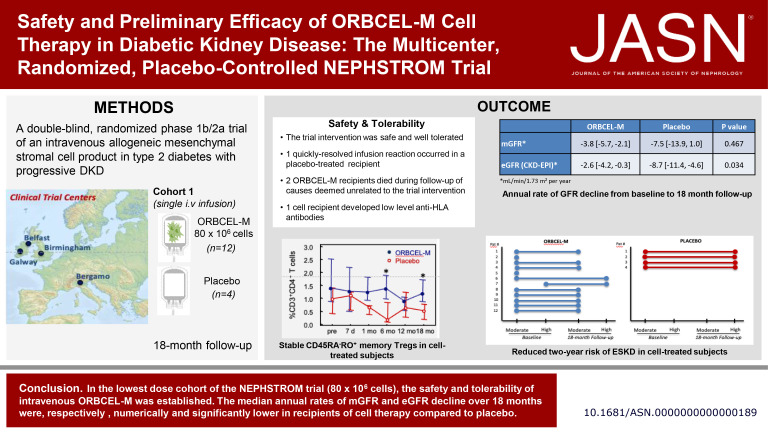
Visual Abstract
Keywords: diabetes, randomized controlled trials, stem cell, diabetic kidney disease
Abstract
Significance Statement
Mesenchymal stromal cells (MSCs) may offer a novel therapy for diabetic kidney disease (DKD), although clinical translation of this approach has been limited. The authors present findings from the first, lowest dose cohort of 16 adults with type 2 diabetes and progressive DKD participating in a randomized, placebo-controlled, dose-escalation phase 1b/2a trial of next-generation bone marrow–derived, anti-CD362 antibody–selected allogeneic MSCs (ORBCEL-M). A single intravenous (iv) infusion of 80×106 cells was safe and well-tolerated, with one quickly resolved infusion reaction in the placebo group and no subsequent treatment-related serious adverse events (SAEs). Compared with placebo, the median annual rate of decline in eGFR was significantly lower with ORBCEL-M, although mGFR did not differ. The results support further investigation of ORBCEL-M in this patient population in an appropriately sized phase 2b study.
Background
Systemic therapy with mesenchymal stromal cells may target maladaptive processes involved in diabetic kidney disease progression. However, clinical translation of this approach has been limited.
Methods
The Novel Stromal Cell Therapy for Diabetic Kidney Disease (NEPHSTROM) study, a randomized, placebo-controlled phase 1b/2a trial, assesses safety, tolerability, and preliminary efficacy of next-generation bone marrow–derived, anti-CD362–selected, allogeneic mesenchymal stromal cells (ORBCEL-M) in adults with type 2 diabetes and progressive diabetic kidney disease. This first, lowest dose cohort of 16 participants at three European sites was randomized (3:1) to receive intravenous infusion of ORBCEL-M (80×106 cells, n=12) or placebo (n=4) and was followed for 18 months.
Results
At baseline, all participants were negative for anti-HLA antibodies and the measured GFR (mGFR) and estimated GFR were comparable between groups. The intervention was safe and well-tolerated. One placebo-treated participant had a quickly resolved infusion reaction (bronchospasm), with no subsequent treatment-related serious adverse events. Two ORBCEL-M recipients died during follow-up of causes deemed unrelated to the trial intervention; one recipient developed low-level anti-HLA antibodies. The median annual rate of kidney function decline after ORBCEL-M therapy compared with placebo did not differ by mGFR, but was significantly lower by eGFR estimated by the Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease equations. Immunologic profiling provided evidence of preservation of circulating regulatory T cells, lower natural killer T cells, and stabilization of inflammatory monocyte subsets in those receiving the cell therapy compared with placebo.
Conclusions
Findings indicate safety and tolerability of intravenous ORBCEL-M cell therapy in the trial's lowest dose cohort. The rate of decline in eGFR (but not mGFR) over 18 months was significantly lower among those receiving cell therapy compared with placebo. Further studies will be needed to determine the therapy's effect on CKD progression.
Clinical Trial registration number
ClinicalTrial.gov NCT02585622.
Introduction
Type 2 diabetes mellitus (DM) is a rapidly increasing global health care challenge, estimated to affect 437 million individuals worldwide in 2019.1 Among its complications, DKD affects 30%–40% of adults living with type 2 diabetes2 and accounts for approximately 40% of people with end stage kidney failure (ESKF) requiring kidney replacement therapy in high-income countries.3
Clinically, DKD is typically characterized by the onset of microalbuminuria, which can further progress to macroalbuminuria, and a subsequent decline in GFR, ultimately leading to uremia.4,5 A wide range of maladaptive processes, predominantly driven by hyperglycemia, contribute to the pathobiology of DKD, including increased oxidative stress, chronic inflammation, accumulation of advanced glycation end products, renal hypoxia, cell apoptosis, and altered renin-angiotensin-aldosterone system activation.6–9
Over the past few decades, medical advances have substantially improved the management of patients with DKD, thereby prolonging their survival.10–15 However, despite optimal treatments of metabolic and BP control, lipid management, and proteinuria, patients with DKD continue to have high renal and cardiovascular risk.16 Although recent clinical trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors and other pharmacological agents have shown that the rate of renal function loss can be slowed in DKD because of type 2 DM,17–20 successful targeting of multiple injurious pathways—such as those mediating inflammation, oxidative stress, renal hypoxia, and fibrosis—may be necessary to halt progression of DKD.
Among novel therapeutic strategies for DKD, cell therapy with MSCs is emerging as an option on the basis of its potential to deliver or induce the production of a wide range of mediators that simultaneously target maladaptive processes contributing to kidney injury.21 Numerous studies in preclinical models of diabetes and diabetic nephropathy have shown that MSCs exert beneficial renoprotective activities by modulating, both locally and systemically, several of the key pathophysiologic pathways that underpin DKD.22–24 Clinical translation of this cell therapy, however, has been limited, although encouraging results were reported by Packham et al. after iv infusion of allogeneic mesenchymal precursor cells (rexlemestrocel-L) in adults with type 2 DM and moderate-to-severe CKD.25
On the basis of this evidence, as part of the European Union (EU) Horizon 2020–funded NEPHSTROM consortium (grant number: 634086), we conducted a phase 1b/2a multicenter, randomized, placebo-controlled clinical trial (NEPHSTROM trial) with the primary aim of investigating the safety and feasibility, and a secondary aim of preliminary assessment of efficacy, of cell therapy with a next-generation human bone marrow–derived, antibody-purified (CD362+) population of MSCs (ORBCEL-M)26,27 in individuals with type 2 DM with progressive DKD. In this study, we report the results of the first ORBCEL-M dose/placebo cohort recruited into the NEPHSTROM trial.
Methods
Trial Design and Participants
The NEPHSTROM (Novel Stromal Cell Therapy for Diabetic Kidney Disease, www.nephstrom.eu) study is a pilot, exploratory, investigator-initiated, European, multicenter, randomized, double-blind, placebo-controlled clinical trial. It was performed at three academic clinical centers in Ireland (University of Galway), Italy (Azienda Socio-Sanitaria Territoriale Papa Giovanni XXIII, ASST-PG23, Bergamo), and the United Kingdom (University Hospital Birmingham NHS Foundation Trust, UHBFT, Birmingham) and was coordinated by the Istituto di Ricerche Farmacologiche Mario Negri IRCCS (IRFMN), Bergamo, Italy. A second clinical center in the United Kingdom (Belfast Health and Social Care Trust, BHSCT, Belfast), initially working as a clinical trial enrollment site, withdrew, but remained in the NEPHSTROM study as the centralized laboratory for screening and monitoring of anti-HLA antibodies in the study participants. The local ethics committees and the national competent authorities approved the study protocol and the Investigational Medicine Product (IMP). For these regulatory approvals, the NEPHSTROM consortium followed the Voluntary Harmonization Procedure ([VHP1038][VHP2017011]). The trial was registered with the European Union Clinical Trial Register (EUDRACT N°2016-000661-23) and at ClinicalTrials.gov (NCT02585622). Written informed consent was obtained from all participants.
Eligible participants were between 40 and 85 years with type 2 DM for 3 or more years under a clinician with mandated responsibility for management to national guidelines. Other inclusion criteria were as follows: (1) urine albumin-to-creatinine ratio (UACR) ≥88 mg/g (≥10 mg/mmol) in a spot morning urine sample; (2) eGFR 25–55 ml/min per 1.73 m2 by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation28 on two or more consecutive measurements at least 30 days apart within the past 6 months; and (3) a documented eGFR decline of ≥10 ml/min per 1.73 m2 over the past 3 years or documented rate of eGFR decline ≥5 ml/min per 1.73 m2 per year on the basis of 3 or more consecutive readings at least 90 days apart, within the past 18 months up to the date of consent, or an intermediate or high 5-year risk of progression to ESKF (dialysis or transplantation) on the basis of the validated Tangri 4-variable (age, sex, eGFR, urinary albumin/creatinine ratio) kidney failure risk equation for patients with CKD stage 3–5.29,30 Key exclusion criteria were (1) resting systolic BP ≥150 mm Hg and diastolic BP ≥90 mm Hg in a clinical setting, despite treatment with three antihypertensive agents of different classes; (2) hemoglobin A1c (HbA1c) >75 mmol/mol (>9%); (3) fasting total cholesterol >7 mmol/L; (4) fasting total triglycerides >3.5 mmol/L; and (5) positive screening test for anti-HLA antibodies (mean fluorescence intensity >1500). Patients with chronic lung or liver disease, with cardiovascular events within 6 months before enrollment, currently enrolled or had a history within 6 months before enrollment of New York Heart Association (NYHA) class III or IV heart failure, and with active malignancy or women of childbearing potential without use of acceptable methods of contraception or women who were pregnant or lactating were also excluded.
In keeping with the large majority of therapeutic trials of interventions for DKD, none of the participants enrolled in the NEPHSTROM trial had a kidney biopsy at study entry to confirm the presence of pathological changes of diabetic nephropathy and to rule out non-DKDs.31,32 One of the 16 enrolled participants had a previously recorded kidney biopsy (which confirmed pathological abnormalities consistent with diabetic nephropathy), and one had a prior biopsy attempt which did not yield diagnostic tissue.
Procedures and Assessments
The NEPHSTROM trial follows a phase 1b/2a dose-escalation design aiming to recruit 48 participants with type 2 diabetes and DKD who have provided written consent. Equal numbers of participants were expected to be enrolled by each center. However, if difficulty in enrollment was to occur in one or more centers, additional recruitment could be implemented in the other participating centers. Study participants were randomly allocated in a 3:1 ratio to a double-blind single iv infusion of one of three ORBCEL-M doses (80, 160, or 240×106 cells) or to placebo infusion. Each of the three cohorts consists of 16 participants (12 receiving ORBCEL-M and 4 receiving placebo [Cryostor CS10]). Because the NEPHSTROM trial is a preliminary safety study, the first cohort of participants received the lowest dose (80×106 cells) or placebo.
If the Data Monitoring Safety Board indicated that the study could proceed beyond this dose, the next 16 participants could have been allocated and randomized to the next ORBCEL-M 160×106 cell dose or placebo in the absence of a dose-limiting toxicity event (cohort 2). Finally, after completion of allocation to cohort 2, the subsequent 16 participants would have been allocated to the last 240×106 cell dose or placebo (cohort 3). As detailed in the Supplemental Results, cohort 2 enrollment was ended prematurely (13 of 16 participants) after discussion among the principal investigators and the study sponsor because of the coronavirus disease 2019 (COVID-19) pandemic, which precluded any further activities related to the NEPHSTROM trial. Moreover, cohort 3 was not performed because of the delay in the trial from the COVID-19 pandemic and the material inability to further extend validation of cell/placebo bags to be used for iv infusion.
Active and placebo treatments were administered in the context of ongoing independent standard-of-care medical management of glycemia, BP, lipid levels, and other clinical issues by specialist physicians blinded to treatment randomization.
At each clinical center, patients with type 2 diabetes were prescreened for potential eligibility by trained study personnel on the basis of ongoing outpatient evaluation and medical record review. After obtaining informed consent from participants who fulfilled inclusion criteria and agreed to participate in the study, screening tests were performed to confirm suitability for randomization. Prerandomization tests consisted of basic blood parameters, UACR, serum anti-HLA antibody screen (Luminex bead assay), and, for women of childbearing potential, a pregnancy test. Participants confirmed as meeting eligibility criteria were then randomized to ORBCEL-M or placebo infusion according to a computer-generated randomization procedure through the Clinical Trial Coordinating Center, IRFMN. The randomization list was prepared by an independent statistician (Giovanni Antonio Giuliano) at the Laboratory of Biostatistics of Clinical Research Center for Rare Diseases Aldo e Cele Daccò, IRFMN (Ranica, Bergamo, Italy).
Each randomized participant was admitted to a clinical research facility (CRF) for baseline evaluations, including systolic and diastolic BP, electrocardiogram, fasting blood glucose, HbA1c, lipid profile, and hematology and biochemistry panels with GFR estimation by CKD-EPI and Modification of Diet in Renal Disease (MDRD) equations. UACR was measured on spot morning urine samples; GFR was measured by plasma iohexol clearance. Blood and urine samples were also processed and stored for profiling of blood immune cell subsets, and biomarkers of inflammation. The trial infusion (ORBCEL-M or placebo) was administered within 48 hours (Figure 1B). The participants were closely monitored during the infusion and thereafter for an 8-hour period in the CRF.
Figure 1.
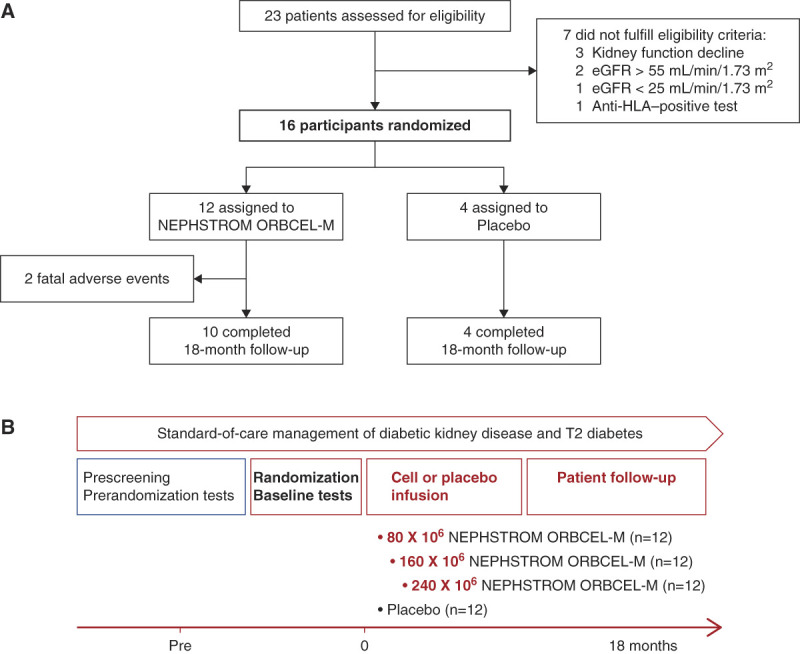
Summary of NEPHSTROM trial design. Study flowchart of the NEPHSTROM cohort trial (A) and the overall NEPHSTROM clinical trial treatment plan and follow-up (B). Figure 1 can be viewed in color online at www.jasn.org.
On days 1 and 7 and at month 1 after infusion, the participants returned to the CRF, where they were interviewed regarding symptoms and adverse events (AEs) (early post-IMP/placebo administration monitoring). At these time points, they had measurements of BP, serum creatinine, fasting blood glucose, hematology and biochemistry panels, and UACR. At the 7-day and 1-month time points, blood and urine samples were collected and processed for immune monitoring and assays of inflammation-related soluble mediators.
Subsequent CRF follow-up visits at 3, 6, 12 and 18 months after ORBCEL-M/placebo infusion included physical examination, interviews for intercurrent AEs, and routine laboratory tests (later postadministration monitoring). At 6, 12, and 18 months, kidney function (GFR by plasma iohexol clearance, and eGFR by CKD-EPI and MDRD equations) was assessed, UACR measured on spot morning urine samples, and blood collected for immune/inflammatory mediator monitoring. Trial follow-up was ended at 18 months after infusion.
The trial imposed no restrictions on concomitant treatments, which were at the discretion of the participants primary and specialist physicians. At randomization and at each follow-up visit thereafter, concomitant treatments were reviewed and recorded.
ORBCEL-M Preparation, Administration, and Postinfusion Monitoring
ORBCEL-M was manufactured from healthy donor bone marrow aspirates under license (Orbsen Therapeutics Ltd, Galway, Ireland) by three Good Manufacturing Practice (GMP) facilities (Centro di Terapia Cellulare Gilberto Lanzani, ASST-PG23, Bergamo, Italy; Center for Cellular Manufacturing CCMI, Galway, Ireland; NHS Blood and Transplant NHSBT, Liverpool, United Kingdom), with a fourth GMP facility—Academish Ziekenhuis Leiden-Leids Universitair Medisch Centrum LUMC, Leiden, the Netherlands—serving as the primary isolation and coordinating site responsible for the manufacturing protocol. Bone marrow aspirates were collected from screened, healthy adult volunteers by a consultant hematologist at the Irish HPRA-approved Galway Blood and Tissue Establishment and were shipped to Leiden University Medical Center. Here, ORBCEL-M cells were antibody-enriched in a CliniMACS separation system using a GMP-grade anti-CD362 antibody and were primarily expanded to passage (P)1 in tissue culture flasks. The P1 cells were lifted, using recombinant enzyme; reseeded into a cryoprecipitate-coated hollow-fiber bioreactor (Quantum Cell Expansion System [Terumo BCT Europe N.V., Belgium]) where they were further expanded for a maximum of 7 days; and collected by enzymatic release. Aliquots of cells were cryopreserved as an intermediate ORBCEL-M product and shipped to the other three GMP teams for a subsequent second round of expansion in a Quantum Cell Expansion System bioreactor, followed by harvest, formulation at the required doses into individual cryobags, and final release of the GMP product. The GMP facilities of Leiden, Bergamo, Galway, and Liverpool each hold a national license for the manufacturing of Advanced Therapy Medicinal Products, including MSC products. On the day of infusion, GMP-released doses of ORBCEL-M or placebo, cryopreserved in 40-ml sterile bags, were transported frozen to the CRFs in Bergamo, Galway, and Birmingham. The GMP facilities released ORBCEL-M according to specific criteria, including (1) cell surface marker positivity ≥95% by FACS analysis for CD73, CD90, and CD105, as well as ≤1% positivity for CD45 and CD34; (2) negative for mycoplasma, gram-positive and gram-negative bacteria, and fungi; (3) endotoxin below 10 EU/ml by a chromogenic method; (4) viability ≥70% by trypan blue and manual counting; (5) viable cell number ≥350×106; and (6) karyotype G banding and Q banding analysis (no clonal abnormalities and no more than three individual abnormalities). This conforms with the criteria for defining multipotent MSCs by the International Society of Cellular Therapy.33
Immediately after thawing and according to randomization, the ORBCEL-M or placebo dose (in a 40-ml volume) was administered intravenously into a peripheral arm vein of each randomized participant over 10–20 minutes using a 200-μm transfusion filter. A premedication regimen was administered consisting of oral acetaminophen (1 g, 1 hour before infusion) and iv chlorphenamine and hydrocortisone (10 and 100 mg, respectively, immediately before infusion). Baseline temperature, pulse rate, BP, respiratory rate, oxygen saturation, and chest auscultation were recorded and thereafter monitored continuously during the infusion, every 15 minutes during the first hour, and hourly during the subsequent 7 hours after infusion. Study participants were also monitored closely for other signs of adverse reactions, such as rash, urticaria, or wheezing. All events were recorded during the 8-hour observation period, after which the participant was allowed to leave the CRF if no AEs had occurred. The doses of ORBCEL-M and placebo were indistinguishable in labeling and instructions for use, but to avoid identification of the active IMP because of cloudiness of cell suspensions, the primary trial physicians and research nurses, as well as participants the were shielded from seeing the infusion bag and tubing to remain blinded throughout the trial while validation, thawing, and setup of the infusions were performed by a separate (unblinded) team consisting of a technician, pharmacist, and research nurse.
Trial Outcomes
The primary trial outcome was the number and severity of prespecified cell infusion–associated events and the overall number and frequency of AEs and unexpected severe AEs during the early (up to 1 month) and late (from 2 to 18 months) follow-up periods among ORBCEL-M recipients compared with placebo recipients. Secondary outcomes included the efficacy of ORBCEL-M compared with placebo to slow the progression of DKD, assessed using the following variables: (1) GFR changes (∆GFR and slope of GFR decline, evaluated by the serial measurements of mGFR by plasma iohexol clearance)34; (2) serum creatinine-based eGFR by CKD-EPI and MDRD equations (∆eGFR and slope of eGFR decline); and (3) absolute and percentage change of UACR on spot urine samples from baseline to month 18 after infusion. Other secondary outcomes were the effects of ORBCEL-M compared with placebo on other relevant clinical parameters, including proportions of study participants within target ranges for glycemic control (fasting blood glucose and HbA1c), lipid control (total cholesterol, LDL cholesterol, and triglycerides), and BP control. Finally, additional secondary outcomes included the effect of ORBCEL-M compared with placebo on immune and inflammatory profiles, assessed by the following variables: (1) anti-HLA antibody development; (2) proportion/total number of circulating lymphocyte (T cells, B cells, and NK cells) and myeloid cell (monocytes and dendritic cells) subsets; and (3) plasma/serum immunoassay-derived concentrations of biomarkers of inflammation.
Laboratory Measurements
Clinical chemistry was performed according to a trial monitoring protocol. Blood and urine samples were analyzed by local clinical laboratories at the three participating centers. Measured GFR (mGFR) was determined by an established protocol for quantifying the plasma clearance of unlabeled iohexol.34 For this, series of plasma samples were collected and initially stored at each CRF at −80°C and then were shipped for centralized measurement to the Laboratory of the Clinical Trial Coordinating Center, IRFMN, Ranica, Italy. Iohexol plasma levels were assayed by high-performance liquid chromatography.34 Measurement of anti-HLA antibodies in serum were also centralized at the Belfast HSC Trust Histocompatibility and Immunogenetics Laboratory, Belfast, United Kingdom. The procedure consisted of two Luminex assays: an initial antibody screen with Luminex multiantigen beads to detect class I and class II MHC antibodies, followed, if necessary, by a Luminex single-antigen bead assay to determine the specificity of any antibody detected. Longitudinal profiling of peripheral blood lymphocyte and myeloid cell subsets were centralized at the Laboratory of Immunology and Organ Transplantation, IRFMN, at the Clinical Trial Coordinating Center, Italy. Three antibody panels were used to analyze the phenotype of (1) CD4+ and CD8+ T-cell subsets, B-cell subsets, NK, and monocytes; (2) regulatory CD4+ T cells (Tregs); and (3) Lin−HLADR+ dendritic cells by the FACS LSR Fortessa X-20 (Becton Dickinson) and FlowJo software (see Supplemental Table 1). For the inflammatory biomarker evaluation, longitudinal samples collected in the three participating clinical centers were centralized at University of Galway, Ireland. Serum concentrations of soluble tumor necrosis factor receptor 1 (sTNFR1), neutrophil gelatinase-associated lipocalin (NGAL), vascular cell adhesion molecule (VCAM-1), and epidermal growth factor (EGF) (biomarkers with well-documented links to DKD severity and prognosis)21 were quantified by using DuoSet ELISA kits (R&D Systems, Minneapolis) according the manufacturer's instructions.
Sample Size Estimation
Although this is a phase 1 study with the primary objective of determining feasibility and safety, a sample size was calculated according to Cocks and Torgerson for the initial efficacy of ORBCEL-M in slowing the rate of decline of GFR.35 It was determined that the study should include at least 36 participants to be powered to detect a change in the rate of decline of GFR from 5.1 (SD 4.3) ml/min per year in the placebo group to 3.4 ml/min per year in the active group (power=80% and α=0.05, two-sided test). This calculation was based on previously reported interim data in study participants with diabetes enrolled in the Preventing ESRD in Overt Nephropathy of Type 2 Diabetes Preventing ESRD in Overt Nephropathy of Type 2 Diabetes (VALID) trial36 and taking into account the 3:1 random allocation. Thus, 48 consenting participants with type 2 DM and with progressive DKD were planned to be recruited.
Statistical Analyses
Safety and efficacy analyses were predetermined to be primarily performed on an “all-treated” basis—i.e., to include all participants randomized into the study who received an infusion of ORBCEL-M or placebo, regardless of whether an infusion was initiated. In addition, a secondary safety analysis was also to be performed on a “safety set” basis—i.e., to include only participants randomized into the study who had received some or all of an ORBCEL-M or placebo infusion. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and STATA version 15 (StataCorp., College Station, TX). A mixed-effect model with random intercepts was used to determine the mGFR and eGFR slopes and was estimated by restricted maximum likelihood. Results were expressed as mean±SD or median (interquartile range) or number (%) as appropriate. All P values were two-sided with significance assigned to P < 0.05.
Results
Participant Enrollment and Baseline Characteristics
The study protocol was planned to include three cohorts: cohort 1, 80×106 cells or placebo; cohort 2, 160×106 cells or placebo; and cohort 3, 240×106 cells or placebo. The results presented here relate to the enrollment, treatment, and completed follow-up of cohort 1. The detailed rationale for unblinding and analyzing the data for this cohort is described in Supplemental Results.
Between March 2018 and January 2020, 23 consenting patients were screened for final eligibility, of which 16 were randomized to cohort 1 of the trial. Of the seven screening failures, three were due to a failure to meet the criteria for eGFR decline, two were due to screening eGFR >55 ml/min per 1.73 m2, one was due to screening eGFR <25 ml/min per 1.73 m2, and one was due to a positive anti-HLA screening assay (see study flowchart, Figure 1A). The number of participants randomized and treated at each of the sites was Bergamo, Italy, n=8; Galway, Ireland, n=4; and Birmingham, United Kingdom, n=4.
Table 1 summarizes key demographic, clinical, and laboratory characteristics at baseline of the 16 randomized Participant, according to treatment with ORBCEL-M or placebo. The median age was 69 (interquartile range, 66–73) years in the cell-treated group and 59 (interquartile range, 54–66) years in the placebo group. All participants were male. Systolic and diastolic BP were well-controlled with antihypertensive treatment in both groups. In keeping with the inclusion criteria, both groups of participants had comparable moderate-to-severe CKD on the basis of both mGFR and eGFR. Glycemic control as determined by HbA1c was closely comparable for the two groups, and median values for fasting blood glucose, lipid parameters, and UACR were not significantly different. Only one of 16 participants was prescribed a SGLT2 inhibitor at the time of randomization.
Table 1.
Participant baseline, demographic, clinical, and laboratory characteristics
| Parameter | ORBCEL-M (n=12) | Placebo (n=4) | P Valuea |
|---|---|---|---|
| Age (yr) | 69 (66–73) | 59 (54–66) | 0.054 |
| Sex (M/F) (n) | 12/0 | 4/0 | — |
| BP | |||
| Systolic (mm Hg) | 137 (128–147) | 139 (129–144) | 0.144 |
| Diastolic (mm Hg) | 70 (66–78) | 79 (72–84) | 0.715 |
| Serum creatinine (mg/dl) | 1.90 (1.78–2.04) | 2.07 (1.80–2.19) | 0.379 |
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | 35.3 (32.4–38.6) | 33.4 (31.9–40.0) | 0.683 |
| eGFR (MDRD) (ml/min per 1.73 m2) | 35.4 (32.6–38.4) | 32.9 (31.2–38.8) | 0.379 |
| mGFR (ml/min per 1.73 m2) | 38.5 (32.4–42.6) | 39.4 (25.5–50.2) | 0.953 |
| HbA1c (mmol/mol) | 54.1 (47.6–66.8) | 56.1 (45.2–65.5) | 0.861 |
| Fasting glucose (mg/dl) | 131 (117–158) | 183 (138–215) | 0.305 |
| Total cholesterol (mg/dl) | 180 (135–201) | 106 (86–166) | 0.101 |
| UACR (mg/g) | 420.5 (289.4–2042.6) | 982.5 (470.8–3860.8) | 0.845 |
| Medication, n (%) | |||
| Antihypertensive medication use | |||
| ACEi | 5 (41.7) | 2 (50) | 1.000 |
| ARB | 4 (33.3) | 1 (25) | 1.000 |
| β-blocker | 6 (50) | 1 (25) | 0.585 |
| Calcium channel blockers | 6 (50) | 1 (25) | 0.585 |
| Diuretics | 5 (41.7) | 1 (25) | 1.000 |
| Metformin | 6 (50) | 2 (50) | 1.000 |
| Insulin | 12 (100) | 4 (100) | — |
| Statin | 9 (75) | 4 (100) | 0.529 |
| SGLT2i | 1 (9.1) | 0 (0) | 1.000 |
Data are median (interquartile range), unless otherwise indicated. eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; mGFR, measured GFR; HbA1c, hemoglobin A1c; UACR, urinary albumin-to-creatinine ratio; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Wilcoxon test or Fisher exact test, as appropriate.
Primary Outcome
Of the 16 randomized participants, 14 completed 18-month follow-up per protocol while follow-up of 2 (both in the ORBCEL-M-treated group) ended early because of SAEs resulting in death (described in more detail below).
Early Safety Monitoring
Per protocol, all randomized participants were in stable health at the time of study treatment. For 15 of 16 participants, no adverse reactions to cell or placebo infusion occurred. Thus, temperature, pulse rate, respiratory rate, BP, and oxygen saturation remained stable during the infusion and the subsequent 8-hour observation period. In one placebo-treated participant, an episode of moderate bronchospasm occurred shortly after completion of the infusion. In this case, the participant recovered fully approximately 50 minutes later after appropriate pharmacologic treatment. No other adverse reactions occurred between the time of infusion and the 1-month follow-up visit (Table 2).
Table 2.
Early (from 0 to 1 month) and late (from 2 to 18 months) serious adverse events
| Parameter | ORBCEL-M/Placebo | Sex | Event | Relationship with Treatment | Intensity | Outcome |
|---|---|---|---|---|---|---|
| Early SAEs | ||||||
| Placebo | Male | Bronchospasm | Yes | Moderate | Recovered | |
| Late SAEs | ||||||
| ORBCEL-M | Male | Acute myocardial infarction | No | Severe | Recovered with sequelae | |
| Congestive heart failure | No | Severe | Fatal | |||
| ORBCEL-M | Male | COVID-19 test positive | No | Mild | Recovered | |
| ORBCEL-M | Male | Anemia with increased dyspnea | No | Moderate | Recovered | |
| Left hip fracture | No | Severe | Unknown | |||
| Respiratory tract infection | No | Moderate | Recovered | |||
| Complicated duodenal diverticulitis | No | Moderate | Unknown | |||
| Respiratory failure | No | Severe | Unknown | |||
| Hyperkalemia | No | Severe | Recovered | |||
| Multiple myeloma | No | Severe | Fatal | |||
| ORBCEL-M | Male | Headache | No | Moderate | Recovered |
SAE, serious adverse event; COVID-19, coronavirus disease 2019.
Late Safety Monitoring
As summarized in Table 2, eleven additional SAEs occurred in a total of four participants (all recipients of cell infusions) between months 2 and 18 of the follow-up period: seven in a single participant, two in another participant, and one each in two others. None of the late SAEs were deemed to be related to the trial investigational product, ORBCEL-M. For two of these participants, the non–treatment-related SAEs culminated in death: in one case from congestive heart failure after 15 months of trial follow-up and in the second from multiple acute complications of newly diagnosed multiple myeloma after 15 months of follow-up.
Overall, in the cell-treated group, 56 AEs were recorded, of which 11 were deemed serious (Table 3). In the placebo-treated group, 13 AEs occurred, of which one was deemed serious and treatment-related (as described above). The 57 AEs that were not classified as serious are summarized in Table 4.
Table 3.
Summary of adverse events
| Events | ORBCEL-M | Placebo |
|---|---|---|
| Participants with at least one AE | 12 | 4 |
| Any AE | 56 | 13 |
| SAEs | 11 | 1 |
| AEs leading to death | 2 | 0 |
| AEs leading to discontinuation of study drug | 0 | 0 |
| AEs leading to discontinuation of study | 2 | 0 |
| Drug-related AEs | 0 | 0 |
| Drug-related SAEs | 0 | 1 |
Data are reported as numbers. Any AE: both not serious and serious adverse events. AE, adverse event; SAE, serious adverse event.
Table 4.
List of nonserious adverse events
| Adverse Event Type | ORBCEL-M Events, n (Participants, n) |
Placebo Events, n (Participants, n) |
|---|---|---|
| Left bundle block | 2 (2) | — |
| First-degree atrioventricular block | 1 (1) | — |
| Inferior Q wave | 1 (1) | — |
| Ejection systolic murmur | 1 (1) | — |
| Chest pain | 2 (2) | — |
| Fatigue | 1 (1) | — |
| Asthenia | 1 (1) | — |
| Increased edema | 4 (1) | — |
| Hypoglycemia | 1 (1) | — |
| Eye disorder | — | 1 (1) |
| Cataract | 1 (1) | — |
| Diarrhea | 3 (3) | 1 (1) |
| Heartburn | 1 (1) | — |
| Vomiting associated with ileus | 1 (1) | — |
| Hemorrhoids | — | 1 (1) |
| Fever of unknown origin | — | 1 (1) |
| Flu-like syndrome | 1 (1) | — |
| Ankle swelling | 1 (1) | — |
| Bruised ribs | 1 (1) | — |
| Stenosing tenosynovitis | 1 (1) | — |
| Laceration to left shin | 1 (1) | — |
| Left knee swelling | 1 (1) | — |
| Traumatic injury left foot | — | 1 (1) |
| Fall | 1 (1) | — |
| Meralgia paresthetica | 1 (1) | — |
| Wound infection | — | 1 (1) |
| Pain associated with SAE hip fracture | 1 (1) | — |
| Pain right heel | — | 1 (1) |
| Knee pain | 1 (1) | — |
| Painful shoulder | 1 (1) | — |
| Headache | 1 (1) | — |
| Low mood | 1 (1) | — |
| Memory impairment | 1 (1) | — |
| Disoriented | 1 (1) | — |
| Cough | 2 (2) | 1 (1) |
| Hoarse | 1 (1) | — |
| Obstructive sleep apnea | — | 1 (1) |
| Abdominal bruising | — | 1 (1) |
| Perianal dermatitis | — | 1 (1) |
| Pressure sore (sacral) | 1 (1) | — |
| Tingling in limb peripheries | 1 (1) | — |
| Benign prostatic hyperplasia | 1 (1) | — |
| Secondary hyperparathyroidism | 1 (1) | — |
| Erythroderma | — | 1 (1) |
| Urinary tract infection | 1 (1) | — |
| AKI | 1 (1) | — |
| Hypereosinophilia | 1 (1) | — |
| Total | 45 (11) | 12 (4) |
SAE, serious adverse event.
Predefined Secondary Comparisons
Effects on Renal Function
As summarized in Table 5, baseline mGFR (by plasma iohexol clearance) was comparable in the ORBCEL-M and placebo-treated groups. In both groups, mGFR declined during the 18-month follow-up period. The mean changes in mGFR at 6, 12, and 18 months compared with baseline were numerically less in ORBCEL-M–treated participants than in placebo-treated participants, but the differences were not statistically different (P = 0.709, P = 0.443, and P = 0.236, respectively). By contrast, the mean changes in eGFR, whether calculated by CKD-EPI or MDRD equations, were significantly less in the ORBCEL-M group than the placebo group (at 12 months, P = 0.015 and P = 0.018 for CKD-EPI and MDRD, respectively; at 18 months, P = 0.012 and P = 0.014, respectively). Very similar results were observed when changes in renal function during trial follow-up were calculated as rate of decline per year (Table 6). For mGFR, the annual rate of decline was numerically but not significantly lower for the group receiving cells compared with the group receiving placebo (P = 0.467) while for eGFR, a significantly lower annual rate of decline occurred in ORBCEL-M–treated participants than in placebo-treated participants (P = 0.034 for both CKD-EPI and MDRD eGFR).
Table 5.
Time course of mGFR and eGFR during the 18-month follow-up period in participants receiving ORBCEL-M or placebo
| Parameter | ORBCEL-M Mean±SD |
Difference versus Baseline Mean (Range) |
Placebo Mean±SD |
Difference versus Baseline Mean (Range) |
P Valuea |
|---|---|---|---|---|---|
| mGFR (ml/min per 1.73 m2) | |||||
| Baseline | 37.24±8.39 | 37.84±14.78 | |||
| 6 mo | 34.82±9.91 | −1.79 (−6.63 to 3.04) | 36.12±12.26 | −4.35 (−16.20 to 7.5) | 0.709 |
| 12 mo | 33.10±8.85 | −2.53 (−6.38 to 1.32) | 31.33±5.09 | −6.50 (−25.32 to 12.32) | 0.443 |
| 18 mo | 31.86±6.66 | −5.51 (−9.89 to −1.13) | 28.11±4.74 | −9.72 (−32 to 12.55) | 0.236 |
| eGFR (CKD-EPI) (ml/min per 1.73 m2) | |||||
| Baseline | 36.35±5.44 | 36.00±7.06 | |||
| 6 mo | 36.16±7.79 | 0.65 (−4.20 to 5.50) | 32.19±7.14 | −5.58 (−13.27 to 2.11) | 0.239 |
| 12 mo | 35.55±6.57 | −0.90 (−3.43 to 1.62) | 28.82±6.53 | −7.18 (−13.06 to −1.31) | 0.015 |
| 18 mo | 35.00±8.85 | −1.88 (−6.04 to 2.28) | 23.23±4.33 | −12.78 (−23.22 to −2.31) | 0.012 |
| eGFR (MDRD) (ml/min per 1.73 m2) | |||||
| Baseline | 36.52±5.27 | 35.01±6.16 | |||
| 6 mo | 36.46±7.62 | 0.64 (−4.06 to 5.34) | 31.49±6.58 | −5.11 (−11.92 to 1.69) | 0.226 |
| 12 mo | 35.93±6.42 | −0.73 (−3.19 to 1.73) | 28.43±6.05 | −6.58 (−11.87 to −1.29) | 0.018 |
| 18 mo | 35.42±8.43 | −1.66 (−5.73 to 2.40) | 23.21±4.23 | −11.80 (−21.18 to −2.42) | 0.014 |
mGFR, measured GFR; eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
aBy ANCOVA adjusted for baseline value. ORBCEL-M versus Placebo, as difference versus baseline.
Table 6.
Annual rate of renal function decline from baseline to 18-month follow-up in participants receiving ORBCEL-M or placebo
| Parameter | ORBCEL-M | Placebo | P Valuea |
|---|---|---|---|
| mGFR (ml/min per 1.73 m2 per year) | −3.8 (−5.7 to −2.1) | −7.5 (−13.9 to 1.0) | 0.467 |
| eGFR (CKD-EPI) (ml/min per 1.73 m2 per year) | −2.6 (−4.2 to −0.3) | −8.7 (−11.4 to −4.6) | 0.034 |
| eGFR (MDRD) (ml/min per 1.73 m2 per year) | −2.4 (−4.0 to −0.1) | −8.1 (−10.4 to −4.4) | 0.034 |
Data are reported as median (interquartile range). mGFR, measured GFR; eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
Wilcoxon rank-sum test: NEPHSTROM ORBCEL-M versus placebo.
Trends in UACR during trial follow-up are summarized in Table 7. As shown, UACR values varied widely in both groups at baseline and at 6, 12, and 18-month follow-ups without significant between-group differences at any time point. Notably, despite the lower rate of eGFR decline for the group receiving cells, there was no evidence of a reduction in UACR in ORBCEL-M–treated participants.
Table 7.
Time course of urinary albumin-to-creatinine ratio during the 18-month follow-up period in participants receiving ORBCEL-M or placebo
| UACR (mg/g) | ORBCEL-M | Placebo | P Valuea |
|---|---|---|---|
| Baseline | 420.5 (289.4–2042.6) | 982.5 (470.8–3860.8) | 0.845 |
| 6 mo | 1250.2 (239.0–1955.3) | 836.7 (423.3–7203.0) | 1.000 |
| 12 mo | 956.3 (403.8–1580.6) | 636.0 (333.6–4227.7) | 0.845 |
| 18 mo | 1004.9 (371.2–1317.4) | 682.8 (273.7–3244.4) | 0.832 |
Data are reported as median (interquartile range). UACR, urine albumin-to-creatinine ratio.
Wilcoxon rank-sum test: NEPHSTROM ORBCEL-M versus placebo.
Effect on Metabolic Parameters and BP
Values for blood glucose, HbA1c, serum total cholesterol, serum triglycerides, and serum C-reactive protein at baseline and after 6, 12, and 18 months of follow-up are summarized for the two groups in Table 8 along with their mean changes from baseline at the three follow-up time points. As shown, these parameters remained generally stable and within clinically acceptable ranges in both groups throughout the trial with no differences observed. Similar observations were made for BP parameters and heart rate (Table 9).
Table 8.
Time course of metabolic parameters during the 18-month follow-up period in participants receiving ORBCEL-M or placebo
| Parameter | ORBCEL-M Mean±SD |
Difference versus Baseline Mean (Range) |
Placebo Mean±SD |
Difference versus Baseline Mean (Range) |
P Valuea |
|---|---|---|---|---|---|
| Glucose (mg/dl) | |||||
| Baseline | 142±41 | 177±47 | |||
| 6 mo | 150±68 | 8 (−23 to 39) | 143±64 | −19 (−64 to 27) | 0.323 |
| 12 mo | 135±40 | −11 (−33 to 11) | 141±38 | −34 (−88 to 16) | 0.482 |
| 18 mo | 144±44 | −1 (−31 to 29) | 159±44 | −18 (−55 to 19) | 0.826 |
| HbA1c (mmol/mol) | |||||
| Baseline | 58±13 | 55±12 | |||
| 6 mo | 55±16 | −2 (−9 to 5) | 49±14 | −2 (−29 to 26) | 0.971 |
| 12 mo | 55±11 | −5 (−13 to 3) | 52±9 | −3 (−24 to 18) | 0.940 |
| 18 mo | 58±13 | −1 (−7 to 5) | 56±12 | 0.27 (−29 to 29) | 0.959 |
| Total cholesterol (mg/dl) | |||||
| Baseline | 170±39 | 119±42 | |||
| 6 mo | 159±48 | −10 (−27 to 8) | 119±46 | −1 (−11 to 9) | 0.645 |
| 12 mo | 165±57 | −8 (−32 to 16) | 122±46 | 2 (−39 to 43) | 0.507 |
| 18 mo | 161±60 | −10 (−39 to 19) | 121±33 | 0.04 (−13 to 13) | 0.695 |
| Triglycerides (mg/dl) | |||||
| Baseline | 206±108 | 101±59 | |||
| 6 mo | 186±86 | −29 (−66 to 9) | 122±78 | 21 (−53 to 95) | 0.619 |
| 12 mo | 167±62 | −42 (−91 to 6) | 130±41 | 20 (−92 to 132) | 0.919 |
| 18 mo | 180±90 | −35 (−69 to −2) | 202±145 | 30 (−104 to 164) | 0.282 |
| C-reactive protein (mg/dl) | |||||
| Baseline | 0.38±0.47 | 0.54±0.31 | |||
| 6 mo | 1.07±2.64 | 0.67 (−1.11 to 2.45) | 0.29±0.20 | −0.23 (−1.25 to 0.78) | 0.625 |
| 12 mo | 0.47±0.69 | 0.15 (−0.41 to 0.70) | 0.74±0.31 | 0.16 (−0.92 to 1.23) | 0.642 |
| 18 mo | 0.25±0.15 | −0.09 (−0.41 to −0.23) | 0.30±0.10 | −0.27 (−1.16 to 0.62) | 0.996 |
By ANCOVA adjusted for baseline value. ORBCEL-M versus Placebo, as difference versus baseline.
Table 9.
Time course of arterial BP and heart rate during the 18-month follow-up period in participants receiving ORBCEL-M or placebo
| Parameter | ORBCEL-M Mean±SD |
Difference versus Baseline Mean (Range) |
Placebo Mean±SD |
Difference versus Baseline Mean (Range) |
P Valuea |
|---|---|---|---|---|---|
| SBP (mm Hg) | |||||
| Baseline | 136±12 | 136±10 | |||
| 6 mo | 144±17 | 8 (−3 to 19) | 151±15 | 18 (0 to 35) | 0.421 |
| 12 mo | 137±16 | 1 (−12 to 20) | 134±9 | −2 (−15 to 11) | 0.761 |
| 18 mo | 135±23 | 0 (−16 to 15) | 135±12 | −1 (−14 to 12) | 0.968 |
| DBP (mm Hg) | |||||
| Baseline | 72±9 | 78±10 | |||
| 6 mo | 75±9 | 3 (−6 to 12) | 79±14 | 1 (−16 to 18) | 0.580 |
| 12 mo | 72±9 | 1 (−8 to 10) | 76±9 | −2 (−12 to 8) | 0.673 |
| 18 mo | 68±7 | −3 (−10 to 3) | 73±15 | −4 (−12 to 3) | 0.812 |
| MAP (mm Hg) | |||||
| Baseline | 93±8 | 97±9 | |||
| 6 mo | 98±7 | 5 (−3 to 12) | 103±13 | 7 (−10 to 24) | 0.377 |
| 12 mo | 94±8 | 1 (−8 to 11) | 96±8 | −2 (−11 to 7) | 0.572 |
| 18 mo | 91±8 | −2 (−10 to 5) | 94±13 | −3 (−12 to 6) | 0.835 |
| Heart rate (beats/min) | |||||
| Baseline | 67±13 | 71±20 | |||
| 6 mo | 66±13 | −1 (−5 to 3) | 68±17 | −6 (−25 to 12) | 0.375 |
| 12 mo | 69±9 | 1 (−7 to 9) | 70±13 | 0 (−12 to 11) | 0.899 |
| 18 mo | 68±15 | 1 (−5 to 7) | 74±8 | 4 (−19 to 26) | 0.461 |
SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure.
aBy ANCOVA adjusted for baseline value. ORBCEL-M versus Placebo, as difference versus baseline.
Effects on Immunological and Inflammatory Parameters
Screening for anti-HLA class I and class II antibodies was negative at all scheduled visits for 15 of 16 participants. One participant treated with ORBCEL-M tested positive for low-level anti-HLA class I antibodies starting at 12 months after infusion, which persisted to the end of trial follow-up without any clinically relevant consequences.
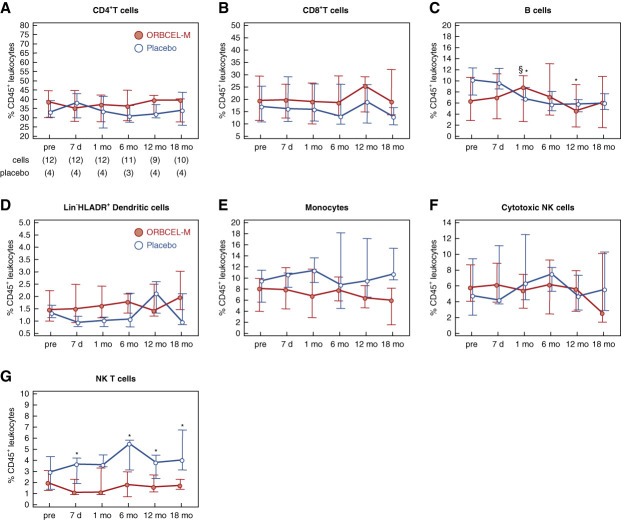
Regarding longitudinal PBMC profiling by flow cytometry, no significant changes or between-group differences were observed in the proportions of CD4+ and CD8+ T cells (Figure 2, A and B). There were small but significant differences in B-cell frequencies between recipients of ORBCEL-M and placebo at 1 and 12 months, although no clear divergence was observed in the trends over time (Figure 2C). The proportions of dendritic cells, total monocytes, and cytotoxic NK cells were similar between the two groups throughout follow-up (Figure 2, D–F). By contrast, the frequency of natural killer T cells, which was similar for the two groups at baseline, was significantly lower in ORBCEL-M–treated participants throughout the postinfusion follow-up period (Figure 2G).
Figure 2.
Frequency of peripheral blood leukocytes during the study period. Percentages of CD4+ T cells (A), CD8+ T cells (B), B cells (C), Lin−HLADR+ dendritic cells (D), monocytes (E), cytotoxic NK cells (F), and natural killer T cells (G) within CD45+ peripheral blood leukocytes in participants randomized to ORBCEL-M or placebo during the follow-up. Values are expressed as median (IQR). *P < 0.05 between the ORBCEL-M and placebo groups (ANCOVA). §P < 0.05 versus preinfusion in the group (Wilcoxon test). Lin−: CD3, CD14, CD16, CD19, CD20, and CD56.
Analysis of the proportions of regulatory T cells (Tregs) among total CD3+CD4+ T cells indicated significantly higher proportions in ORBCEL-M–treated participants at 6 months (Figure 3A). Furthermore, a subanalysis demonstrated that the proportions of memory Tregs (defined as CD45RA−RO+) declined over time in placebo-treated participants, but remained stable in ORBCEL-M–treated participants—in association with significantly different proportions at 6 and 18 months (Figure 3B). This was particularly evident for the subpopulation of memory Tregs defined as Helios+CD95+HLA-DR− (Figure 3C). Proportions of naïve Tregs (defined as CD45RA+RO− Tregs) remained comparably low throughout the trial in both groups (Figure 3D).
Figure 3.
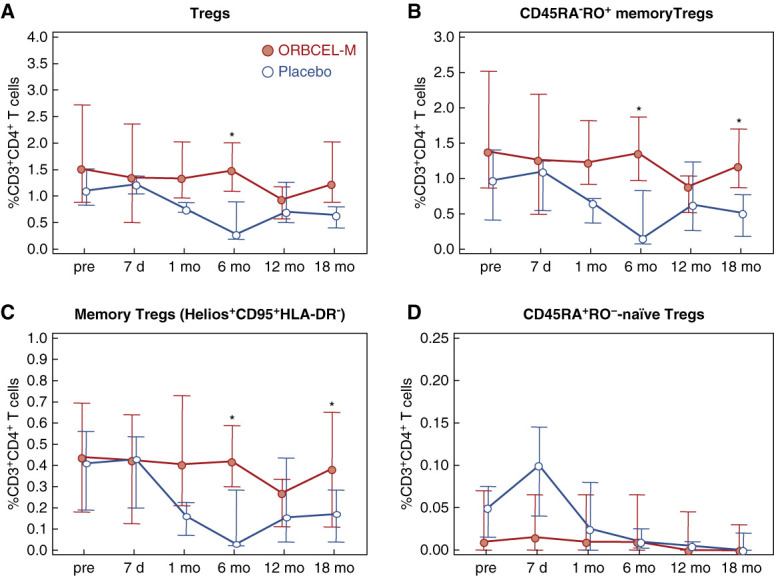
Frequency of peripheral blood Tregs and Treg subpopulations during the study period. Percentages of Tregs (A), CD45RA−RO+ memory Tregs (B), Helios+CD95+HLA-DR− memory Tregs (C), and CD45RA+RO−-naïve Tregs (D) within peripheral blood CD3+CD4+ T cells in participants randomized to ORBCEL-M or placebo during the follow-up. Values are expressed as median (IQR). Tregs, regulatory T cells. *P < 0.05 between the ORBCEL-M and placebo groups (ANCOVA). Tregs, regulatory T cells.
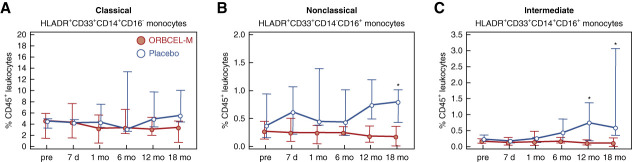
An analysis of monocyte repertoire was performed using the well-accepted 3-subset classification of classical, nonclassical, and intermediate monocytes, expressed as proportions of total CD45+ cells.37 As shown in Figure 4A, the proportions of the most numerous, classical monocyte subset remained stable and very similar between the two groups through the trial follow-up period. By contrast, proportions of both the nonclassical and intermediate subsets tended to increase in the placebo-treated group between 6 and 18 months while remaining stable throughout the trial in the ORBCEL-M–treated group—being significantly lower at 18 months for nonclassical monocytes and at 12 and 18 months for intermediate monocytes (Figure 4, B and C).
Figure 4.
Frequency of peripheral blood monocyte subpopulations during the study period. Percentages of HLADR+CD33+CD14+CD16− monocytes (A), HLADR+CD33+CD14−CD16+ monocytes (B), and HLADR+CD33+CD14+CD16+ monocytes (C) within CD45+ peripheral blood leukocytes in participants randomized to ORBCEL-M or placebo during the follow-up period. Values are expressed as median (IQR). *P < 0.05 between the ORBCEL-M and placebo groups (ANCOVA).
Longitudinal analyses of the serum concentrations of inflammatory biomarkers sTNFR1, NGAL, and VCAM-1 indicated trends of increasing levels during the 18-month follow-up period with no between-group differences (Figure 5, A–C). The serum concentration of EGF remained stable in both groups (Figure 5D).
Figure 5.
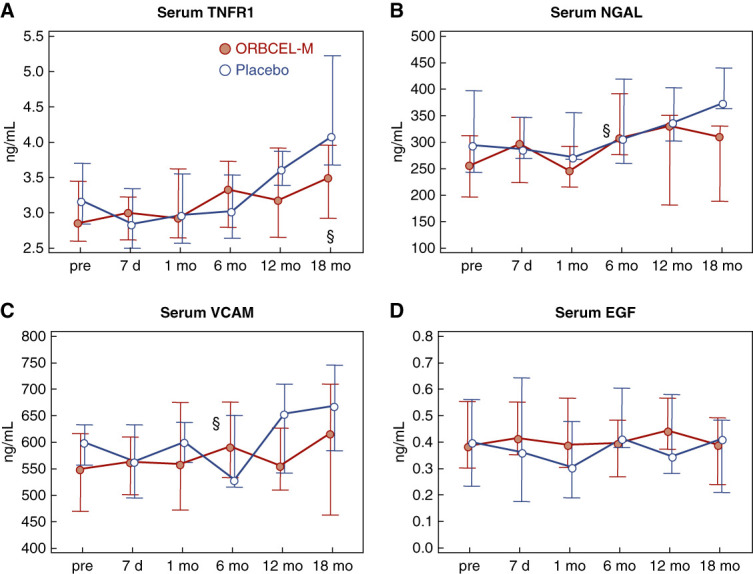
Proinflammatory mediators. Serum concentrations of TNFR1 (A), NGAL (B), VCAM-1 (C), and EGF (D) in participants randomized to ORBCEL-M or placebo during the follow-up. Values are expressed as median (IQR). §P < 0.05 versus preinfusion in the group (Wilcoxon test). TNFR1, soluble tumor necrosis factor 1; NGAL, neutrophil gelatinase-associated lipocalin; VCAM-1, vascular cell adhesion molecule 1; EGF, epidermal growth factor.
Other Exploratory Comparisons
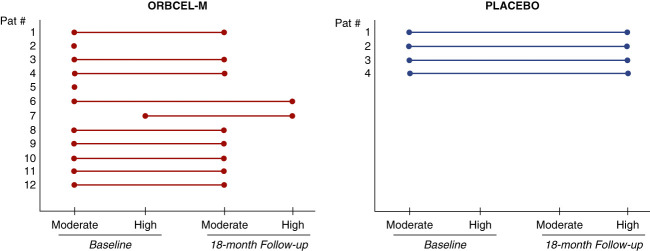
As a post hoc analysis, the progression of CKD for transitions from baseline to 18 months in the two-year risk category for reaching ESKD was compared for the two groups using the kidney failure risk equation.29,30 As shown in Figure 6, the baseline 2-year risk category was moderate for 11 of 12 participants of the ORBCEL-M–treated group and 4 of 4 of the placebo-treated group. One participant who received ORBCEL-M was categorized as being at high risk at baseline. Of the ten participants who received cell therapy and completed 18 months of follow-up, eight remained in the moderate-risk category, one had transitioned from moderate to high risk, and one remained in the high-risk category. By contrast, all four participants who receive placebo had progressed from the moderate to high-risk category.
Figure 6.
Progression of diabetic kidney disease in the two study groups. Risk of diabetic kidney disease progression in participants randomized to ORBCEL-M or placebo for change in the 2-year risk of achieving end stage kidney failure from baseline to 18 months on the basis of the validated Tangri 4-variable kidney failure risk equation. Total number of participants at baseline, ORBCEL-M n=12 and placebo n=4, and at 18 months ORBCEL-M n=10 (2 died before the end of the study) and placebo n=4.
In correlative analyses of the combined immune/inflammatory profiling dataset, it was observed that peripheral blood proportions of total Tregs and CD45RA−RO+ memory Tregs for the whole cohort correlated significantly with the coincident serum concentrations of sTNFR1 (Supplemental Figure 1, A and B). In a subgroup analysis, these correlations remained statistically significant for the group that received ORBCEL-M (P < 0.002 for both cell subsets), but not for placebo-treated participants (P = 0.364 and P = 0.063 for Tregs and CD45RA−RO+ memory Tregs, respectively). Negative correlations were also observed between proportions of total Tregs or CD45RA−RO+ memory Tregs and coincident serum NGAL concentrations (Supplemental Figure 1, C and D). In the cohort as a whole, there were significant positive correlations between the total Treg or CD45RA−RO+ memory Treg proportions and coincident GFR estimated by both CKD-EPI and MDRD equations (Supplemental Figure 2, A–D). Finally, serum sTNFR1 and NGAL concentrations negatively correlated with both mGFR and eGFR (CKD-EPI and MDRD) in the overall cohort (Supplemental Figure 3, A–F) and in the ORBCEL-M–treated group (sTNFR1: P < 0.001 versus CKD-EPI and MDRD and P = 0.003 versus mGFR; NGAL: P < 0.001 versus CKD-EPI and MDRD and mGFR).
Discussion
In the first-dose cohort of this phase 1b/2a multicenter, randomized, double-blind, placebo-controlled clinical trial, we observed that a single iv infusion of 80×106 next-generation, bone marrow–derived, anti-CD362–selected, allogeneic MSCs (ORBCEL-M) was well-tolerated in participants with type 2 diabetes and progressive DKD. This acceptable safety profile was sustained in the weeks and months thereafter up to the end of the 18-month follow-up period. Importantly, SAEs, which occurred at longer time intervals after cell administration, resulted in the death of two patients. These events, however, were deemed not to be related to the trial investigational product, on the basis of the well-recognized short in vivo persistence of intravenously administered MSCs as well as the likelihood of alternative causal factors linked to medical comorbidities associated with type 2 diabetes and DKD.38 Indeed, the participant who died because of an acute cardiovascular event had received the cell infusion more than 14 months previously. This late time point would preclude a role for the activation of the coagulation system because rarely associated acute thromboembolic events have only been described at the end of or a few days after iv administration of MSC-based products.39,40 In this regard, a recent meta-analysis of the reported outcomes of 55 randomized clinical trials, which enrolled more than 2600 participants with a range of significant medical conditions (e.g., cardiovascular, neurological, kidney and liver diseases), showed that MSC administration was associated with an increased risk of fever, but not non-fever acute infusional toxicity, thrombotic/embolic events, malignancy, or death compared with a control group which did not receive cell therapy.41 None of the included clinical trials was ended prematurely because of safety concerns.41
One theoretical concern with culture-expanded progenitor cell therapies, such as MSC therapy, is the possibility that such cells transform during culture and acquire the potential to give rise to tumors in the recipient after infusion. Critically, whereas malignant transformation has been demonstrated with murine MSCs, no such event has been reported for human MSC-based therapies.42 Furthermore, an autopsy study performed on 18 patients who had received allogeneic MSCs for hematological malignancies or solid tumors and had died between 3 and 408 days after the last MSC infusion found no ectopic tissue formation or malignant tumors of MSC origin by macroscopic or histological examination.43 In addition, despite their immunomodulatory properties, MSC therapies have not been associated with increased risk of developing malignancies in solid-organ transplant recipients receiving long-term immunosuppressive drugs.44 For the trial participant who died because of myeloma, the lack of prior reports of new or accelerated cancers among thousands of recipients of MSC therapies for diverse clinical indications (including many immunosuppressed patients with allogeneic hematopoietic stem cells transplant for bone marrow malignancies as well organ transplants and autoimmune diseases), the short duration of the culture expansion protocol for ORBCEL-M manufacture and the strict criteria for GMP release of the cell product lead us to conclude that the condition was highly unlikely to have been caused or exacerbated by the trial intervention. Nonetheless, it is possible, if not likely, that this participant had nonclinically detected plasma cell dyscrasia with circulating monoclonal protein before enrollment. This highlights the potential for non–diabetes-associated renal pathology to be present in patients participating in clinical trials for DKD (which typically relies on clinical rather than biopsy diagnosis of diabetic nephropathy) as well as the need to consider specifically screening for monoclonal gammopathy in trials of cell and other immune modulatory therapies for kidney disease.
A limited number of previously reported clinical trials have addressed the safety, tolerability, and potential benefits of MSC-based therapies in participants with type 2 DM.45 Such trials used autologous or allogeneic MSCs from different tissue sources; were infused into a peripheral vein or through the pancreatic artery; and, in most cases, involved cells isolated and manufactured by plastic adherence, which results in a heterogeneous/unselected stromal cell product. Extensively characterized MSC products manufactured from more selected primary tissue precursors may provide superior and more consistent therapeutic effects and may also be better suited to meet future regulatory criteria for advanced cell products. Only the early-phase trial reported by Packham et al. tested the effects of an antibody-purified allogeneic stromal cell product—specifically mesenchymal precursor cells selected for expression of the surface marker Stro3 (MPC, rexlemestrocel-L, from Mesoblast Ltd)—in participants with established DKD.25 The ORBCEL-M product investigated in the NEPHSTROM trial was also manufactured from primary marrow stromal cells, but was selected for expression of a different surface marker (CD362, also referred to as syndecan 2) and was culture-expanded in a hollow-fiber bioreactor such that the cells were at an early passage number when administered. Thus, the product we tested is novel and well-characterized with potential for therapeutic benefits that are distinct from those of other stromal cell therapies tested in people with type 2 diabetes. The results we report here indicate ORBCEL-M and rexlemestrocel-L to be equally safe and tolerable with preliminary evidence of clinically relevant efficacy. Whether ORBCEL-M may provide advantages, such as superior potency, greater efficacy, or lower cost, than other MSC-based cell therapies that have been investigated in diabetes and DKD cannot be determined until larger or comparative studies have been performed. As for the metabolic effects of ORBCEL-M, there were no notable changes in glycemic parameters (fasting plasma glucose, HbA1c) during the 18 months after cell infusion. Although preclinical and some clinical studies have suggested the potential for MSC therapy to improve glycemic control,45 our results are consistent with those of other early-phase trials in similar participants with type 2 DM.25,46 This may reflect, in part, the fact that the trial participants had very good glycemic control at the time of enrollment. Notably, administration of ORBCEL-M, an allogeneic cell product, proved to have minimal capacity for immunological sensitization, as evidenced by the lack of emergence of detectable anti-HLA antibodies over 18 months of follow-up in all but one participant who developed low-level anti-HLA class I antibodies from 12 months after infusion. These observations are in keeping with findings of other clinical trials which have reported that allogeneic bone marrow–derived MSC products can be safely administered to humans without eliciting clinically relevant immunological reactions.47–49 Of direct relevance to the results we report here, two reported results of clinical trials of the allogeneic mesenchymal precursor cell product, rexlemstrocel-L, in participants with type 2 DM or diabetic nephropathy, also indicated a lack of development of persistent de novo donor-specific antibodies.25,46 The observed lack of frequent or high-level sensitization against allogeneic HLA is of particular importance for the future role of ORBCEL-M, or other allogeneic MSC-derived products, in the setting of kidney disease, particularly from the perspective of the potential subsequent need for kidney transplantation.
The completed 18-month follow-up of NEPHSTROM cohort 1 also showed that the rate of decline of mGFR was numerically but not significantly lower for recipients of ORBCEL-M than for recipients of placebo while similar trends for eGFR did reach statistical significance. This occurred in the setting of comparable relevant baseline characteristics and similarly acceptable metabolic and BP control between the two treatment groups. The further observation that the KRFE-based 2-year risk category for reaching ESKF worsened in all placebo-treated participants but remained stable in most of the evaluable recipients of ORBCEL-M also favors a cautious conclusion that divergent post-treatment renal functional trajectories occurred in these two groups of participants with preexisting rapidly progressive DKD.
Regarding mechanism of action, MSCs are now considered to mediate their therapeutic benefits predominantly through inducible secretion of paracrine mediators and reprogramming of myeloid and lymphoid immune cells.50,51 For example, several lines of evidence have shown that MSCs promote IL-10 production by T cells through inhibition of the differentiation of Th1 and Th17 cells, thereby inducing the generation of Tregs.52 Furthermore, MSCs may act indirectly to promote the induction and expansion of Tregs as well as other anti-inflammatory mediators by modulating the activities of monocytes, macrophages, and dendritic cells.26,53 It is of interest, therefore, that we observed a divergence in the trends for circulating memory Tregs between the two trial groups—with recipients of placebo (who experienced greater rates of decline of eGFR) having diminishing proportions over time while recipients of ORBCEL-M retained more stable proportions. Although a cause–effect relationship cannot be concluded from these findings, the positive correlations observed between Tregs/memory Tregs and eGFR as well as the inverse correlations between the coincident serum concentrations of well-established DKD-associated inflammatory biomarkers sTNFR1 and NGAL and eGFR for the cohort as a whole (and in the ORBCEL-M-treated group) tend to support the hypothesis that infusion of the allogeneic MSC product is associated with a sustained immunomodulatory/anti-inflammatory effect with potential for modulating aspects of the pathophysiology of progressive DKD.50 Also consistent with this hypothesis is the divergence that occurred between cell therapy and placebo recipients at later time points in the trial for proportions of circulating intermediate monocytes, which have proinflammatory properties and have been linked to cardiovascular disease and rate of eGFR decline in CKD.54,55 It remains possible that clinically applicable assays of serum, plasma, and urine biomarkers linked to systemic and intrarenal inflammation or fibrosis will provide value as indicators of response to MSC therapy. However, the interindividual variability we observed for selected serum biomarkers in this cohort suggests that larger participant numbers will be required to meaningfully investigate this question. Finally, it should be acknowledged that the potential anti-inflammatory/immune-modulating effects observed in recipients of ORBCEL-M and documented in many other preclinical and clinical studies bring at least a theoretical risk of promoting or worsening infection and cancer and that this potential must continue to be investigated in a careful and unbiased manner in trials such as ours.
A number of limitations to this study should be acknowledged. Clearly, the small sample size, although appropriate for a first-in-human trial, cannot exclude the potential for rarer AEs. Moreover, the trial duration of 18 months may be too brief to evaluate the long-term clinical effect of a proregenerative therapeutic intervention for a chronic disease with a variable progression rate and risk of complications, such as DKD. Importantly, this challenge was carefully considered in the design of the trial through the selection of participants with documented relatively rapid progression of DKD and a threshold UACR before enrollment who, we believed, were more likely to manifest a detectable effect of the cell therapy. It should also be noted that important new drug classes (SGLT2 inhibitors and nonsteroidal mineralocorticoid receptor antagonists) with renoprotective effects in DKD have emerged since this cohort was enrolled. Inclusion of these agents and/or focusing on participants unable to tolerate them will be critical issues for future trial designs, as well as for the health economic assessment of ORBCEL-M and other novel therapies for this condition.56 Moreover, we do not believe that the unintended 10-year difference in median age between placebo and ORBCEL-M recipient groups introduced a major bias because mGFR and eGFR values at enrollment as well as the calculated risk of progression of DKD at enrollment were closely comparable between the two groups. Regarding the fact that only male participants were recruited, this was also unintentional because both male and female participants attending diabetes and nephrology clinics at the three enrollment sites were screened without bias for eligibility. Importantly, however, this limitation precludes a direct demonstration of the safety/tolerability and preliminary efficacy issues of ORBCEL-M in female participants, which should be addressed in future studies. Regarding the exploratory PBMC profiling and serum biomarker analyses, while the results provide some intriguing observations with credible links to MSC mechanisms of action and renal functional trajectory in the setting of DKD, we acknowledge that substantially larger numbers of participants per group will be necessary to validate these findings in future trials. Finally, it was not possible to directly determine the bio-distribution of administered cells in a trial such as this. In particular, we cannot determine whether some of the administered cells migrated to the affected kidney and, if so, how long they persisted. However, extensive animal studies and a limited number of human studies have consistently shown that intravenously administered MSCs can be expected to become predominantly localized within the lungs for 24–48 hours after administration where the majority are cleared by processes which have been directly linked to their mechanism of action in inflammatory conditions while a minority may redistribute to the liver, spleen, and other organs.57 Some preclinical studies have specifically demonstrated migration of systemically injected MSCs to the kidneys with variable duration of engraftment, raising the possibility that they also elicit repair through localized paracrine mechanisms that modulate the intrarenal immune/inflammatory responses.21,58,59
In conclusion, the results reported here for a completed cohort of the multisite, randomized, double-blind, placebo-controlled NEPHSTROM trial document the safety and tolerability of a single infusion of 80×106 ORBCEL-M. In addition, our findings confirm low potential for this MSC-based cell therapy product to sensitize recipients against allogeneic HLA and reveal preliminary evidence for potential renoprotective and immune modulatory effects over an 18-month postinfusion observation period. These results, as well as the continued need for new, disease-modulating therapies to preserve renal function in people with progressive DKD, support further investigation of ORBCEL-M in an appropriately sized and powered phase 2b study.
Supplementary Material
Acknowledgments
We thank all patients for participation in our study. In addition, we are grateful to all medical, nursing, and laboratory personnel in the clinical centers involved in the NEPHSTROM trial for their support. The materials presented and views expressed are the responsibility of the authors only. The EU Commission takes no responsibility for any use made of the information set out.
Footnotes
N.P., G.R., and M.D.G. contributed equally to this work.
Members of the NEPHSTROM Trial Consortium include: Norberto Perico, Giuseppe Remuzzi, Matthew D. Griffin, Paul Cockwell, Alexander P. Maxwell, Federica Casiraghi, Nadia Rubis, Tobia Peracchi, Alessandro Villa, Marta Todeschini, Fabiola Carrara, Bernadette A. Magee, Piero L. Ruggenenti, Stefano Rota, Laura Cappelletti, Veronica McInerney, Tomás P. Griffin, Md Nahidul Islam, Martino Introna, Olga Pedrini, Josée Golay, Andrew A. Finnerty, Jon Smythe, Willem E. Fibbe, Stephen J. Elliman, Timothy O'Brien, Valentina Portalupi, Eliana Gotti, Elena Perticucci, Grazia Natali, Alessandro Rambaldi, Giuseppe Gritti, Anna Maria Barbui, Silvia Ferrari, Silvia Mariani, Gianmaria Borleri, Michelle Hennessy, Sarah Cormican, Nathan Devaney, Cassandra Phan, Amy Hanson, Sara Martyn, Joy Buckley, Sean Naughton, Julie Woods, Caroline Kelly, Amjad Hayat, Dimitrios Chanouzas, Lesley Fifer, Kulli Kuningas, Natalie Walmsley-Allen, Amisha Desai, Lucy Atchinson, Sinead White, Vijayan Suresh, Katie Kirkham, Fiona Evans, Mark Little, Piergiorgio Messa, Christina Yap, Olimpia Diadei, Paola Boccardo, Oleksii Noreiko, Davide Martinetti, Giovanni Antonio Giuliano, Annalisa Perna, Daniela Cugini, Silvia Ferrari, Nadia Stucchi, Marilena Mister, Lisa O'Flynn, Yuka Shimizu, Helene Roelofs, Esther Steeneveld, Brigitte Wieles, Chiara Capelli, Eric Austen, Helen Murray, Vivien Hanson, Jenny Chan, Aoife Duffy, Miriam Holohan, Janusz Krawczyk, Matthew Duggan, Lauren Connolly, Amjad Hyatt, Janusz Krawczyk, Margaret Tarpey, Sean Naughton, Joy Buckley, Layka Abbasi Asbagh, Brent Rice, Stefano Baila, Grace Davey, Michael Creane, Ciaran Clissmann, and Mark Sweetnam. Additional information can be found in the Supplemental File.
Contributor Information
Collaborators: Norberto Perico, Giuseppe Remuzzi, Matthew D. Griffin, Paul Cockwell, Alexander P. Maxwell, Federica Casiraghi, Nadia Rubis, Tobia Peracchi, Alessandro Villa, Marta Todeschini, Fabiola Carrara, Bernadette A. Magee, Piero L. Ruggenenti, Stefano Rota, Laura Cappelletti, Veronica McInerney, Tomás P. Griffin, Md Nahidul Islam, Martino Introna, Olga Pedrini, Josée Golay, Andrew A. Finnerty, Jon Smythe, Willem E. Fibbe, Stephen J. Elliman, Timothy O'Brien, Valentina Portalupi, Eliana Gotti, Elena Perticucci, Grazia Natali, Alessandro Rambaldi, Giuseppe Gritti, Anna Maria Barbui, Silvia Ferrari, Silvia Mariani, Gianmaria Borleri, Michelle Hennessy, Sarah Cormican, Nathan Devaney, Cassandra Phan, Amy Hanson, Sara Martyn, Joy Buckley, Sean Naughton, Julie Woods, Caroline Kelly, Amjad Hayat, Dimitrios Chanouzas, Lesley Fifer, Kulli Kuningas, Natalie Walmsley-Allen, Amisha Desai, Lucy Atchinson, Sinead White, Vijayan Suresh, Katie Kirkham, Fiona Evans, Mark Little, Piergiorgio Messa, Christina Yap, Olimpia Diadei, Paola Boccardo, Oleksii Noreiko, Davide Martinetti, Giovanni Antonio Giuliano, Annalisa Perna, Daniela Cugini, Silvia Ferrari, Nadia Stucchi, Marilena Mister, Lisa O'Flynn, Yuka Shimizu, Helene Roelofs, Esther Steeneveld, Brigitte Wieles, Chiara Capelli, Eric Austen, Helen Murray, Vivien Hanson, Jenny Chan, Aoife Duffy, Miriam Holohan, Janusz Krawczyk, Matthew Duggan, Lauren Connolly, Amjad Hyatt, Janusz Krawczyk, Margaret Tarpey, Sean Naughton, Joy Buckley, Layka Abbasi Asbagh, Brent Rice, Stefano Baila, Grace Davey, Michael Creane, Ciaran Clissmann, and Mark Sweetnam
Disclosures
P. Cockwell reports Advisory or Leadership Role: UK Kidney Association (President and Trustee); Speakers Bureau: Amgen; and Other Interests or Relationships: Boehringer Ingleheim—non-remunerated research collaboration, AstraZeneca—non-remunerated clinical development program, and Glaxo Smith Kline—non-remunerated sponsored session chair at ISN 2020. S.J. Elliman is an employee of and equity holder in Orbsen Therapeutics. He was not involved in recruitment or follow-up of participants enrolled in the trial, nor was involved in the collection, analysis, and interpretation of the data presented herein. S.J. Elliman also reports Research Funding: Orbsen Therapeutics; Patents or Royalties: Orbsen Therapeutics; and Speakers Bureau: Orbsen Therapeutics. W.E. Fibbe reports Consultancy: Boost Pharma, Glycostem Therapeutics (iDMC), and Starfish Innovations; and Advisory or Leadership Role: Starfish Innovations. M.D. Griffin reports honoraria from the American Society of Nephrology, Hebei Medical University in China, Novo Nordisk, and Théa Pharma Ltd. in Ireland, as well as advisory roles as an Editorial Board member for the journals Frontiers in Pharmacology and Transplantation and an Associate Editor for JASN and Mayo Clinic Proceedings. M.D. Griffin also reports Research Funding: Orbsen Therapeutics Ltd.; Honoraria: American Society of Nephrology and National Institutes of Health (NIDDK). T.P. Griffin reports Consultancy: Abbvie, AztraZeneca, Dexcom, Eli Lilly, and Novo Nordisk; Research Funding: Hardiman Scholarship from the College of Medicine, Nursing and Health Science at the National University of Ireland in Galway, a bursary from the Irish Endocrine Society/Royal College of Physicians of Ireland, and a grant from the European Commission Horizon 2020 Collaborative Health Project NEPHSTROM (grant number 634086); Honoraria: Abbvie, AztraZeneca, Dexcom, Eli Lilly, and Novonordisk; Advisory or Leadership Role: Irish Diabetes Technology Network—no fee, Diabetes Ireland Research Alliance—no fee; Speakers Bureau: Dexcom, Eli Lilly, and Novonordisk; and Other Interests or Relationships: Member of Diabetes UK, Irish Endocrine Society, ADA, EASD, TG and spouse have pensions that are invested by pension providers. TG and spouse not aware of the stocks purchased by these pension providers but could include pharmaceutical company stocks. TG collaborated with RANDOX Teronta and was a subinvestigator for studies conducted by Medtronic and Abbot. M. Introna reports Research Funding: Associazione Italiana Ricerca sul Cancro (AIRC), Fondazione Regionale per la Ricerca Biomedica Regione Lombardia (FRBB), and European Horizion 2017. T. O’Brien is a Director and Equity holder in Orbsen Therapeutics. He was not involved in recruitment or follow-up of participants enrolled in the trial, nor was involved in the collection, analysis, and interpretation of the data presented herein. T. O’Brien also reports Employer: University of Galway; Consultancy: AstraZeneca and Novo Nordisk; and Advisory or Leadership Role: Board member of Orbsen Therapeutics. G. Remuzzi reports Consultancy: Consulting fees: Alexion Pharmaceuticals, AstraZeneca Pharmaceuticals, Otsuka, and Silence Therapeutics; and Advisory or Leadership Role: member of numerous Editorial Boards of Scientific Medical Journals. J. Smythe reports Other Interests or Relationships: JACIE Inspector. M. Todeschini reports Employer: Recordati S.p.A. (spouse). All remaining authors have nothing to disclose. Because Matthew Griffin is an editor of the Journal of the American Society of Nephrology, he was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript.
Funding
This study was supported by EU-Horizon 2020 Framework Programme grant 634086. The funder had no role in design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit it for publication. MDG, TPG, MdNI, and TOB were also funded for work relevant to this report by a Science Foundation Ireland Research Centres grant (CÚRAM, grant number 13/RC/2073_P2), a Hardiman Scholarship from the University of Galway College of Medicine, Nursing and Health Science, a bursary from the Irish Endocrine Society/Royal College of Physicians of Ireland, and by the European Regional Development Fund.
Author Contributions
Conceptualization: Paul Cockwell, Matthew D. Griffin, Alexander P. Maxwell, Timothy O’Brien, Norberto Perico, Giuseppe Remuzzi.
Trial organization and data management: Veronica McInerney, Nadia Rubis, Alessandro Villa.
Formal analysis: Tobia Peracchi.
Funding acquisition and project administration: Timothy O’Brien.
Investigation: Laura Cappelletti, Paul Cockwell, Matthew D. Griffin, Tomás P. Griffin, Norberto Perico, Stefano Rota, Piero L. Ruggenenti.
Methodology: Fabiola Carrara, Federica Casiraghi, Stephen J. Elliman, Willem E. Fibbe, Andrew A. Finnerty, Matthew D. Griffin, Tomás P. Griffin, Josée Golay, Martino Introna, Md Nahidul Islam, Bernadette A. Magee, Alexander P. Maxwell, Olga Pedrini, Norberto Perico, Jon Smythe, Marta Todeschini.
Software: Nadia Rubis
Supervision: Norberto Perico, Giuseppe Remuzzi.
Writing – original draft: Norberto Perico, Giuseppe Remuzzi, Matthew D. Griffin.
Writing – review & editing: Federica Casiraghi, Paul Cockwell, Matthew D. Griffin, Alexander P. Maxwell, Norberto Perico, Giuseppe Remuzzi.
Data Sharing Statement
Sharing of individual participant data with third parties was not specifically included in the informed consent of the study, and unrestricted diffusion of such data may pose a potential threat of revealing participants' identities, as permanent data anonymization was not carried out (participants records were instead deidentified per protocol during the data retention process). To minimize this risk, individual participant data that underlie the results reported in this article will be available after three months and up to five years from article publication. Researchers shall submit a methodologically sound proposal to Dr. Annalisa Perna (annalisa.perna@marionegri.it), head of the Laboratory of Biostatistics of the Department of Renal Medicine of the Istituto di Ricerche Farmacologiche Mario Negri IRCCS. To gain access, data requestors will need to sign a data access agreement and obtain the approval of the local ethics committee.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E500.
Supplemental Results. Considerations for cohort 1 final analysis.
Supplemental Table 1. List of antibodies and the relative fluorochrome (as Format).
Supplemental Figure 1. Correlations between percentages of Tregs or CD45RA−RO+ memory Tregs and serum concentrations of inflammatory mediators in the overall study cohort.
Supplemental Figure 2. Correlations between percentages of Tregs or CD45RA−RO+ memory Tregs and eGFR in the overall study cohort.
Supplemental Figure 3. Correlations between serum concentrations of inflammatory mediators and measured or eGFR in the overall study cohort.
NEPHSTROM Trial Consortium. Members, coordinating centers and contributions.
References
- 1.Safiri S, Nejadghaderi SA, Karamzad N, Kaufman JS, Carson-Chahhoud K, Bragazzi NL. Global, regional and national burden of cancers attributable to high fasting plasma glucose in 204 countries and territories, 1990-2019. Front Endocrinol (Lausanne). 2022;13:879890. doi: 10.3389/fendo.2022.879890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes. 2006;55(6):1832–1839. doi: 10.2337/db05-1620 [DOI] [PubMed] [Google Scholar]
- 3.Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(suppl 2):64–78. doi: 10.2337/diab.32.2.s64 [DOI] [PubMed] [Google Scholar]
- 5.Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3(5):382–391. doi: 10.1016/s2213-8587(15)00094-7 [DOI] [PubMed] [Google Scholar]
- 6.Kanwar YS, Sun L, Xie P, Liu F-Y, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol. 2011;6(1):395–423. doi: 10.1146/annurev.pathol.4.110807.092150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124(6):2333–2340. doi: 10.1172/jci72271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley CD. Role of senescent renal cells in pathophysiology of diabetic kidney disease. Curr Diab Rep. 2020;20(8):33. doi: 10.1007/s11892-020-01314-y [DOI] [PubMed] [Google Scholar]
- 9.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011 [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6(6):319–330. doi: 10.1038/nrneph.2010.58 [DOI] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351(19):1941–1951. doi: 10.1056/nejmoa042167 [DOI] [PubMed] [Google Scholar]
- 12.Parving HH Lehnert H Bröchner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi: 10.1056/nejmoa011489 [DOI] [PubMed] [Google Scholar]
- 13.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/nejmoa011303 [DOI] [PubMed] [Google Scholar]
- 14.Heart Outcomes Prevention Evaluation Study Investigators, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 15.de Zeeuw D, Remuzzi G, Parving H-H, Keane WF, Zhang Z, Shahinfar S. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.cir.0000139860.33974.28 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Perticucci E, Cravedi P, Gambara V, Costantini M, Sharma SK. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol. 2008;19(6):1213–1224. doi: 10.1681/ASN.2007090970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou F-F. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947. doi: 10.1016/s0140-6736(19)30772-x [DOI] [PubMed] [Google Scholar]
- 18.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/nejmoa1811744 [DOI] [PubMed] [Google Scholar]
- 19.Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/nejmoa2025845 [DOI] [PubMed] [Google Scholar]
- 20.The EMPA-KIDNEY Collaborative Group, Herrington WG Staplin N Wanner C, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/nejmoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin TP, Martin WP, Islam N, O’Brien T, Griffin MD. The promise of mesenchymal stem cell therapy for diabetic kidney disease. Curr Diab Rep. 2016;16(5):42. doi: 10.1007/s11892-016-0734-6 [DOI] [PubMed] [Google Scholar]
- 22.An X, Liao G, Chen Y, Luo A, Liu J, Yuan Y. Intervention for early diabetic nephropathy by mesenchymal stem cells in a preclinical nonhuman primate model. Stem Cell Res Ther. 2019;10(1):363. doi: 10.1186/s13287-019-1401-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv S-S, Liu G, Wang J-P, Wang W-W, Cheng J, Sun A-L. Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration. Int Immunopharmacol. 2013;17(2):275–282. doi: 10.1016/j.intimp.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 24.Xu N, Liu J, Li X. Therapeutic role of mesenchymal stem cells (MSCs) in diabetic kidney disease (DKD). Endocr J. 2022;69(10):1159–1172. doi: 10.1507/endocrj.ej22-0123 [DOI] [PubMed] [Google Scholar]
- 25.Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–269. doi: 10.1016/j.ebiom.2016.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36(4):602–615. doi: 10.1002/stem.2779 [DOI] [PubMed] [Google Scholar]
- 27.Pappritz K, Dong F, Miteva K, Kovacs A, El-Shafeey M, Kerim B. Impact of syndecan-2-selected mesenchymal stromal cells on the early onset of diabetic cardiomyopathy in diabetic db/db mice. Front Cardiovasc Med. 2021;8:632728. doi: 10.3389/fcvm.2021.632728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangri N Stevens LA Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 30.Tangri N, Grams ME, Levey AS, Coresh J, Appel LJ, Astor BC. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders H-J, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–377. doi: 10.1038/s41581-018-0001-y [DOI] [PubMed] [Google Scholar]
- 32.Gambara V, Mecca G, Remuzzi G, Bertani T. Heterogeneous nature of renal lesions in type II diabetes. J Am Soc Nephrol. 1993;3(8):1458–1466. doi: 10.1681/ASN.v381458 [DOI] [PubMed] [Google Scholar]
- 33.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 34.Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6(2):257–263. doi: 10.1681/ASN.v62257 [DOI] [PubMed] [Google Scholar]
- 35.Cocks K, Torgerson DJ. Sample size calculations for pilot randomized trials: a confidence interval approach. J Clin Epidemiol. 2013;66(2):197–201. doi: 10.1016/j.jclinepi.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 36.Ruggenenti P, Trillini M, Barlovic D, Cortinovis M, Pisani A, Parvanova A. Effects of valsartan, benazepril and their combination in overt nephropathy of type 2 diabetes: a prospective, randomized, controlled trial. Diabetes Obes Metab. 2019;21(5):1177–1190. doi: 10.1111/dom.13639 [DOI] [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 38.Tancredi M, Rosengren A, Svensson A-M, Kosiborod M, Pivodic A, Gudbjörnsdottir S. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373(18):1720–1732. doi: 10.1056/nejmoa1504347 [DOI] [PubMed] [Google Scholar]
- 39.Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk H-D. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–163. doi: 10.1016/j.molmed.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 40.Coppin L, Najimi M, Bodart J, Rouchon M-S, van der Smissen P, Eeckhoudt S. Clinical protocol to prevent thrombogenic effect of liver-derived mesenchymal cells for cell-based therapies. Cells. 2019;8(8):846. doi: 10.3390/cells8080846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson M, Mei SHJ, Wolfe D, Champagne J, Fergusson D, Stewart DJ. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19:100249. doi: 10.1016/j.eclinm.2019.100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casiraghi F, Remuzzi G, Abbate M, Perico N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev Rep. 2013;9(1):65–79. doi: 10.1007/s12015-011-9345-4 [DOI] [PubMed] [Google Scholar]
- 43.von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–1578. doi: 10.1002/stem.1118 [DOI] [PubMed] [Google Scholar]
- 44.Perico N, Casiraghi F, Todeschini M, Cortinovis M, Gotti E, Portalupi V. Long-term clinical and immunological profile of kidney transplant patients given mesenchymal stromal cell immunotherapy. Front Immunol. 2018;9:1359. doi: 10.3389/fimmu.2018.01359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao S, Zhang Y, Liang K, Bi R, Du Y. Mesenchymal stem cells (MSCs): a novel therapy for type 2 diabetes. Stem Cells Int. 2022;22:8637493. doi: 10.1155/2022/8637493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skyler JS, Fonseca VA, Segal KR, Rosenstock J. Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo-controlled, dose-escalation safety and tolerability pilot study. Diabetes Care. 2015;38(9):1742–1749. doi: 10.2337/dc14-2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129(22):2287–2296. doi: 10.1161/circulationaha.113.007412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hickson LJ, Herrmann SM, McNicholas BA, Griffin MD. Progress toward the clinical application of mesenchymal stromal cells and other disease-modulating regenerative therapies: examples from the field of nephrology. Kidney360. 2021;2(3):542–557. doi: 10.34067/kid.0005692020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22(6):824–833. doi: 10.1016/j.stem.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345–2360. doi: 10.1007/s00018-017-2473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: current understanding and clinical relevance. Stem Cells. 2020;38(5):596–605. doi: 10.1002/stem.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogacev KS, Zawada AM, Emrich I, Seiler S, Böhm M, Fliser D. Lower Apo A-I and lower HDL-C levels are associated with higher intermediate CD14++CD16+ monocyte counts that predict cardiovascular events in chronic kidney disease. Arterioscler Thromb Vasc Biol. 2014;34(9):2120–2127. doi: 10.1161/atvbaha.114.304172 [DOI] [PubMed] [Google Scholar]
- 55.Naicker SD, Cormican S, Griffin TP, Maretto S, Martin WP, Ferguson JP. Chronic kidney disease severity is associated with selective expansion of a distinctive intermediate monocyte subpopulation. Front Immunol. 2018;9:2845. doi: 10.3389/fimmu.2018.02845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barry LE, Crealey GE, Cockwell P, Elliman SJ, Griffin MD, Maxwell AP. Mesenchymal stromal cell therapy compared to SGLT2-inhibitors and usual care in treating diabetic kidney disease: a cost-effectiveness analysis. PLoS One. 2022;17(11):e0274136. doi: 10.1371/journal.pone.0274136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Diaz M, Quiñones-Vico MI, Sanabria de la Torre R, Montero-Vílchez T, Sierra-Sánchez A, Molina-Leyva A. Biodistribution of mesenchymal stromal cells after administration in animal models and humans: a systematic review. J Clin Med. 2021;10(13):2925. doi: 10.3390/jcm10132925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee RH Seo MJ Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Li Y, Zhao J, Zhang J, Huang Y. Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant. 2013;19(4):538–546. doi: 10.1016/j.bbmt.2013.01.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sharing of individual participant data with third parties was not specifically included in the informed consent of the study, and unrestricted diffusion of such data may pose a potential threat of revealing participants' identities, as permanent data anonymization was not carried out (participants records were instead deidentified per protocol during the data retention process). To minimize this risk, individual participant data that underlie the results reported in this article will be available after three months and up to five years from article publication. Researchers shall submit a methodologically sound proposal to Dr. Annalisa Perna (annalisa.perna@marionegri.it), head of the Laboratory of Biostatistics of the Department of Renal Medicine of the Istituto di Ricerche Farmacologiche Mario Negri IRCCS. To gain access, data requestors will need to sign a data access agreement and obtain the approval of the local ethics committee.