Abstract
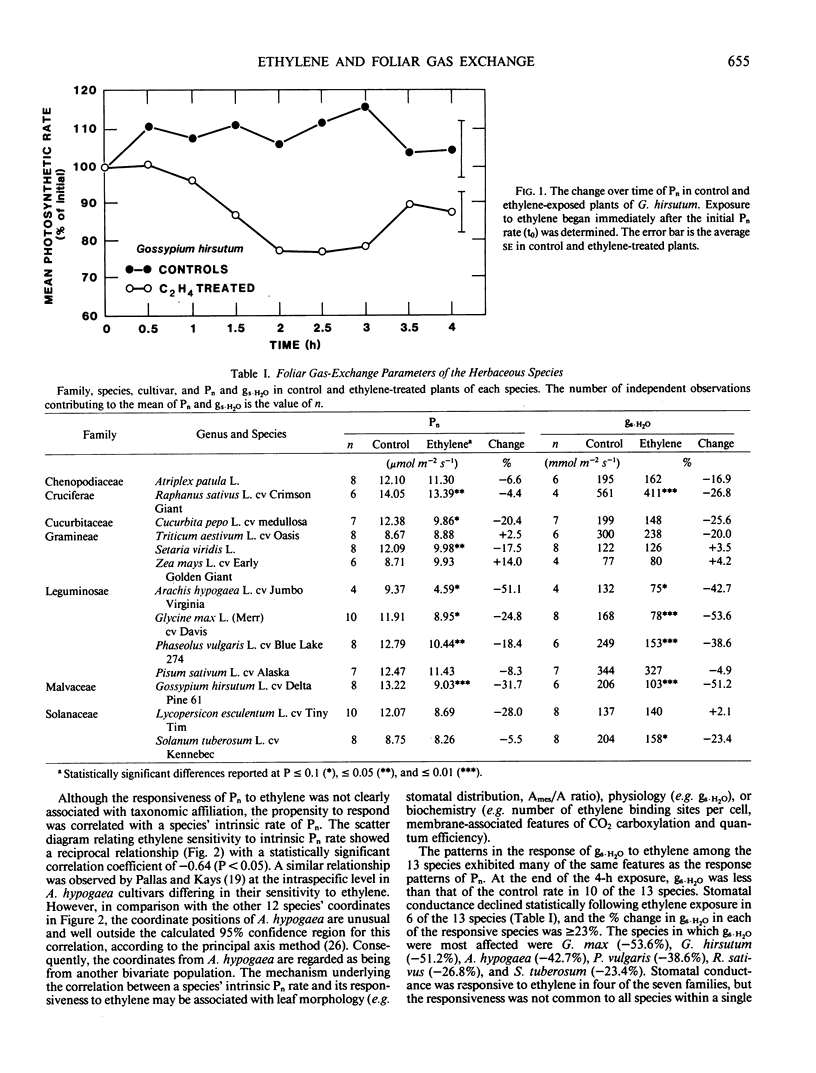
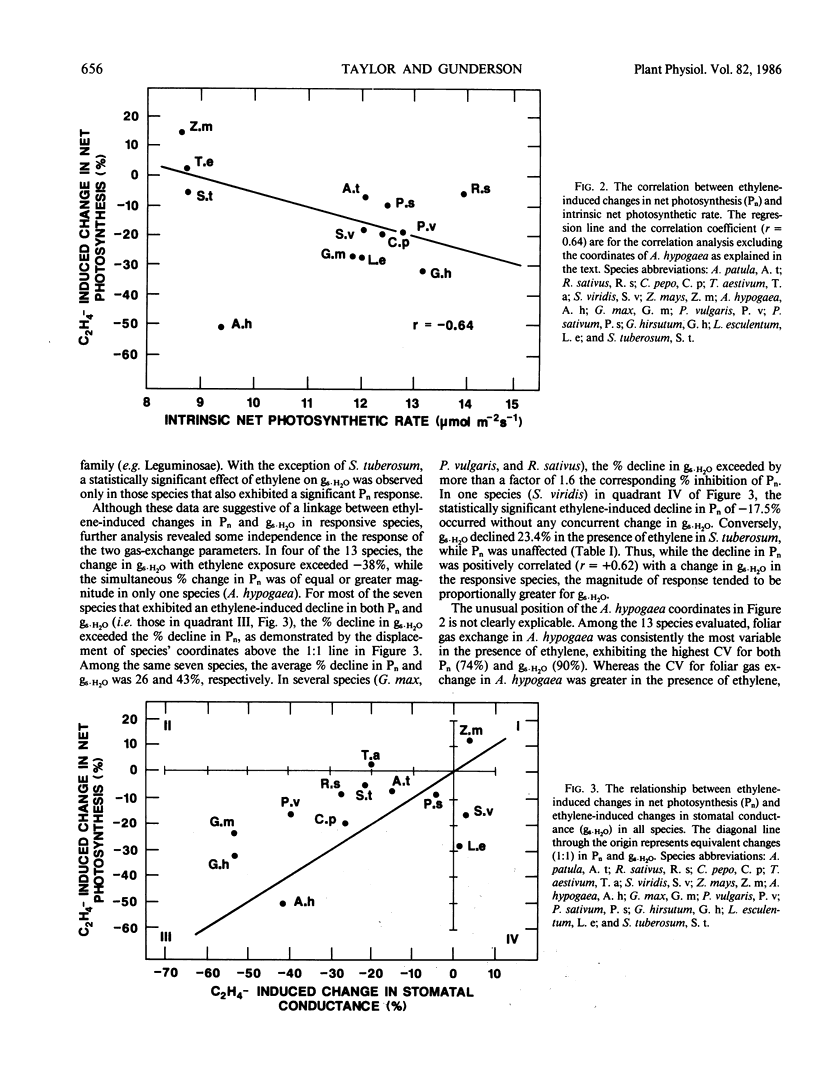
The responsiveness to ethylene of net photosynthesis and stomatal conductance to water vapor in intact plants was investigated in 13 herbaceous species representing seven plant families. Exposures were conducted in an open, whole-plant exposure system providing controlled levels of irradiance, air temperature, CO2, relative humidity, and ethylene concentration. Net photosynthesis and stomatal conductance to water vapor in units of moles per square meter per second were measured on recently expanded leaves in control and ethylene-treated plants using a remotely operated single-leaf cuvette. The ethylene concentration was either 0 or 210 micromoles per cubic meter and was maintained for 4 hours. Species varied substantially in the response of their foliar gas exchange to ethylene. In 7 of the 13 species, net photosynthesis was inhibited statistically by 4 hours of ethylene exposure. As a function of the rate in control plants, the responses were most pronounced and statistically significant in Arachis hypogaea (−51.1%), Gossypium hirsutum (−31.7%), Glycine max (−24.8%), Cucurbita pepo (−20.4%), Phaseolus vulgaris (−18.4%), Setaria viridis (−17.5%), and Raphanus sativus (−4.4%). Whereas the responsiveness of net photosynthesis to ethylene among the 13 species showed no specific taxonomic associations, the responsiveness was positively correlated with the intrinsic rate of net photosynthesis. Stomatal conductance to water vapor after 4 hours of ethylene exposure declined statistically in 6 of the 13 species. As a function of control rates, the most marked and statistically significant responses of stomatal conductance were in Glycine max (−53.6%), Gossypium hirsutum (−51.2%), Arachis hypogaea (−42.7%), Phaseolus vulgaris (−38.6%), Raphanus sativus (−26.8%), and Solanum tuberosum (−23.4%). Although ethylene-induced changes in net photosynthesis and stomatal conductance were positively correlated, there were species-specific exceptions in which net photosynthesis declined after 4 hours of exposure without a concurrent change in stomatal conductance, stomatal conductance declined without a change in net photosynthesis, and the decline in stomatal conductance substantially exceeded the corresponding decline in net photosynthesis. Thus, the responsiveness to ethylene of net photosynthesis and stomatal conductance to water vapor were not consistently synchronous or equivalent among the 13 species. It is concluded that foliar gas exchange is responsive to exogenously applied ethylene in many plant species. The sensitivity of foliar gas exchange to ethylene may play a role in general plant response to environmental stress in which one of the physiological sites of action for endogenously produced stress ethylene in the leaf is the plant's photosynthetic capacity and/or stomatal conductance to water vapor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N. Relationship between Leaf Water Status and Endogenous Ethylene in Detached Leaves. Plant Physiol. 1978 Apr;61(4):658–662. doi: 10.1104/pp.61.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerer T. W., Kozlowski T. T. Ethylene, Ethane, Acetaldehyde, and Ethanol Production By Plants under Stress. Plant Physiol. 1982 Apr;69(4):840–847. doi: 10.1104/pp.69.4.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallaghy C. K., Raschke K. No stomatal response to ethylene. Plant Physiol. 1972 Feb;49(2):275–276. doi: 10.1104/pp.49.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E. An apparent anomaly in peanut leaf conductance. Plant Physiol. 1980 May;65(5):848–851. doi: 10.1104/pp.65.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas J. E., Kays S. J. Inhibition of photosynthesis by ethylene-a stomatal effect. Plant Physiol. 1982 Aug;70(2):598–601. doi: 10.1104/pp.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Yang S. F. Ethylene and Ethane Production from Sulfur Dioxide-injured Plants. Plant Physiol. 1979 Jan;63(1):142–145. doi: 10.1104/pp.63.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey D. T., Standley C., Field R. W. Stress ethylene evolution: a measure of ozone effects on plants. Atmos Environ. 1976;10(11):969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]


