Abstract
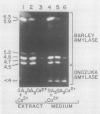
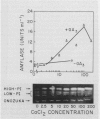
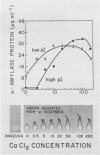
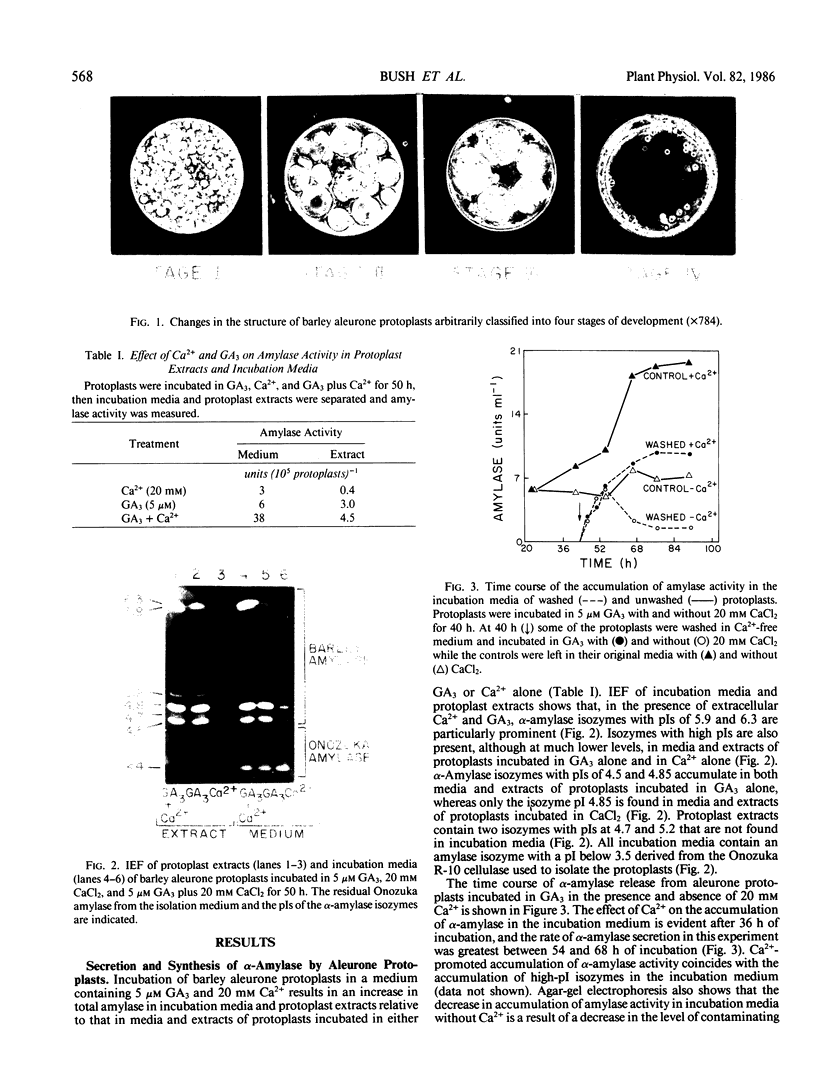
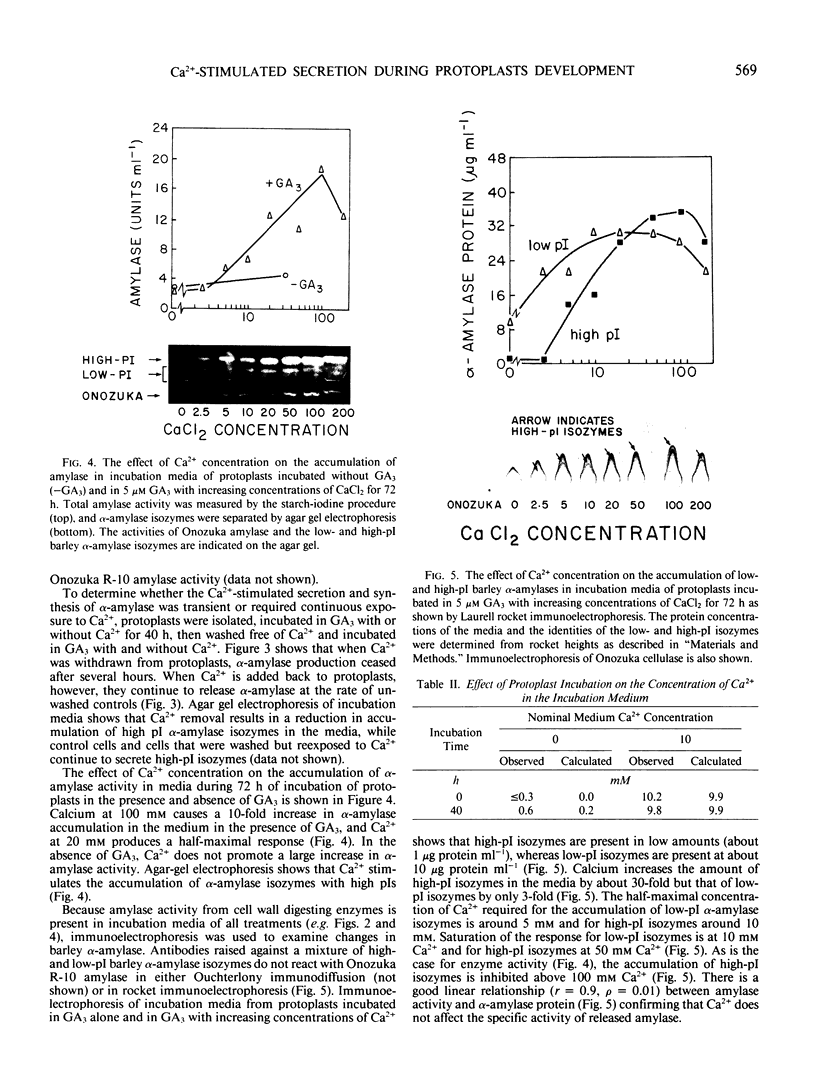
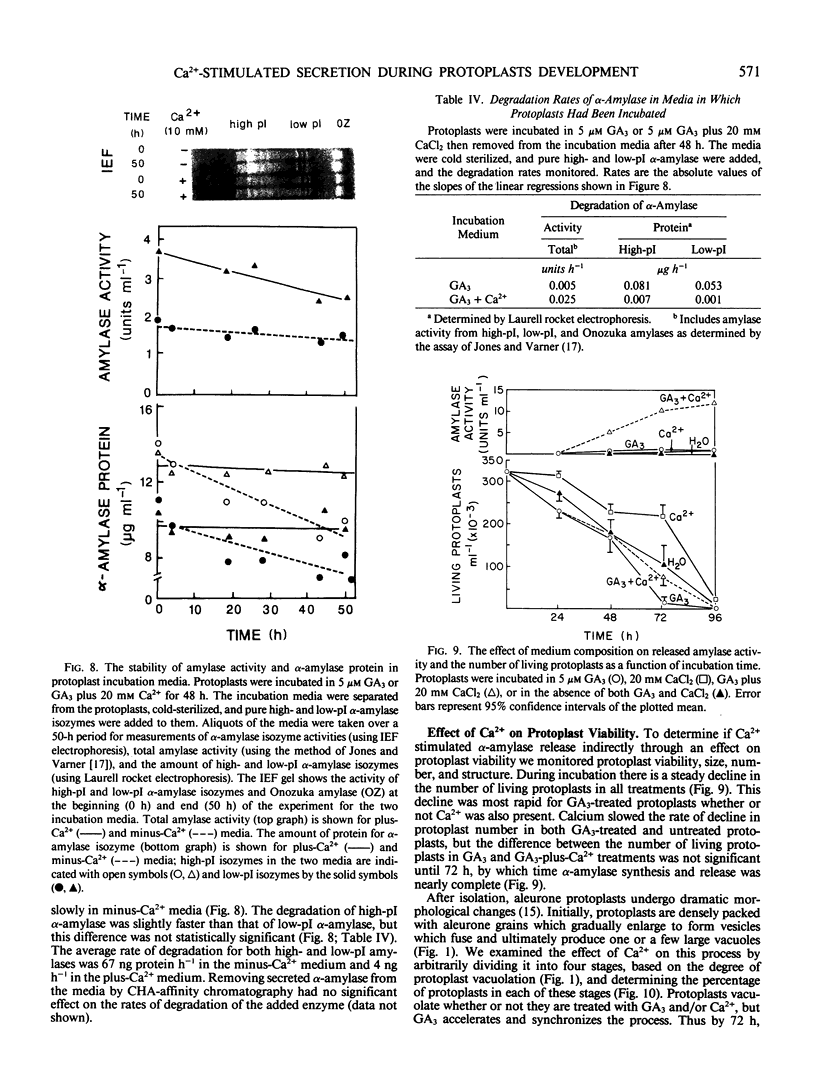
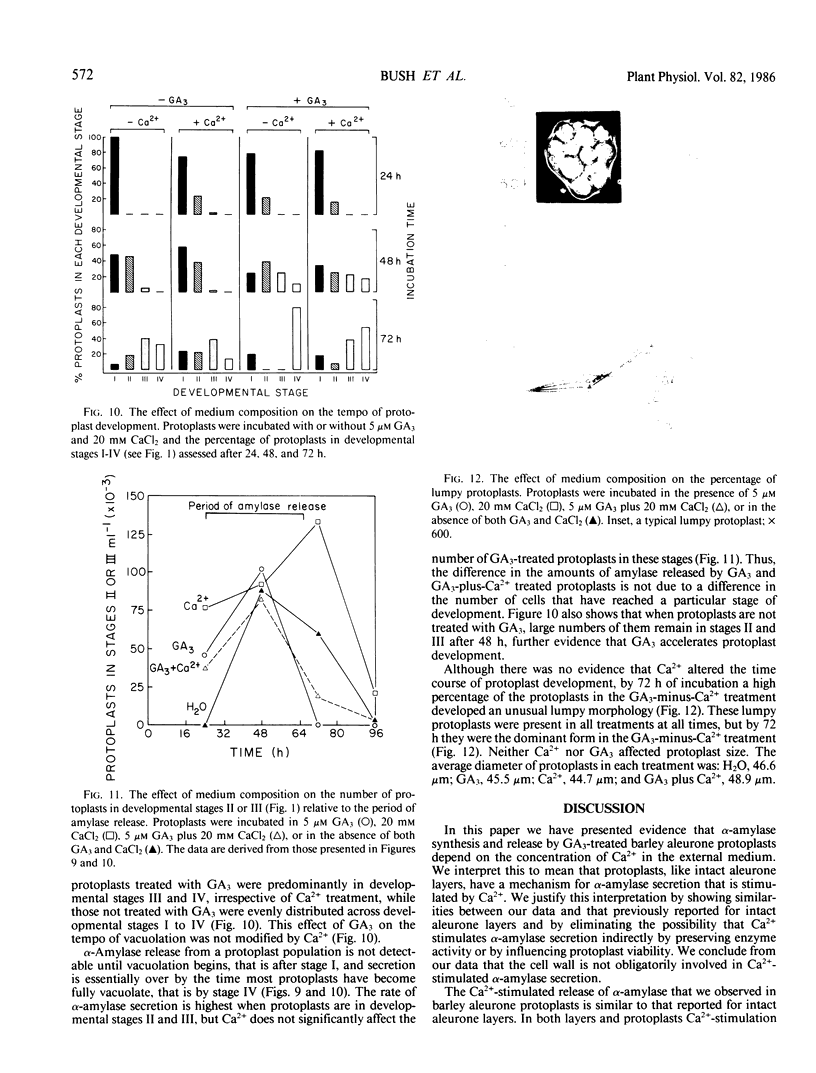
The effects of gibberellic acid (GA3) and Ca2+ on the synthesis and secretion of α-amylase from protoplasts of barley (Hordeum vulgare L. cv Himalaya) aleurone were studied. Protoplasts undergo dramatic morphological changes whether or not the incubation medium contains GA3, CaCl2, or both. Incubation of protoplasts in medium containing both GA3 and Ca2+, however, causes an increase in the α-amylase activity of both incubation medium and tissue extract relative to controls incubated in GA3 or Ca2+ alone. Isoelectric focusing shows that adding Ca2+ to incubation media containing GA3 increases the levels of α-amylase isozymes having high isoelectric points (pI). In the presence of GA3 alone, only isozymes with low pIs accumulate. The increase in α-amylase activity in the incubation medium begins after 36 hours of incubation, and secretion is complete after about 72 hours. Protoplasts require continuous exposure to Ca2+ to maintain elevated levels of α-amylase release. Immunoelectrophoresis shows that Ca2+ stimulates the release of low-pI α-amylase isozymes by 3-fold and high-pI isozymes by 30-fold over controls incubated in GA3 alone. Immunochemical data also show that the half-maximum concentration for this response is between 5 and 10 millimolar CaCl2. The response is not specific for Ca2+ since Sr2+ can substitute, although less effectively than Ca2+. Pulse-labeling experiments show that α-amylase isozymes produced by aleurone protoplasts in response to GA3 and Ca2+ are newly synthesized. The effects of Ca2+ on the process of enzyme synthesis and secretion is not mediated via an effect of this ion on α-amylase stability or on protoplast viability. We conclude that Ca2+ directly affects the process of enzyme synthesis and transport. Experiments with protoplasts also argue against the direct involvement of the cell wall in Ca2+-stimulated enzyme release.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippen R. W., Perrier J. L. The use of neutral red and Evans blue for live-dead determinations of marine plankton (with comments on the use of rotenone for inhibition of grazing). Stain Technol. 1974 Mar;49(2):97–104. doi: 10.3109/10520297409116949. [DOI] [PubMed] [Google Scholar]
- Deikman J., Jones R. L. Control of alpha-amylase mRNA accumulation by gibberellic Acid and calcium in barley aleurone layers. Plant Physiol. 1985 May;78(1):192–198. doi: 10.1104/pp.78.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deikman J., Jones R. L. Regulation of the accumulation of mRNA for alpha-amylase in barley aleurone. Plant Physiol. 1986 Mar;80(3):672–675. doi: 10.1104/pp.80.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Scandalios J. G., Varner J. E. Multiple forms of amylase induced by gibberellic acid in isolated barley aleurone layers. Plant Physiol. 1970 Apr;45(4):367–371. doi: 10.1104/pp.45.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Armstrong J. E. Evidence for osmotic regulation of hydrolytic enzyme production in germinating barley seeds. Plant Physiol. 1971 Aug;48(2):137–142. doi: 10.1104/pp.48.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. L., Carbonell J. Regulation of the synthesis of barley aleurone alpha-amylase by gibberellic Acid and calcium ions. Plant Physiol. 1984 Sep;76(1):213–218. doi: 10.1104/pp.76.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moll B. A., Jones R. L. Alpha-amylase secretion by single barley aleurone layers. Plant Physiol. 1982 Oct;70(4):1149–1155. doi: 10.1104/pp.70.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S. T. Characterization of the murexide method: dual-wavelength spectrophotometry of cations under physiological conditions. Anal Biochem. 1978 Mar;85(1):165–179. doi: 10.1016/0003-2697(78)90287-7. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Mense R. M. Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 1972 Feb;49(2):187–189. doi: 10.1104/pp.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Rocket immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:37–46. doi: 10.1111/j.1365-3083.1973.tb03777.x. [DOI] [PubMed] [Google Scholar]