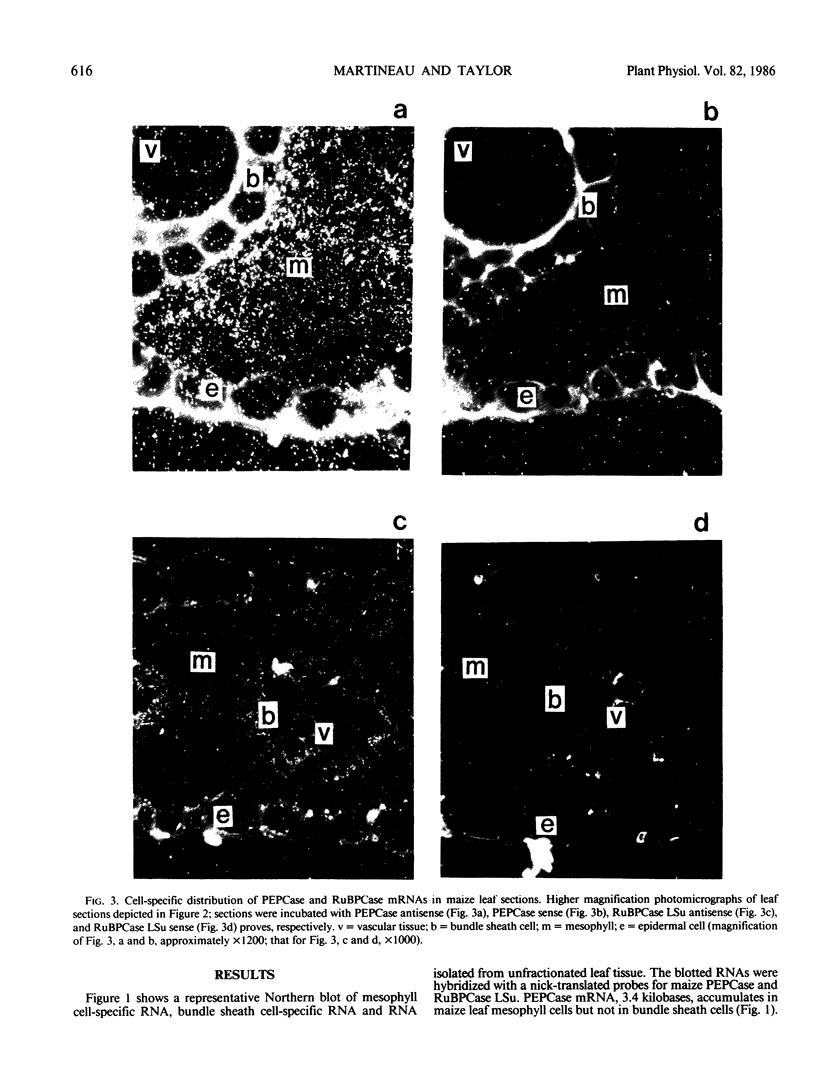
Abstract
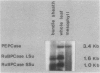
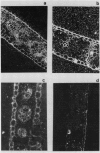
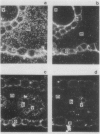
Bundle sheath strands and mesophyll cell extracts have been isolated from maize (Zea mays L.) leaves using a mechanical disruption-filtration technique. Northern blot analysis showed that phosphoenolpyruvate carboxylase (PEPCase; EC 4.1.1.31) mRNA accumulates only in mesophyll cells. The mechanisms regulating the cell-specific expression of this gene must, therefore, be at either the level of RNA transcription or that of mRNA turnover. The first successful application of hybridization to mRNA molecules in photosynthetic plant tissue sections is described. Results obtained from this in situ study corroborate our finding that PEPCase mRNA accumulates only in mesophyll cells as well as the previously reported (Link, G, DM Coen, L Bogorad 1978 Cell 15: 725-731) finding that the accumulation of ribulose 1,5-bisphosphate carboxylase (RuBPCase; EC 4.1.1.39) large subunit mRNA is restricted to bundle sheath cells. Demonstrating the differential accumulation of PEPCase mRNA and RuBPCase mRNA by utilizing the in situ hybridization technique paves the way for its use as a powerful tool in relating cellular differentiation to gene expression during plant development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Bedbrook J. R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M. L., Melis A. Localization of photosynthetic electron transport components in mesophyll and bundle sheath chloroplasts of Zea mays. Arch Biochem Biophys. 1983 Jul 1;224(1):19–28. doi: 10.1016/0003-9861(83)90186-8. [DOI] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpster M. H., Taylor W. C. Maize phosphoenolpyruvate carboxylase. Cloning and characterization of mRNAs encoding isozymic forms. J Biol Chem. 1986 May 5;261(13):6132–6136. [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Link G., Coen D. M., Bogorad L. Differential expression of the gene for the large subunit of ribulose bisphosphate carboxylase in maize leaf cell types. Cell. 1978 Nov;15(3):725–731. doi: 10.1016/0092-8674(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Martineau B., Taylor W. C. Photosynthetic gene expression and cellular differentiation in developing maize leaves. Plant Physiol. 1985 Jun;78(2):399–404. doi: 10.1104/pp.78.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai W. S. Simple method for differential staining of paraffin embedded plant material using toluidine blue o. Stain Technol. 1973 Sep;48(5):247–249. doi: 10.3109/10520297309116632. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Bartlett S. G., Grossman A. R., Cashmore A. R., Chua N. H. Biosynthetic pathways of two polypeptide subunits of the light-harvesting chlorophyll a/b protein complex. J Cell Biol. 1981 Nov;91(2 Pt 1):468–478. doi: 10.1083/jcb.91.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G., Ohad I., Martineau B., Taylor W. C. Differentiation and development of bundle sheath and mesophyll thylakoids in maize. Thylakoid polypeptide composition, phosphorylation, and organization of photosystem II. J Biol Chem. 1985 Sep 25;260(21):11866–11873. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]