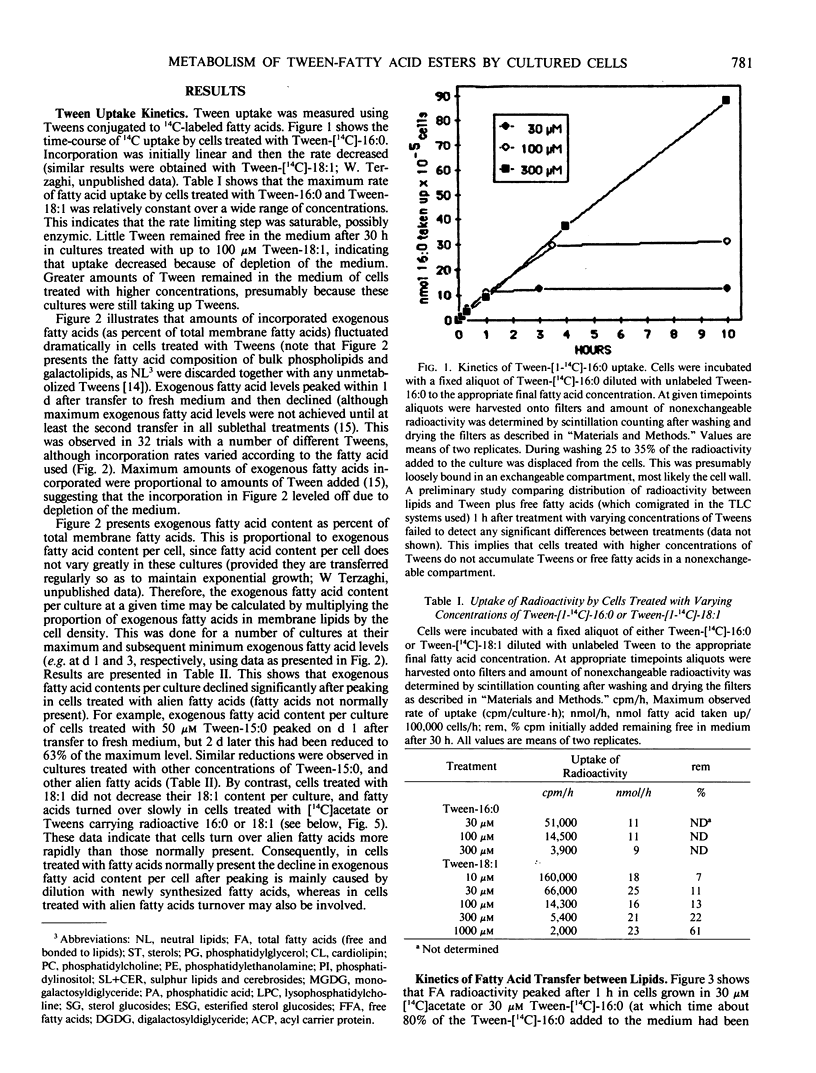
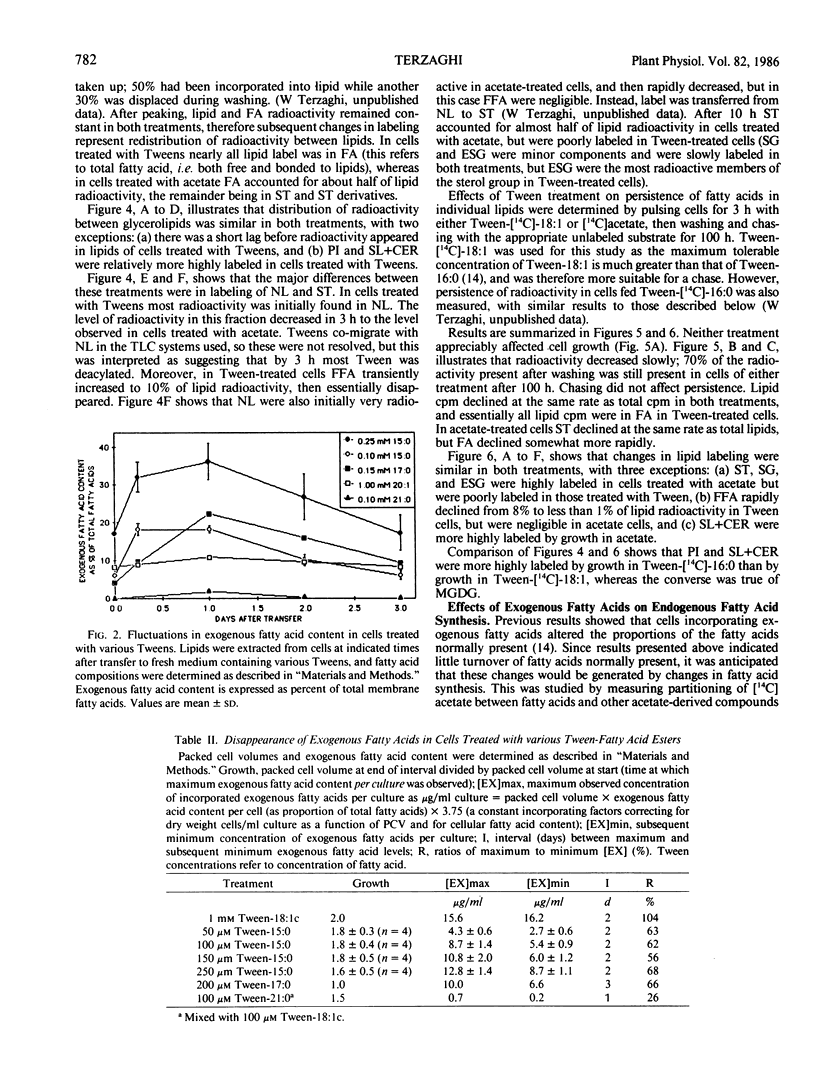
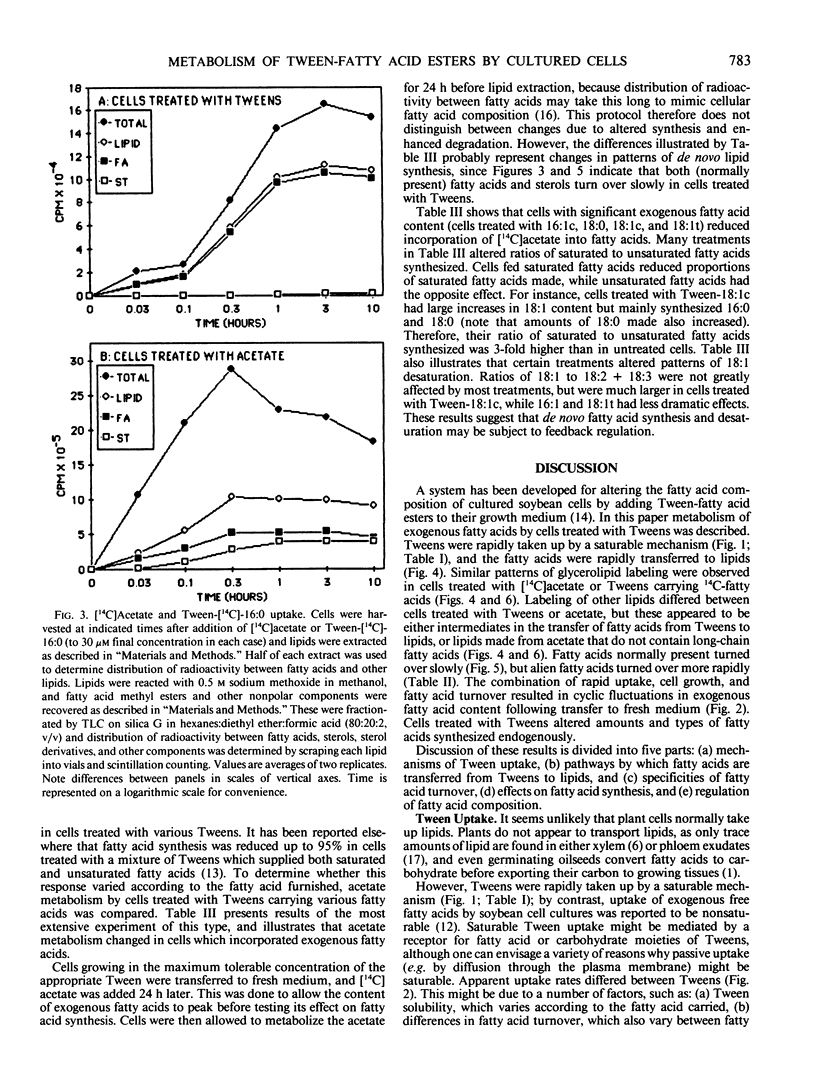
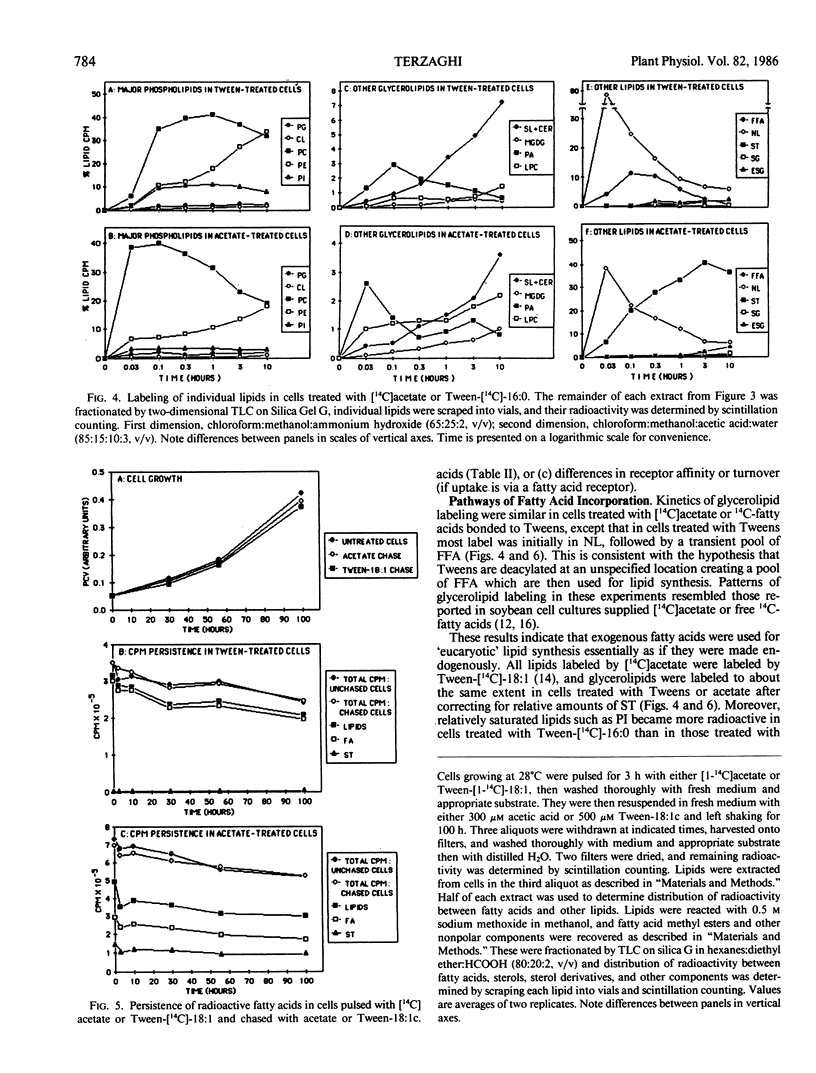
Abstract
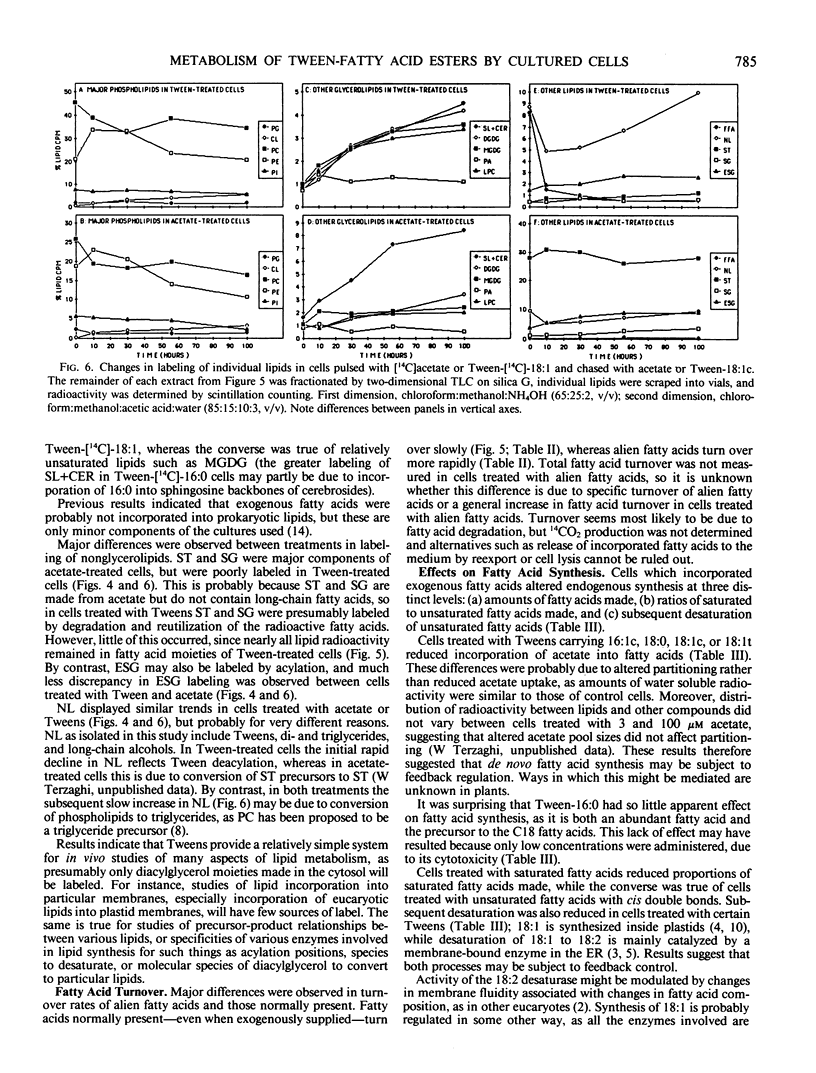
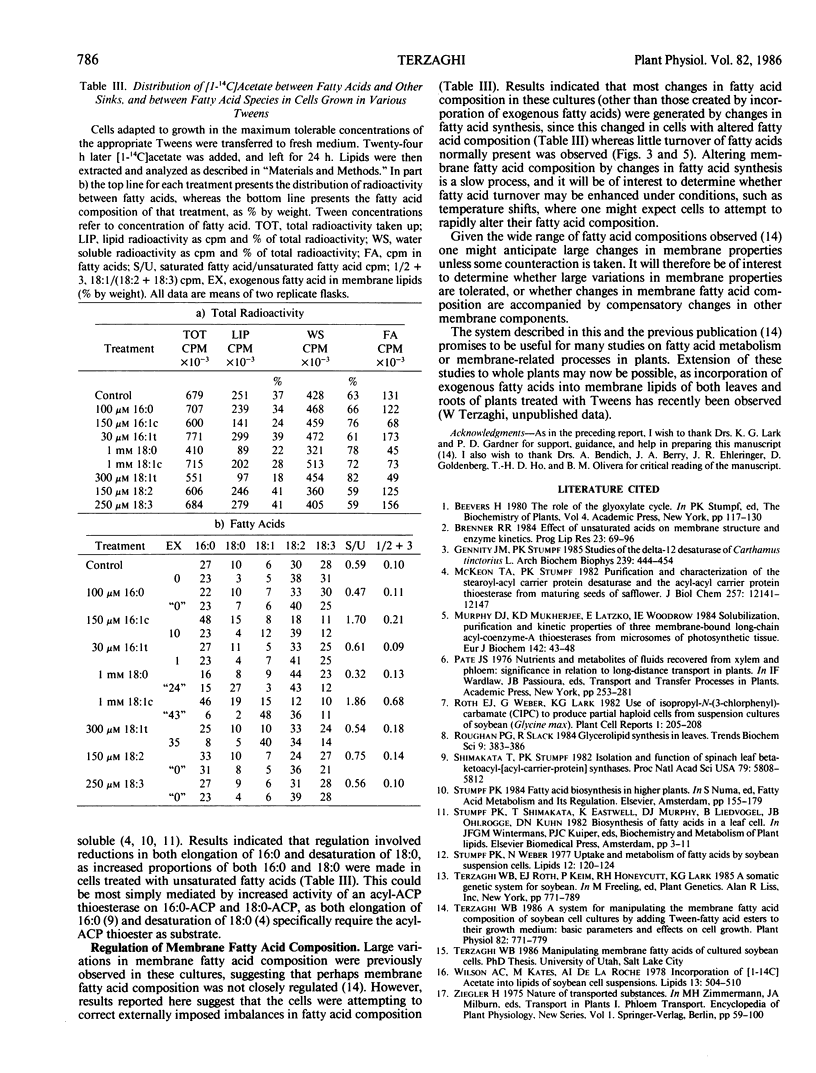
Uptake of Tween-fatty acid esters and incorporation of the fatty acids into lipids by soybean (Glycine max [L.] Merr.) suspension cultures was investigated, together with subsequent turnover of the incorporated fatty acids and associated changes in endogenous fatty acid synthesis. Tween uptake was saturable, and fatty acids were rapidly transferred from Tweens to all acylated lipids. Patterns of incorporation into glycerolipids were similar in cells treated with Tweens carrying [1-14C]-fatty acids and in cells treated with [1-14C]acetate, indicating that exogenous fatty acids were used for glycerolipid synthesis essentially as if they had been made by the cell. In Tween-treated cells neutral lipids (which include Tweens) initially accounted for the majority of lipid radioactivity. Radioactivity was then rapidly transferred to glycerolipids. A transient pool of free fatty acids accounting for up to 10% of lipid radioactivity was observed. This was consistent with the hypothesis that fatty acids are transferred from Tweens to lipids by deacylation of the Tweens, creating a pool of free fatty acids which are then used for lipid synthesis. Sterols were only slightly labeled in cells treated with Tweens, but accounted for nearly 50% of lipid radioactivity in cells treated with acetate. This suggested very little degradation and reutilization of the radioactive fatty acids in cells treated with Tweens. In cells treated with either [1-14C]acetate or Tween-[1-14C]-18:1, 70% of the initial fatty acid radioactivity remained in fatty acids after a 100 hour chase. By contrast, fatty acids not normally present disappeared more rapidly, suggesting differential treatment of such fatty acids compared with those normally present. Cells which had incorporated large amounts of exogenous fatty acids altered fatty acid synthesis in three distinct ways: (a) amounts of [1-14C]acetate incorporated into fatty acids were reduced; (b) cells incorporating exogenous unsaturated fatty acids increased the proportion of [1-14C]acetate partitioned into saturated fatty acids, while the converse was true of cells which had incorporated exogenous saturated fatty acids; (c) desaturation of 18:1 to 18:2 and 18:3 was reduced in cells which had incorporated unsaturated fatty acids. These results suggest that Tween-fatty acid esters will be useful for supplying fatty acids to cells for a variety of studies related to fatty acid or membrane metabolism.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner R. R. Effect of unsaturated acids on membrane structure and enzyme kinetics. Prog Lipid Res. 1984;23(2):69–96. doi: 10.1016/0163-7827(84)90008-0. [DOI] [PubMed] [Google Scholar]
- Gennity J. M., Stumpf P. K. Studies of the delta 12 desaturase of Carthamus tinctorius L. Arch Biochem Biophys. 1985 Jun;239(2):444–454. doi: 10.1016/0003-9861(85)90710-6. [DOI] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Murphy D. J., Mukherjee K. D., Latzko E., Woodrow I. E. Solubilization, purification and kinetic properties of three membrane-bound long-chain acyl-coenzyme-A thioesterases from microsomes of photosynthetic tissue. Eur J Biochem. 1984 Jul 2;142(1):43–48. doi: 10.1111/j.1432-1033.1984.tb08248.x. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Isolation and function of spinach leaf beta-ketoacyl-[acyl-carrier-protein] synthases. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5808–5812. doi: 10.1073/pnas.79.19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Weber N. Uptake and metabolism of fatty acids by soybean suspension cells. Lipids. 1977 Jan;12(1):120–124. doi: 10.1007/BF02532983. [DOI] [PubMed] [Google Scholar]
- Terzaghi W. B. A system for manipulating the membrane Fatty Acid composition of soybean cell cultures by adding tween-Fatty Acid esters to their growth medium : basic parameters and effects on cell growth. Plant Physiol. 1986 Nov;82(3):771–779. doi: 10.1104/pp.82.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]


