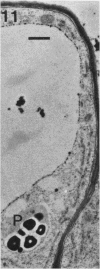
A variety of red pepper (Capsicum annuum L., cv Yaglik) responds to Fe deficiency stress with simultaneously enhanced H+ extrusion, reduction of ferric ions and synthesis of malic and citric acid in a swollen subapical root zone densely covered with root hairs. It is demonstrated that these stress responses temporally coincide with the development of rhizodermal and hypodermal transfer cells in this root zone. During stress response the transfer cells show a marked autofluorescence which could arise from endogenous iron chelators of the phenolic acid type. The presence of organelle-rich cytoplasm which often exhibits rotational cytoplasmic streaming points to high physiological activity and makes these cells, with their increased plasmalemma surface, particularly well suited for the entire stress response mechanism. Since Fe stress-induced acidification is diminished by vanadate and erythrosin B, both specific inhibitors of plasmalemma ATPases, it seems reasonable to suppose that H+ pumping from transfer cells is activated by an ATPase located in their plasmamembrane. H+ extrusion is also shown to be inhibited by abscisic acid. Raised phosphoenolpyruvate carboxylase activity and simultaneous accumulation of malate in the swollen root zone point to the action of a pH stat preventing a detrimental rise in cytoplasmic pH of transfer cells during enhanced H+ extrusion. The simultaneous increase in citric acid concentration favors chelation of iron at the site of its uptake and thus ensures long distance transport to the areas of metabolic demand. A direct link between citrate accumulation and ferric ion reduction as proposed in recent literature further supports the crucial role of transfer cells in the response to Fe deficiency stress.
Full text
PDF
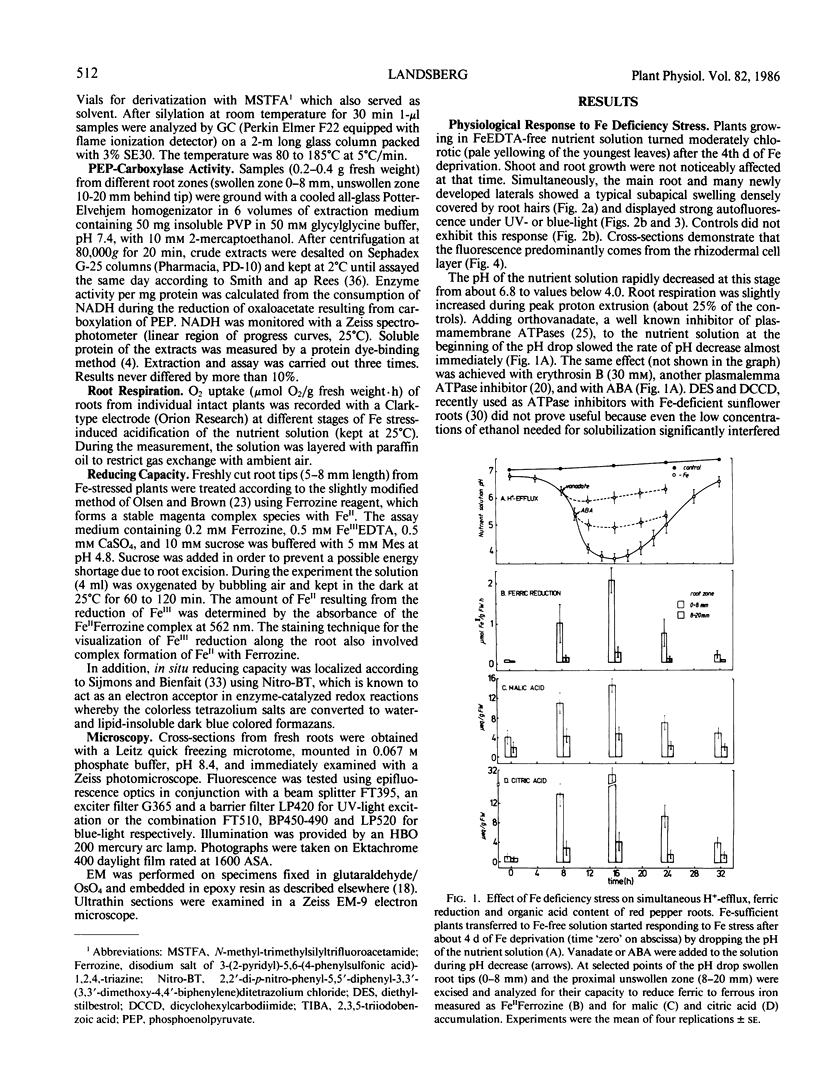
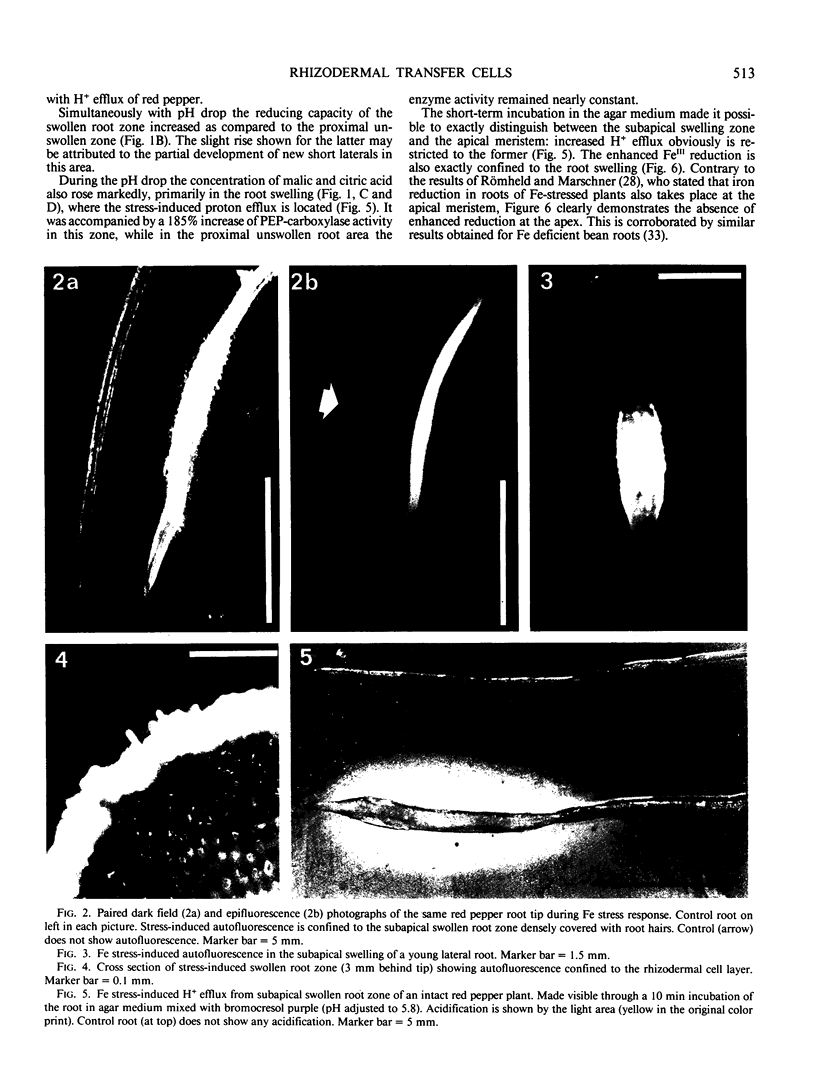
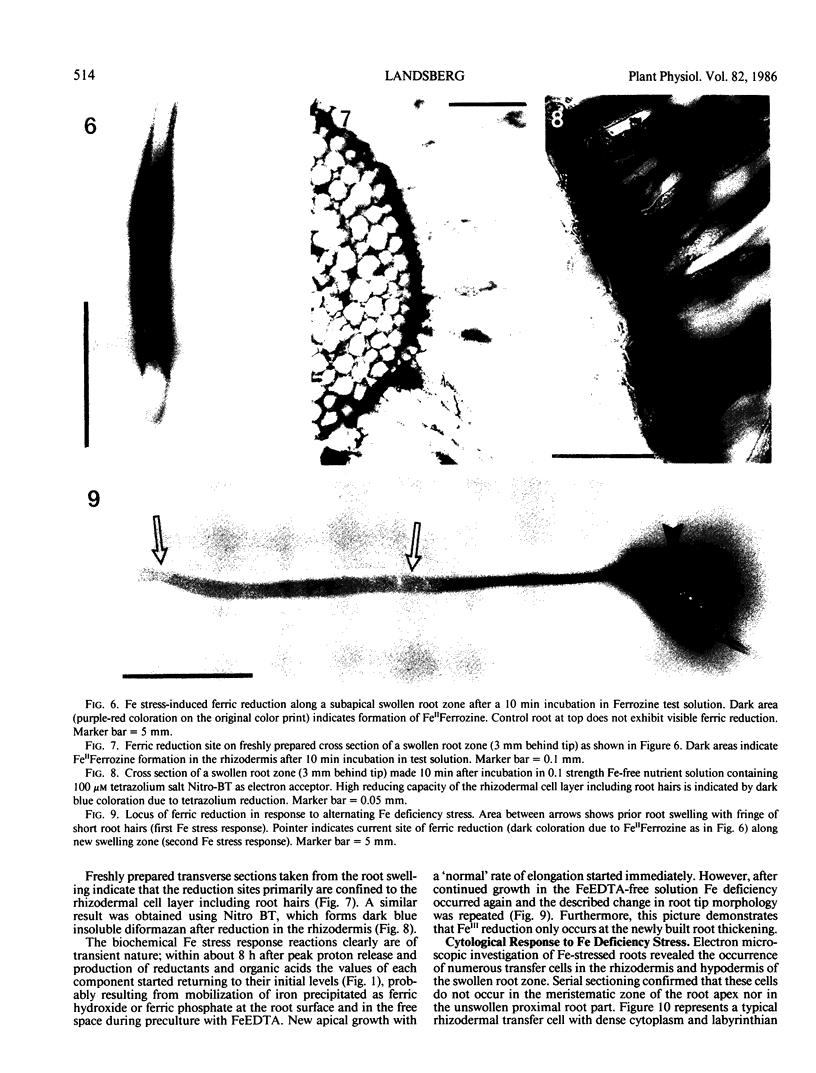


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON J. S., DEKOCK P. C., PALMER M. J. Aconitase levels in the leaves of iron-deficient mustard plants (Sinapis alba). Biochem J. 1961 Jul;80:64–70. doi: 10.1042/bj0800064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fekete F. A., Spence J. T., Emery T. A rapid and sensitive paper electrophoresis assay for the detection of microbial siderophores elicited in solid-plating culture. Anal Biochem. 1983 Jun;131(2):516–519. doi: 10.1016/0003-2697(83)90207-5. [DOI] [PubMed] [Google Scholar]
- Gabathuler R., Cleland R. E. Auxin regulation of a proton translocating ATPase in pea root plasma membrane vesicles. Plant Physiol. 1985 Dec;79(4):1080–1085. doi: 10.1104/pp.79.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Spanswick R. M. Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol. 1984 Jul;75(3):586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Marschner H. Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol. 1986 Jan;80(1):175–180. doi: 10.1104/pp.80.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Marschner H. Mechanism of iron uptake by peanut plants : I. Fe reduction, chelate splitting, and release of phenolics. Plant Physiol. 1983 Apr;71(4):949–954. doi: 10.1104/pp.71.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Müller C., Marschner H. Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol. 1984 Nov;76(3):603–606. doi: 10.1104/pp.76.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijmons P. C., Lanfermeijer F. C., de Boer A. H., Prins H. B., Bienfait H. F. Depolarization of Cell Membrane Potential during Trans-Plasma Membrane Electron Transfer to Extracellular Electron Acceptors in Iron-Deficient Roots of Phaseolus vulgaris L. Plant Physiol. 1984 Dec;76(4):943–946. doi: 10.1104/pp.76.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant R. P. Dose-independent clearance rates of blood ethanol in the rat. Naturwissenschaften. 1978 Jun;65(6):339–340. doi: 10.1007/BF00368379. [DOI] [PubMed] [Google Scholar]
- Tiffin L. O. Iron Translocation II. Citrate/Iron Ratios in Plant Stem Exudates. Plant Physiol. 1966 Mar;41(3):515–518. doi: 10.1104/pp.41.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]