Abstract
The murine monoclonal antibody (MAb) 18B7 [immunoglobulin G1(κ)] is in preclinical development for treatment of Cryptococcus neoformans infections. In anticipation of its use in humans, we defined the serological and biological properties of MAb 18B7 in detail. Structural comparison to the related protective MAb 2H1 revealed conservation of the antigen binding site despite several amino acid differences. MAb 18B7 was shown by immunofluorescence and agglutination studies to bind to all four serotypes of C. neoformans, opsonize C. neoformans serotypes A and D, enhance human and mouse effector cell antifungal activity, and activate the complement pathway leading to deposition of complement component 3 (C3) on the cryptococcal capsule. Administration of MAb 18B7 to mice led to rapid clearance of serum cryptococcal antigen and deposition in the liver and spleen. Immunohistochemical studies revealed that MAb 18B7 bound to capsular glucuronoxylomannan in infected mouse tissues. No reactivity of MAb 18B7 with normal human, rat, or mouse tissues was detected. The results show that both the variable and constant regions of MAb 18B7 are biologically functional and support the use of this MAb in human therapeutic trials.
Cryptococcus neoformans is unusual among the pathogenic fungi because it has a polysaccharide capsule that is antiphagocytic (32). C. neoformans infection occurs in 6 to 8% of patients with late-stage human immunodeficiency virus infection (9). During human C. neoformans infection, capsular polysaccharide accumulates in tissue, where it mediates a variety of deleterious effects on the host immune function (6). Most patients with C. neoformans infections have a high concentration of serum polysaccharide antigen and low titers of serum antibodies to the capsular polysaccharide (11). Several groups have shown that antibody administration can modify the course of murine cryptococcal infection to the benefit of the host in intravenous (i.v.) (13, 40, 44, 47, 53, 54), intraperitoneal (17, 22, 37), intratracheal (14), and intracerebral (36) models of infection. Antibodies to the capsular polysaccharide are opsonic (31, 39, 41, 48, 56) and can enhance the therapeutic efficacy of amphotericin B (12, 21, 38), fluconazole (34), and flucytosine (15). These observations, together with an association between rapid clearance of capsular polysaccharide after antibody administration in humans (20), mice (38), and rats (19), suggest antibody therapy may have a role in the therapy of human cryptococcosis. In the past, antibody administration was used for therapy of human cryptococcal infection, but too few patients were treated to draw conclusions regarding the efficacy of antibody therapy (for a review see reference 20).
Monoclonal antibodies (MAbs) to the polysaccharide capsule are potential therapeutic reagents for use in C. neoformans infections. MAb 2H1 (immunoglobulin G1 [IgG1]) is a protective antibody that has been extensively characterized, and it was our leading candidate for clinical development until we encountered problems of aggregation during purification. Hence, we have opted to develop another IgG1 murine MAb, known as 18B7, that is closely related to 2H1 in variable region structure, but not identical (33). Like 2H1, MAb 18B7 was generated from a BALB/c mouse immunized with an investigational glucuronoxylomannan (GXM)-tetanus toxoid vaccine (3, 10). The 18B7 heavy chain (VH) and light chain (VL) antibody regions are composed of VH7183, JH2, and an unidentified D gene element, and Vκ5.1 and Jκ1, respectively (33). This molecular structure defines 18B7 as a class II anticryptococcal MAb (2). MAb 18B7 prolongs the survival of mice with lethal C. neoformans infections (33). Other factors that contributed to the selection of MAb 18B7 for additional preclinical development were (i) high affinity for C. neoformans GXM (33), (ii) having been the parent antibody for a protective chimeric mouse-human antibody (55), (iii) absence of toxicity in monkeys when used as the control antibody in a therapeutic trial of an anti-endotoxin MAb (30), and (iv) lack of significant problems during purification. The information available on MAb 18B7 is listed in Table 1. In this study, the biological activity of MAb 18B7 was characterized further as a prelude to its use in a phase I trial. An important goal was to compare MAbs 2H1 and 18B7 to determine whether we could extend the experience gained for MAb 2H1 to MAb 18B7.
TABLE 1.
Summary of published information on MAb 18B7
| Parameter | Description | Comment and reference(s) |
|---|---|---|
| Origin | Mouse hybridoma | The hybridoma was generated by fusion of BALB/c splenocytes and NSO myeloma. For a description of hybridoma generation see reference 3. Although MAb 18B7 is not mentioned by name, it is one of the IgG1 MAbs described. |
| Isotype | IgG1 | The isotype was determined with rabbit alkaline phosphatase-conjugated isotype-specific antibodies (3). |
| Immunizing agent | GXM-TTa conjugate vaccine | GXM-TT vaccine was made and provided by the laboratory of John Robbins (Bethesda, Md.). For a description of GXM-TT see reference 10. |
| Apparent affinity | 5 × 109 M−1 | Apparent affinity was measured by the method of Nieto et al. (42). |
| GXM serotype reactivity | A > B > C > D | Relative serotype reactivity was determined by inhibition ELISA (3). |
| Serological studies | Reactivity with strain 24067 GXM | MAb 18B7 antibody was shown to react with strain 24067 GXM by direct-binding ELISA, competition ELISA, and agglutination reaction with whole cells (55). MAb 18B7 was also shown to enhance the phagocytic and antifungal activity of J774.16 cells for strain 24067 (55). |
| Protection data | Murine intraperitoneal model | Mice given MAb 18B7 lived significantly longer than control mice (33). The protective efficacy of 18B7 was comparable to that of 2H1 in the same mouse model (37). |
| VH | VH7183 and JH2 | Heavy chain variable region usage was determined by sequencing of VH mRNA (33). |
| VL | Vκ5.1 and Jκ1 | Light chain variable region usage was determined by sequencing of LH mRNA (33). |
| Construction of mouse-human chimeric antibody | ch18B7 | Chimeric mouse-human antibody was opsonic for human microglia and mouse J774.16 cells and prolonged survival of lethally infected mice (55). |
| Peptide mimotope | GLQYTPSWMLVG | MAb 18B7 has been shown to bind a peptide mimotope of GXM in its antigen binding site (51). |
| Toxicity in primates | None | MAb 18B7 was used as an isotype-matched control in studies of a therapeutic antibody that binds LPS (30). |
TT, tetanus toxoid.
MATERIALS AND METHODS
MAbs.
MAbs 18B7 (IgG1), 2H1 (IgG1), and 2D10 (IgM) have been described previously (3). For some experiments, the murine IgG1 MAbs 3665 and 3671 or pooled murine IgG (Sigma Chemical Co., St. Louis, Mo.) were used as isotype-matched controls. MAbs 3665 and 3671 have specificity for phenylarsonate (49). MAbs 18B7 and 3665 were purified by protein G affinity chromatography. Antibody concentration was determined by either enzyme-linked immunosorbent assay (ELISA) relative to isotype-matched standards or absorbance at 280 nm.
C. neoformans strains and GXM.
The C. neoformans strains 28957, 24067, and 32068 were obtained from the American Type Culture Collection (Rockville, Md.). Strains CN110, CN98, and CN15 were obtained from Stuart Levitz (Boston, Mass.). Strains 62066, H99, 388, and 371 were obtained from Robert Cherniak (Atlanta, Ga.), John Perfect (Durham, N.C.), Kjung J. Kwon-Chung (Bethesda, Md.), and John Bennett (Bethesda, Md.), respectively. Strains J9 and J22 are clinical isolates (4). The strain serotypes are listed in Table 2. The strains were maintained at 4°C on Sabouraud dextrose agar or frozen in a solution of 50% glycerol and culture medium. C. neoformans capsular GXM was isolated from culture supernatant of strain 24067 as described previously (5).
TABLE 2.
MAb 18B7 binding to C. neoformans strains
| Strain | Serotype | Agglutination (μg/ml) |
|---|---|---|
| H99 | A | 6.25 |
| CN98 | A | 1.56 |
| 62066 | A | >50 |
| CN110 | A | 3.12 |
| 371 | A | 3.12 |
| CN15 | A | 0.39 |
| J22 | D | 0.78 |
| 28957 | D | 0.78 |
| 28958 | D | 6.25 |
| J9 | D | 12.5 |
| 24067 | D | 12.5 |
| 24065 | B | 3.12 |
| 32068 | C | 50.0 |
| NIH 34 | C | 12.5 |
Structural comparison of MAb 18B7 and 2H1 binding sites.
The location of the amino acid differences between MAbs 18B7 and 2H1 was mapped on the 2H1 Fab structure with the software program InsightII (Biosym Technologies, San Diego, Calif.). The structure of the 2H1 Fab has been solved to 2.4 Å resolution alone and in complex with the peptide mimetic PA1 (GLQYTPSWMLVG) (50, 51) and is accessible on the Protein Data Bank (Brookhaven National Library, Brookhaven, N.Y.) under accession no. 2h1p.
Immunofluorescence, agglutination, and immunohistochemistry studies.
Indirect immunofluorescence of MAb 18B7 binding to C. neoformans strains was done as described previously (7). The agglutination endpoint of MAb 18B7 binding for various C. neoformans strains was determined as described previously (8) and is the lowest concentration of the antibody that induced cell aggregation. MAb 18B7 binding to normal and C. neoformans-infected tissues was studied by immunohistochemistry as described previously (14), except that color was developed with fast red (Sigma). Human tissue sections were obtained from Sunhee Lee and Steve Factor (Bronx, N.Y.) from autopsy specimens under a protocol approved by the institutional review board of our institution. For negative controls, MAb 18B7 incubation was omitted from the immunohistochemistry protocol. Normal brain, lung, and kidney tissues from noninfected rats were used to assess for reactivity of MAb 18B7 for normal rat tissues.
Capture spot ELISA.
Microtiter polystyrene plates (Corning, Corning, N.Y.) were coated with goat anti-mouse IgM (1 μg/ml) and blocked with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Next, the IgM GXM-binding MAb 2D10 (10 μg/ml) was added, and the plate was incubated for 1.5 h. A suspension of 3.1 × 107 cells of strain 24067/ml in PBS was then added, serially diluted on the plate, and incubated overnight. The ELISA was completed by adding, in successive steps, MAb 18B7 (10 μg/ml) in PBS, 1 μg of biotin-labeled goat anti-mouse IgG1 (Southern Biotechnology Associates)/ml, Vectastain ABC-AP (Vector Laboratories, Burlingame, Calif.), and 50 μl of 5-bromo-4-chloro-3-indolylphosphate (BCIP; Amersco, Solon, Ohio) in AMP buffer (95.8 ml of 2 amino-2-methyl-1-propanol, 0.1 ml of Triton X-405, 0.2 g of MgCl2-6H2O in 800 ml of double-distilled water, pH 8.6). Between every 2 steps the wells were washed with 0.05% Tween 20 in Tris-buffered saline. Washing was done manually after the initial capture step. All incubations were done at 37°C except cell capture, which was done at 4°C, and the Vectastain and BCIP steps, which were done at room temperature. After BCIP staining the chambers were washed. MAb 2H1 was used as a positive control. For negative control, 1% BSA was substituted for GXM binding antibodies.
Phagocytosis and antifungal activity assays.
The murine macrophage-like cell line J774.16 was used to determine the ability of MAb 18B7 to opsonize C. neoformans (39, 41). The phagocytic index is the total number of attached and internalized yeast cells divided by the number of J774.16 cells per microscope field. The ability of MAb 18B7 to enhance J774.16 antifungal efficacy was studied as described previously (39, 41), with an effector-to-target (E/T) ratio of 20:1 and an incubation time of 24 h. Human polymorphonuclear leukocytes (PMNs) and monocytes were used to study the opsonic efficacy of MAb 18B7 for human cells. The effect of MAb 18B7 on human PMN phagocytosis of C. neoformans was measured by a fluorescence-activated cell sorter assay as described previously (56). The PMNs associated with fluorescein isothiocyanate (FITC)-labeled yeast cells were identified by their size and their acquisition of fluorescence as described previously (46). Phagocytosis was measured as the percentage of the total number of PMNs that fluoresced after incubation with FITC-labeled yeast cells and fluorescence quenching. Measurement of the effect of MAb 18B7 on human peripheral blood mononuclear cell (PBMC) phagocytosis of C. neoformans was done by counting internalized yeast. Monolayers were used after 8 days of culture, and only wells with confluent monolayers were used. All experiments used cells from a single immunocompetent donor. Cells from strains 24067 and 371 were treated with PBS, 100 μg of MAb 18B7/ml, or 100 μg of MAb 3665/ml and incubated for 1 h at 37°C. The yeast cells were then added to monolayers of human PBMCs at an E/T ratio of 1:10 and incubated for 4 h at 37°C. After incubation, the monolayers were washed three times in PBS, stained with Giemsa stain, and visualized by light microscopy. The number of PBMCs in the monolayers was confirmed by counting Giemsa-stained cells on the slide at the end of the experiment.
Complement activation studies.
The sites of C3 deposition in C. neoformans were determined by immunofluorescence microscopy as described previously (27). C3 binding studies were done with formalin-killed cells of strain 388 (serotype A) as described previously (52). The kinetics of activation and binding of C3 in the presence (50 μg/ml) and absence of MAb 18B7 was done exactly as described previously (27).
Effect of MAb 18B7 on serum antigen.
The ability of MAb 18B7 to reduce serum antigen in A/J mice (National Cancer Institute, Frederick, Md.) injected i.v. with 50 μg of strain 24067 GXM was done as described previously (29). MAb 3665 or an equivalent volume of PBS intraperitoneally was used as a negative control. Tissue GXM levels were determined by antigen capture ELISA after protease and heat treatment of tissue homogenate, as described previously (38).
Immunogold electron microscopy.
C57B1/6 mice (National Cancer Institute) were infected intratracheally with strain 24067 as described previously (14) and killed on day 28 by cervical dislocation, and the lungs were removed and placed in Trump’s fixative (4% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer) overnight. Tissue blocks were postfixed with 1% osmium for 1 h, dehydrated in solutions with increasing ethanol concentrations (50 to 100%), cleared in two changes of acetonitrile, and infiltrated with and embedded in araldite-Epon. Ultrathin lung tissue sections were then placed on nickel grids and incubated in 3% H2O2 for 10 min, washed in PBS, etched in a saturated solution of sodium periodate for 10 min, washed in PBS, and blocked with 2% goat serum for 1 h. The grids were then incubated overnight with 5 μg of MAb 18B7/ml in 2% goat serum or murine IgG (Sigma) as a control. The grids were washed in PBS with 2% goat serum with 0.01% Tween 20 and 0.1% gelatin (60 Bloom units) and then incubated in 2.5 μg of biotin-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates)/ml for 1 h. After being washed, the grids were incubated with 10-nm-diameter gold particles conjugated to streptavidin (Goldmark Biologicals, Phillipsburg, N.J.) diluted 1:30 in 1% BSA for 2 h at room temperature, washed, and fixed in 2% glutaraldehyde. The grids were then stained briefly in uranyl acetate followed by lead citrate for 20 s and examined with a JEOL (Tokyo, Japan) 100S electron microscope.
Statistical analysis.
Several statistical tests were used to analyze the data, as described in the text. P values were obtained by using the Primer of Biostatistics (17a).
RESULTS
Structural and serological studies of MAb 18B7. (i) Structural comparison of MAbs 18B7 and 2H1.
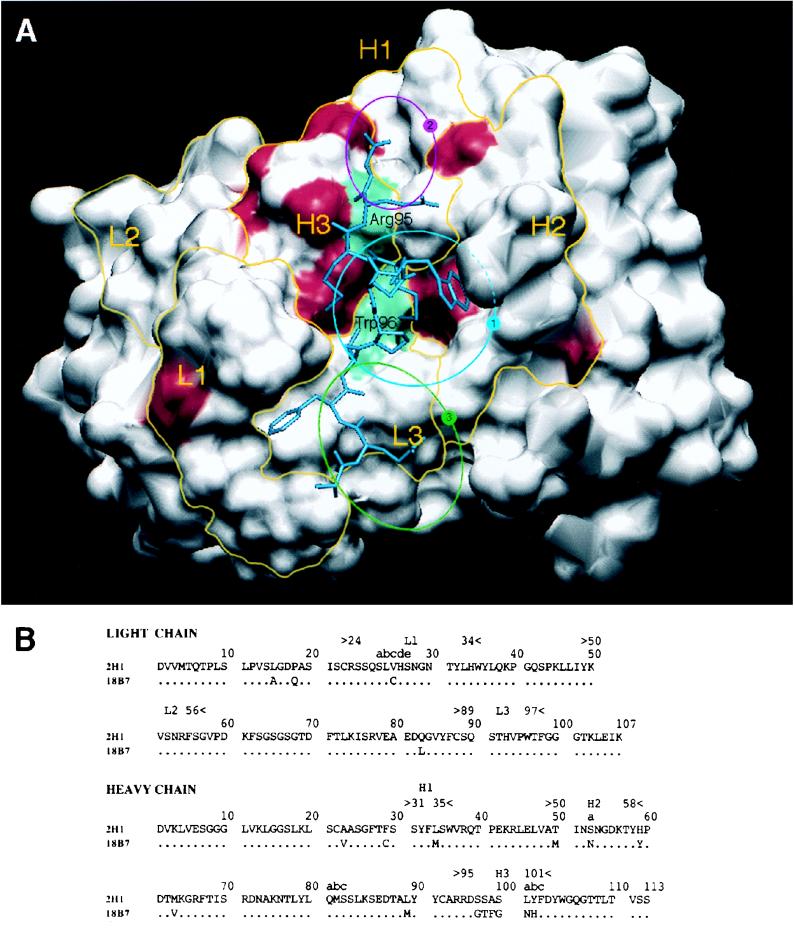
MAbs 18B7 and 2H1 are closely related class II MAbs (2) which differ at 18 amino acid positions (33) (Fig. 1). Since MAb 2H1 has been extensively studied, we compared the sequence differences of MAbs 2H1 and 18B7 on the structure of the 2H1 Fab (51). This mapping revealed that only 4 of the 18 amino acid differences between MAbs 18B7 and 2H1 are in the putative GXM binding site (Fig. 1).
FIG. 1.
(A) Solvent-accessible surfaces of the MAb 2H1 binding site with the amino acid differences between MAbs 2H1 and 18B7 highlighted in red. L1, L2, and L3 refer to the three light chain CDRs. H1, H2, and H3 refer to the three heavy chain CDRs. Yellow lines denote the structural area associated with CDRs. The positions of arginine H95 in the VH CDR3 and tryptophan L96 in the VL CDR3 are shown in green. The position of the peptide mimotope PA1 is drawn in blue (50). The sequence of PA1 is GLQYTPSWMLVG. The blue circle labeled “1” denotes the location of the central hydrophobic pocket delimited by VH CDR1, CDR2, and CDR3 and VL CDR1 and -3. The magenta circle labeled “2” denotes the location of a small hydrophobic pocket containing arginine H95. The green circle labeled “3” denotes the location of a potential extension of the binding groove that is highly conserved in both MAbs 2H1 and 18B7. (B) Sequence comparison of heavy and light chain variable regions of MAbs 2H1 and 18B7 (numbering according to Kabat et al. [24]).
(ii) Reactivity of MAb 18B7 with C. neoformans strains.
To confirm that MAb 18B7 had broad reactivity with different cryptococcal strains, we studied its binding to 13 C. neoformans strains by indirect immunofluorescence and its ability to promote yeast cell agglutination (Table 2). MAb 18B7 bound to all strains with an annular immunofluorescence pattern (data not shown), associated with antibody-mediated protection (43).
(iii) Spot capture ELISA study.
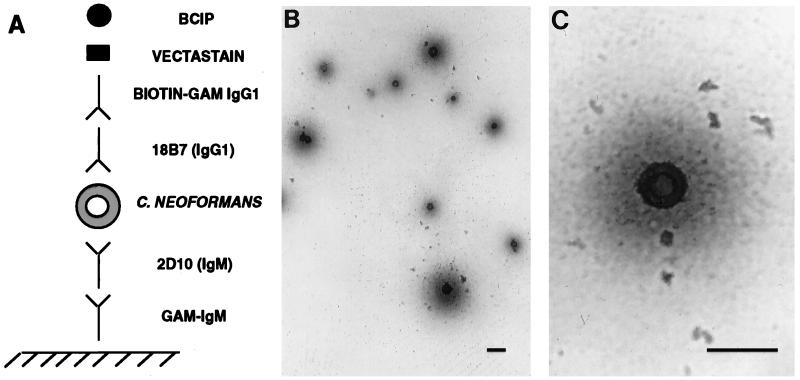
A MAb-based capture ELISA designed for Mycobacterium tuberculosis (18) was modified for C. neoformans cells by using MAb 18B7 (Fig. 2). The assay showed that C. neoformans cells can be captured from solution by MAb binding, immobilized to the polystyrene plate, and detected with a second MAb to GXM that differs in isotype from the capture MAb.
FIG. 2.
Spot ELISA for C. neoformans with MAb 18B7. (A) Graphic representation of the ELISA configuration. GAM, goat anti-mouse. (B and C) Light microscopy images of C. neoformans 24067 captured and detected by the assay. In this assay the C. neoformans cells stain blue. Magnification, ×200 (B) and ×400 (C). Bars, 20 μm.
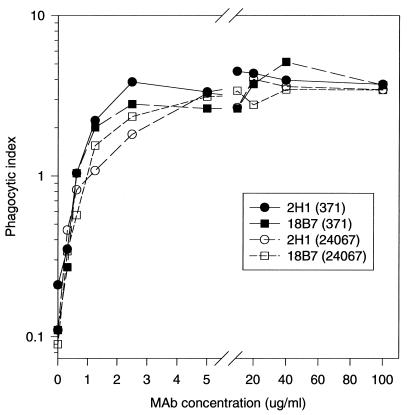
Functional assays with phagocytic cells and complement. (i) Effect of MAb 18B7 on phagocytosis of C. neoformans by J774.16 murine macrophage-like cells.
Addition of MAb 18B7 to gamma interferon- and lipopolysaccharide (LPS)-stimulated J774.16 cells significantly increased phagocytosis of C. neoformans 24067 (serotype A) and 371 (serotype D) cells (Fig. 3). The IgG1 MAb 2H1, a potent opsonin (39, 41), was used as a positive control. The phagocytic indices resulting from the addition of MAb 18B7 to J774.16 cell monolayers and C. neoformans cells were comparable to those observed with MAb 2H1. In contrast, there was little or no phagocytosis of C. neoformans in the absence of MAb 18B7 or after addition of the irrelevant isotype-matched control IgG1 MAb 3665 (not shown).
FIG. 3.
Phagocytosis of C. neoformans 24067 and 371 by J774.16 cells in the presence and absence of MAbs 18B7 and 2H1. Each point is the average of three measurements.
(ii) Effect of MAb 18B7 on J774.16 murine macrophage-like cells’ antifungal efficacy against C. neoformans.
Addition of MAb 18B7 to gamma interferon- and LPS-stimulated J774.16 cell monolayers and C. neoformans cells at an E/T ratio of 20:1 significantly enhanced the killing of fungal cells. (This was demonstrated previously for strain 24067 [55]. That result is confirmed here and extended to strain 371.) For strain 24067 (serotype D) the percent survival without J774.16 cells, with J774.16 cells alone, with J774.16 cells plus MAb 2H1, and with J774.16 cells plus 18B7 was 100, 77.1 ± 3.6, 15.6 ± 2.7, and 21.5 ± 7.8, respectively (n = 4 for all means; P < 0.001 for reduction in CFU relative to the condition of J774.16 cells alone). For strain 371 (serotype A) the percent survival without J774.16 cells, with J774.16 cells alone, with J774.16 cells plus MAb 2H1, and with J774.16 cells plus 18B7 was 100, 77.7 ± 3.4, 35.4 ± 3.2, and 39.1 ± 5.8, respectively (n = 4 for all means; P < 0.001 for reduction in CFU relative to the condition of J774.16 cells alone). Hence, MAb 18B7 enhanced the antifungal efficacy of J774.16 cells, and the relative efficacies of MAbs 2H1 and 18B7 were comparable.
(iii) Effect of MAb 18B7 on phagocytosis of C. neoformans by human neutrophils.
The percentage of PMNs that phagocytosed C. neoformans cells after the addition of MAb 18B7-treated 24067 (serotype D) yeast cells was (29.2 ± 2.2)%. In contrast, the percentage of PMNs that phagocytosed C. neoformans after the addition of the irrelevant control IgG1 MAb 3665-treated 24067 yeast cells was only (0.275 ± 0.17)% (n = 3 for each group; P < 0.001 [Mann-Whitney test]). For strain 371 (serotype A), the percent PMN phagocytosis after addition of MAb 18B7-treated and 3665-treated cryptococci 39.8 ± 1.84 and 0.35 ± 0.05, respectively (n = 3 for each group; P < 0.001 [Mann-Whitney test]). Hence, MAb 18B7 is a potent opsonin for serotype A and D C. neoformans strains with human PMNs.
(iv) Effect of MAb 18B7 on phagocytosis of C. neoformans by human PBMCs.
For strain 24067 (serotype D), the phagocytosis indices in the presence of MAbs 18B7 and 3665 were 2.71 ± 1.2 and 0.48 ± 0.01, respectively (n = 3 for each group; P < 0.002 [Mann-Whitney test]). For strain 371 (serotype A), the phagocytosis indices in the presence of MAbs 18B7 and 3665 were 3.25 ± 0.19 and 0.052 ± 0.011, respectively (n = 3 for each group; P < 0.001 [Mann-Whitney test]). The number of attached or internalized yeast cells for the PBS-treated cells was similar to that of MAb 3665-treated yeast for both strains. Hence, MAb 18B7 is a potent opsonin for C. neoformans strains of A and D serotypes with human PBMCs.
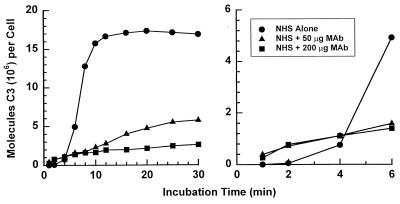
(v) Effect of MAb 18B7 on activation and binding of C3 to encapsulated cryptococci.
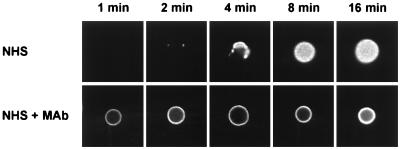
The C3 deposition assay in normal human serum (NHS) alone showed the expected lag of 4 to 6 min (2) in binding of C3 when the yeast cells were incubated with NHS alone (Fig. 4). When MAb 18B7 was added there was a slight, but readily detectable, enhancement of early (<4 min) C3 binding. Consistent with previous studies of antibody-mediated complement activation by group II MAbs to GXM, there was a major reduction in the overall rate of accumulation of C3 on the yeast cells over time (27). Immunofluorescence microscopy of yeast cells incubated with NHS in the presence or absence of MAb 18B7 for 1, 2, 4, 8, or 16 min confirmed the findings with radiolabeled C3. In contrast to NHS, where C3 deposition began focally at 2 min and expanded with time to fill the capsule by 8 min, yeast cells incubated with MAb 18B7 had homogeneous C3 deposition at 1 min (Fig. 5). Thus, both the kinetic analysis and the immunofluorescence microscopy showed that MAb 18B7 promoted earlier C3 binding than NHS, consistent with antibody-mediated complement activation.
FIG. 4.
Kinetics for activation and binding of C3 fragments to serotype A C. neoformans cells incubated with 40% NHS in the presence and absence of MAb 18B7. The binding of C3 fragments was determined by incorporation of trace amounts of 125I-labeled C3 into the reaction mixture.
FIG. 5.
Immunofluorescence analysis of the sites for binding of C3 to serotype A C. neoformans cells incubated for 1, 2, 4, 8, and 16 min with 40% NHS in the presence (50 μg/ml) or absence of MAb 18B7. Sites of C3 deposition were determined by the use of FITC-labeled antiserum to C3. All images were collected under identical conditions.
Tissue binding and removal of antigen in vivo. (i) Immunohistochemistry of human, rat, and mouse tissues.
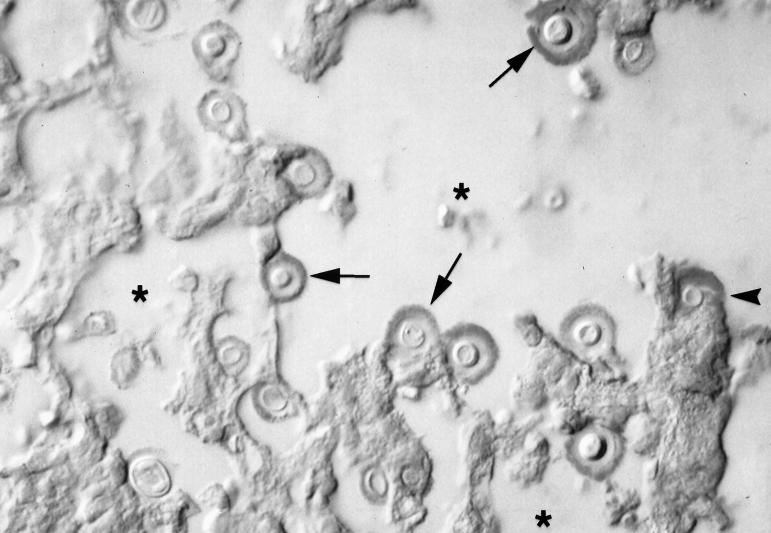
Tissue immunohistochemistry was used to evaluate whether MAb 18B7 bound to normal human tissue and to cryptococci in infected murine tissue. Immunohistochemistry revealed that MAb 18B7 did not bind to normal human brain, lung, heart, liver, and kidney tissues (not shown). MAb 18B7 had no reactivity with normal rat brain, kidney, and lung tissues (not shown). In contrast, MAb 18B7 had strong reactivity with cryptococcal cells in infected tissue and in areas adjacent to inflammatory foci, consistent with shed polysaccharide (Fig. 6). No reactivity was observed with normal mouse lungs. Hence, MAb 18B7 reacts with GXM made in vivo by C. neoformans but not with normal mouse, rat, or human tissues.
FIG. 6.
Immunohistochemistry for 18B7 by light microscopy shows antibody binding to yeast cell capsules in a mouse lung during experimental infection. Arrows point to several immunostained cryptococci. The asterisks denote air spaces. The arrowhead points to shed polysaccharide inside an alveolar macrophage. In this assay the polysaccharide stains red. Magnification, ×400.
(ii) Immunogold studies of MAb 18B7 binding to C. neoformans.
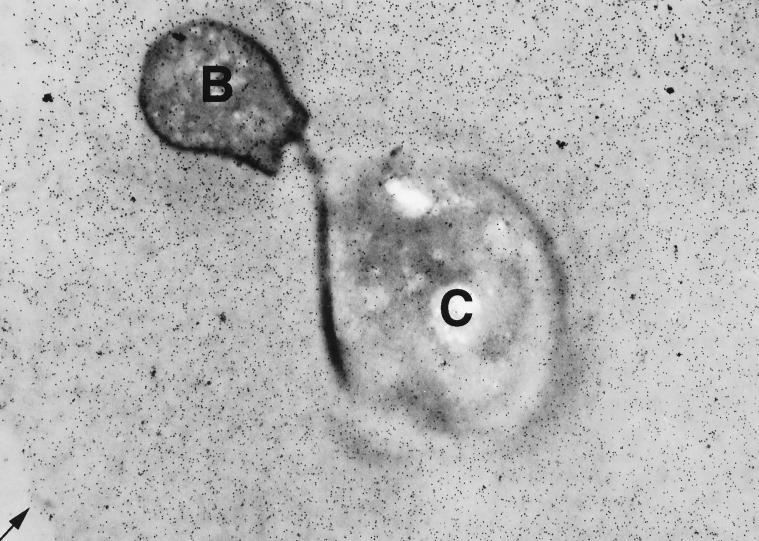
Electron microscopy with immunogold labeling was used to determine the location of the 18B7 epitope in the capsule of cryptococci in vivo and in vitro. Immunogold studies following MAb 18B7 binding to C. neoformans showed localization of gold particles to the polysaccharide capsule. Figure 7 shows gold particles localizing to the capsule of yeast cells in mouse pulmonary tissue. Similar results were obtained for C. neoformans grown in Sabouraud dextrose broth (not shown). MAb 18B7 epitope was distributed throughout the capsule. Control studies with polyclonal murine IgG revealed no binding to the C. neoformans capsules in vitro or in vivo. Polyclonal murine IgG was observed to bind to the yeast cell walls in vitro (not shown), consistent with reports that cell wall-reactive antibodies are common in nonimmune sera (23, 25).
FIG. 7.
Immunogold labeling shows that the 18B7 epitope is evenly distributed throughout the C. neoformans capsule in a mouse lung during experimental infection. C, cryptococcus cell body; B, bud. The arrow points to the edge of the capsule. No immunogold labeling was apparent in mouse tissues or alveolar spaces (not shown). Magnification, ×10,000.
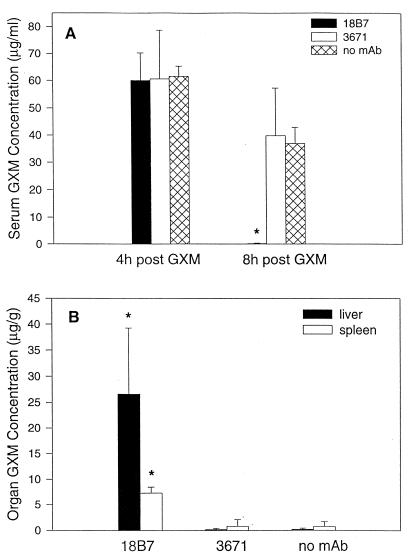
(iii) Effect of MAb 18B7 on serum GXM level and organ deposition of GXM.
To establish whether MAb 18B7 could mediate the clearance of serum GXM, mice were given 50 μg of strain 24067 GXM i.v. followed by administration of MAb 18B7 4 h later. Administration of MAb 18B7 led to nearly complete clearance of serum GXM and antibody-mediated deposition of GXM in hepatic and splenic tissue (Fig. 8). In contrast, administration of neither the irrelevant isotype-matched control MAb 3671 nor PBS had a significant effect on serum GXM level or GXM deposition in the liver or spleen.
FIG. 8.
Effect of MAb administration on serum GXM level and organ deposition of GXM. Mice were given 50 μg of strain 24067 GXM i.v. followed by one of the following: 1 mg of 18B7, 1 mg of 3671 (isotype-matched irrelevant MAb), or an equivalent volume of PBS. The bars denote the average GXM concentrations (n = 4). The error bars denote standard deviations. The asterisks denote P values of <0.05 compared with conditions where MAb 3671 or PBS was administered.
DISCUSSION
MAb 18B7 has a molecular structure that is very similar to that of MAb 2H1, another class II MAb that has been extensively studied. However, MAbs 18B7 and 2H1 differ by 18 of the 230 amino acids that constitute their Fabs. These differences are a result of somatic mutations and variable region gene rearrangements during the ontogeny of the antibody response (33). To determine the locations of the amino acid differences between MAbs 2H1 and 18B7, we mapped them on the structure of the 2H1 Fab (51). The MAb 2H1 antigen binding site has a central hydrophobic pocket delimited by VH CDR1, CDR2, and CDR3 and VL CDR1 and CDR3 (Fig. 1A). Two differences in amino acid sequence mapped to the heavy chain part of this pocket at positions H50 and H100a, according to the antibody nomenclature of Kabat et al. (24). No differences were noted between the 2H1 and 18B7 VL CDR3s, which form most of the floor of the cavity. The VL CDR3 contains tryptophan L96 (Fig. 1A), which is intimately involved in GXM binding, as shown by fluorescence emission studies in class II MAbs (45). This pocket is also the main binding site of the peptide mimotope PA1 (GLQYTPSWMLVG), which competes with GXM for binding to 2H1 (50). The fact that PA1 binds to MAb 18B7 (50) provides additional evidence that the binding pockets of MAbs 2H1 and 18B7 are similar.
Analysis of the 2H1 Fab crystal structure suggested two possible additional locations for the GXM binding site in class II MAbs. The VH CDR3 domain functions to provide a wall to the main pocket. Class II MAbs have an 11-amino-acid VH CDR3 domain composed in part of a 7-amino-acid D/N segment and 4 amino acids from JH2. The amino acid compositions of VH CDR3 are variable among class II MAbs except for the first two amino acids, arginine H95 (Fig. 1A) and glutamate H96 (33). Arginine H95 is part of a small hydrophobic pocket (Fig. 1A). Two differences between the amino acid sequences of MAbs 18B7 and 2H1 mapped to the heavy chain part of this small pocket, at positions H52a and H97. While clearly delimited by VL CDR1 and VH CDR2 and -3, the main pocket is largely open on the side delimited by VL CDR3 and is followed by a large groove (Fig. 1A), which is remarkably conserved between MAbs 2H1 and 18B7 and is a potential extension of the GXM binding site. In conclusion, MAbs 2H1 and 18B7 are characterized by an extended potential antigen binding site. This binding site is centered on a well-conserved hydrophobic pocket. The amino acid differences between MAbs 18B7 and 2H1 are presumably responsible for the higher apparent affinity of 18B7 for GXM (33). The molecular similarities of MAbs 18B7 and 2H1 provide a rationale for extrapolating observations made with MAb 2H1 to MAb 18B7.
An ideal therapeutic MAb against C. neoformans should bind to all clinical strains to eliminate the need for establishing reactivity with individual patient isolates before clinical use. Group II antibodies, such as MAb 18B7, have broad reactivity with strains of all four serotypes (1, 2). In this study we show that MAb 18B7 has reactivity with C. neoformans strains representative of the four serotypes and with several recently isolated clinical strains. MAb 18B7 bound to each C. neoformans strain, as shown by indirect immunofluorescence and agglutination assays (Table 2). The amount of antibody required to agglutinate C. neoformans varied with the strain used. The highest agglutination endpoint was >50 μg/ml for strain 62066. This strain has a giant capsule in vitro, and the high agglutination endpoint may reflect the need to saturate capsule epitopes before agglutination occurs. The immunogold study revealed that the 18B7 epitope is found throughout the capsule. For all of the strains studied, the indirect immunofluorescence pattern was annular. All protective MAbs studied thus far produce annular immunofluorescence patterns on C. neoformans, whereas nonprotective MAbs often show punctate patterns (35, 43). Hence, MAb 18B7 binds C. neoformans strains of the four serotypes with an immunofluorescence pattern like that associated with protective antibodies.
Another desirable characteristic for therapeutic antibodies is high-affinity binding to the targeted antigen with no binding to host tissues. Cross-reactivity between human brain tissue and bacterial antigens has been a concern in the development of vaccines and antibody-based therapies against some pathogens that cause meningitis (16). The exact epitope for MAb 18B7 on C. neoformans GXM is unknown, but antibodies of similar specificity do not react with de-O-acetylated GXM (3). Although GXM-like polysaccharide structures have not been reported in mammalian tissues, establishing whether MAb 18B7 has cross-reactivity for such tissue was important. MAb 18B7 showed no reactivity with human brain, heart, lung, liver, and kidney tissues by immunohistochemistry. There was also no reactivity with rat brain, kidney, and lung tissues. Similarly, MAb 18B7 had no reactivity with mouse lung tissue. In contrast, there was strong reactivity of MAb 18B7 with polysaccharide antigens in lung tissue from C. neoformans-infected mice by immunogold electron microscopy, and immunohistochemistry shows that MAb 18B7 binds to GXM produced during infection.
MAb 18B7 is a potent opsonin for C. neoformans, which shows the functional integrity of its Fc region. Addition of MAb 18B7 to C. neoformans and monolayers of J774.16 murine macrophage-like cells, human PBMCs, and human PMNs resulted in a sharp increase in yeast cell phagocytosis. The concentrations of MAb 18B7 that promoted human and mouse effector cell phagocytosis were comparable to those observed for other GXM binding IgG1 MAbs (39, 41). Administration of antibodies to mice and rats with antigenemia results in clearance of serum antigen (19, 29, 38). In this study, MAb 18B7 administration was shown to clear serum GXM by promoting deposition in the spleen and liver tissues. In contrast, an isotype-matched control MAb did not affect serum GXM clearance or tissue GXM deposition compared to the effect observed by administering PBS alone. The efficacy of MAb 18B7 as an opsonin and in clearing serum GXM in mice provides evidence that both the Fab and Fc regions of this antibody are functionally intact in vivo.
Another important function of antibody Fc regions is activation of the classical complement pathway that promotes early deposition of C3 on microbial surfaces. The C. neoformans capsule can activate the alternate complement pathway without a requirement for specific antibodies, but this process is slow and requires several minutes before significant C3 deposition occurs on the yeast surface (26). The addition of MAb 18B7 to C. neoformans significantly enhanced the kinetics of C3 deposition on the yeast capsule at the early incubation times. However, after longer incubation times the total deposition of C3 on antibody-treated cells was lower than that resulting from activation of the alternative complement pathway alone. This phenomenon has been observed for other class II MAbs, and its relationship to the antibody protective efficacy is poorly understood (27). For the purposes of MAb 18B7 preclinical development, these studies show that MAb 18B7 can activate the human complement pathway, promoting early deposition of C3 fragments in the capsule.
The feasibility of designing an ELISA spot assay with MAbs of different isotypes was demonstrated in our laboratory for M. tuberculosis (18). It was not certain whether such an assay could be adapted for C. neoformans because the polysaccharide capsule is not covalently bound to the yeast cell (28). Here we describe an ELISA spot assay that uses one MAb to capture and hold yeast cells on polystyrene plates and a second biotin-labeled antibody to promote deposition of an insoluble dye on the yeast cell capsule. In contrast to the prior M. tuberculosis study, where the bacterial cells were sharply demarcated (18), we noted a blue halo around each of the cryptococcal cells that probably reflects shed polysaccharide. For the purposes of MAb 18B7 preclinical development, this assay provides additional confirmation of the specificity of this antibody for capsular polysaccharide, and it may be useful to study cryptococcal cells from MAb 18B7-treated patients. By omitting the 18B7 incubation step this assay may be adapted to capture, concentrate, and immobilize cryptococci from cerebrospinal fluid and determine whether they have antibodies on their capsules.
In summary, MAb 18B7 binds to the capsular polysaccharide of C. neoformans isolates from the four serotypes and is biologically active, as demonstrated by its ability to promote phagocytosis by human and murine cells, activate complement, and enhance clearance of polysaccharide in mice. These results show that both the antigen binding site and the constant region of the 18B7 IgG1 molecule are competent and functional. The structural and functional similarities between MAbs 18B7 and 2H1 strongly suggest that previous observations made with MAb 2H1 are applicable to MAb 18B7. This study supports the selection of MAb 18B7 for phase I studies in patients with human cryptococcosis.
ACKNOWLEDGMENTS
This research was supported in part by Public Health Service grants AI133774 (A.C.), AI13342 (A.C.), CA-39838 (M.D.S.), AI-35370 (L.-A. P.), AI-14209 (T.R.K.), AI-31696 (T.R.K.), AI-37194 (T.R.K.), AI-01341 (M.F.), 5732GM07491 (A.L.R), and AI-01300 (D.L.G.); a Burroughs Wellcome Fund Developmental Therapeutics Award (A.C.); the Harry Eagle Chair provided by the Women’s Division of the Albert Einstein College of Medicine (M.D.S.), and an Aaron Diamond Award (A.G.-F.).
We thank Yvonne Kress for help with electron microscopy. We thank Sunhee Lee and Steve Factor for providing pathological specimens.
REFERENCES
- 1.Belay T, Cherniak R, Kozel T R, Casadevall A. Reactivity patterns and epitope specificities of anti-Cryptococcus neoformans monoclonal antibodies by enzyme-linked immunosorbent assay and dot enzyme assay. Infect Immun. 1997;65:718–728. doi: 10.1128/iai.65.2.718-728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, DeShaw M, Fan M, Dromer F, Kozel T R, Pirofski L-A. Molecular and idiotypic analysis of antibodies to Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:3864–3872. doi: 10.1128/iai.62.9.3864-3872.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans glucuronoxylomannan-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;65:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 4.Cherniak R, Morris L C, Belay T, Spitzer E D, Casadevall A. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent cryptococcal meningitis. Infect Immun. 1995;63:1899–1905. doi: 10.1128/iai.63.5.1899-1905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherniak R, Reiss E, Turner S. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239–250. [Google Scholar]
- 6.Cherniak R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleare W, Casadevall A. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlates with serotype classification and differences in functional assays. Clin Diagn Lab Immunol. 1998;5:125–129. doi: 10.1128/cdli.5.2.125-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleare W, Mukherjee S, Spitzer E D, Casadevall A. Prevalence in Cryptococcus neoformans strains of a polysaccharide epitope which can elicit protective antibodies. Clin Diagn Lab Immunol. 1994;1:737–740. doi: 10.1128/cdli.1.6.737-740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among HIV infected individuals in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 10.Devi S J N, Schneerson R, Egan W, Ulrich T J, Bryla D, Robbins J B, Bennett J E. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991;59:3700–3707. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond R D, Bennett J E. Prognostic factors in cryptococcal meningitis. Ann Intern Med. 1974;80:176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- 12.Dromer F, Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991;163:1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 13.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldmesser M, Casadevall A. Effect of serum IgG1 against murine pulmonary infection with Cryptococcus neoformans. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 15.Feldmesser M, Mukherjee J, Casadevall A. Combination of 5-flucytosine and capsule binding monoclonal antibody in therapy of murine Cryptococcus neoformans infections and in vitro. J Antimicrob Chemother. 1996;37:617–622. doi: 10.1093/jac/37.3.617. [DOI] [PubMed] [Google Scholar]
- 16.Finne J, Leinonoen M, Makela P H. Antigenic similarities between brain components and bacteria causing meningitis. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 17.Gadebusch H H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958;98:611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- 17a.Glantz S A. Primer of biostatistics. 3rd ed. New York, N.Y: McGraw-Hill, Inc.; 1992. [Google Scholar]
- 18.Glatman-Freedman A, Martin J M, Riska P F, Bloom B R, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman D L, Lee S C, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–3453. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon M A, Casadevall A. Serum therapy of cryptococcal meningitis. Clin Infect Dis. 1995;21:1477–1479. doi: 10.1093/clinids/21.6.1477. [DOI] [PubMed] [Google Scholar]
- 21.Gordon M A, Lapa E. Serum protein enhancement of antibiotic therapy in cryptococcosis. J Infect Dis. 1964;114:373–378. doi: 10.1093/infdis/114.4.373. [DOI] [PubMed] [Google Scholar]
- 22.Graybill J R, Hague M, Drutz D J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981;19:237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- 23.Houpt D C, Pfrommer G S T, Young B J, Larson T A, Kozel T R. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62:2857–2864. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Proteins of immunological interest. Publication No. 91-3242. Bethesda, Md: National Institutes of Health; 1991. [Google Scholar]
- 25.Keller R G, Pfrommer G S, Kozel T R. Occurrences, specificities, and functions of ubiquitous antibodies in human serum that are reactive with the Cryptococcus neoformans cell wall. Infect Immun. 1994;62:215–220. doi: 10.1128/iai.62.1.215-220.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel T R. Activation of the complement system by pathogenic fungi. Clin Microbiol Rev. 1996;9:34–46. doi: 10.1128/cmr.9.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozel T R, deJong B C H, Grinsell M M, MacGill R S, Wall K K. Characterization of anti-capsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect Immun. 1998;66:1538–1546. doi: 10.1128/iai.66.4.1538-1546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozel T R, Gotschlich E. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 29.Lendvai, N., A. Casadevall, Z. Liang, D. L. Goldman, J. Mukherjee, and L. Zuckier. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis., in press. [DOI] [PubMed]
- 30.Leturcq D J, Moriarty A M, Talbott G, Winn R K, Martin T R, Ulevitch R J. Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest. 1996;98:1533–1538. doi: 10.1172/JCI118945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Invest. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee J, Casadevall A, Scharff M D. Molecular characterization of the antibody responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993;177:1105–1106. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukherjee J, Feldmesser M, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:1398–1405. doi: 10.1128/aac.39.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mukherjee J, Nussbaum G, Scharff M D, Casadevall A. Protective and non-protective monoclonal antibodies to Cryptococcus neoformans originating from one B-cell. J Exp Med. 1995;181:405–409. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukherjee J, Pirofski L, Scharff M D, Casadevall A. Antibody mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci USA. 1993;90:3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee J, Scharff M D, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee J, Zuckier L, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee S, Feldmesser M, Casadevall A. J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J Infect Dis. 1996;173:1222–1231. doi: 10.1093/infdis/173.5.1222. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee S, Lee S, Mukherjee J, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994;62:1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee S, Lee S C, Casadevall A. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun. 1995;63:573–579. doi: 10.1128/iai.63.2.573-579.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieto A, Goya A, Jansa M, Moreno C, Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. J Immunol Methods. 1984;21:537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 43.Nussbaum G, Cleare W, Casadevall A, Scharff M D, Valadon P. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J Exp Med. 1997;185:685–697. doi: 10.1084/jem.185.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nussbaum G, Yuan R, Casadevall A, Scharff M D. Immunoglobulin G3 blocking antibodies to Cryptococcus neoformans. J Exp Med. 1996;183:1905–1909. doi: 10.1084/jem.183.4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otteson E W, Welch W H, Kozel T R. Protein-polysaccharide interactions. A monoclonal antibody specific for the capsular polysaccharide of Cryptococcus neoformans. J Biol Chem. 1994;269:1858–1864. [PubMed] [Google Scholar]
- 46.Plasman N, Vray B. Quantification of bacterial phagocytosis by flow cytometry and spectrofluorimetry. J Immunol Methods. 1994;174:195–202. doi: 10.1016/0022-1759(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 47.Sanford J E, Lupan D M, Schlagetter A M, Kozel T R. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun. 1990;58:1919–1923. doi: 10.1128/iai.58.6.1919-1923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlagetter A M, Kozel T R. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun. 1990;58:1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siekevitz M, Huang S Y, Gefter M L. The genetic basis for antibody production: a single heavy chain variable region gene encodes all molecules bearing the dominant anti-arsonate idiotype in the strain A mouse. Eur J Immunol. 1983;13:123–132. doi: 10.1002/eji.1830130207. [DOI] [PubMed] [Google Scholar]
- 50.Valadon P, Nussbaum G, Boyd L F, Margulies D H, Scharff M D. Peptide libraries define the fine specificity of anti-polysaccharide antibodies to Cryptococcus neoformans. J Mol Biol. 1996;261:11–22. doi: 10.1006/jmbi.1996.0438. [DOI] [PubMed] [Google Scholar]
- 51.Young A C M, Valadon P, Casadevall A, Scharff M D, Sacchettini J C. The three-dimensional structures of a polysaccharide binding antibody to Cryptococcus neoformans and its complex with a peptide from a phage display library: implication for the identification of peptide mimotopes. J Mol Biol. 1997;274:622–634. doi: 10.1006/jmbi.1997.1407. [DOI] [PubMed] [Google Scholar]
- 52.Young B J, Kozel T R. Effects of strain variation, serotype, and structural modification on kinetics for activation and binding of C3 to Cryptococcus neoformans. Infect Immun. 1993;61:2966–2972. doi: 10.1128/iai.61.7.2966-2972.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a non-protective murine antibody to C. neoformans into a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 55.Zebedee S L, Koduri R K, Mukherjee J, Mukherjee S, Lee S, Sauer D F, Scharff M D, Casadevall A. Mouse-human immunoglobulin G1 chimeric antibodies with activity against Cryptococcus neoformans. Antimicrob Agents Chemother. 1994;38:1507–1514. doi: 10.1128/aac.38.7.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Z, Pirofski L. Antifungal activity of a human anti-glucuronoxylomannan antibody. Clin Diagn Lab Immunol. 1998;5:58–64. doi: 10.1128/cdli.5.1.58-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]