Abstract
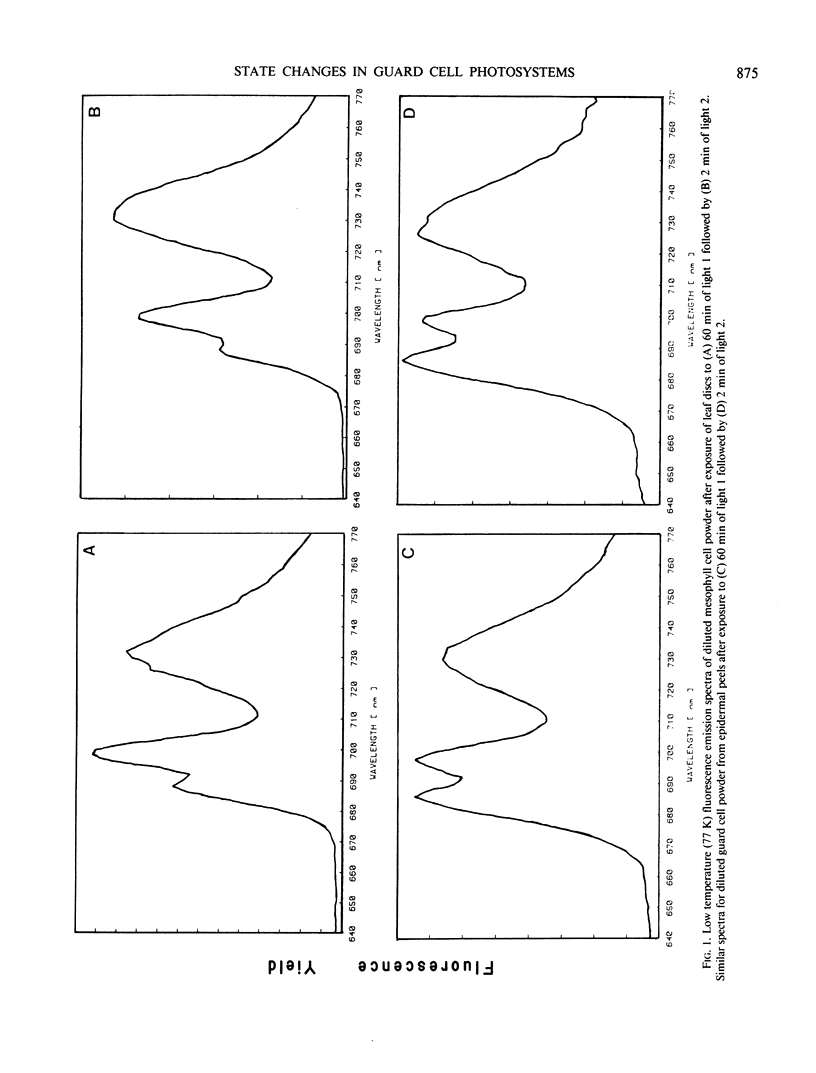
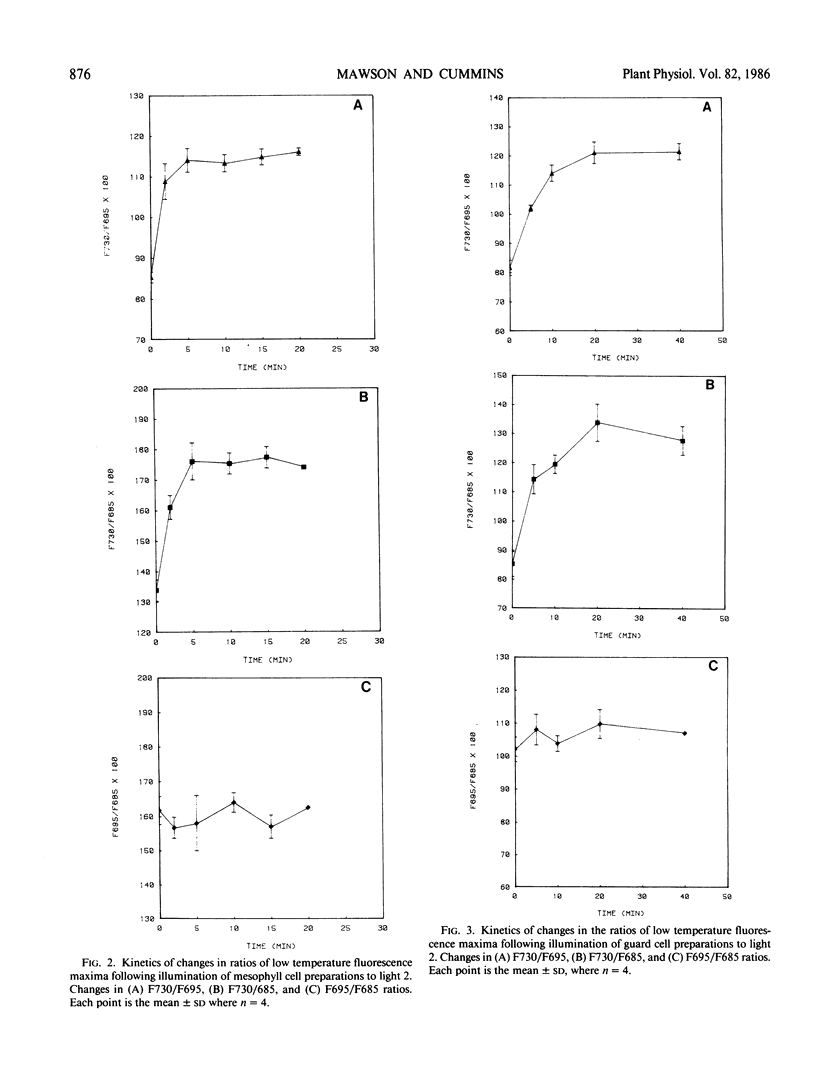
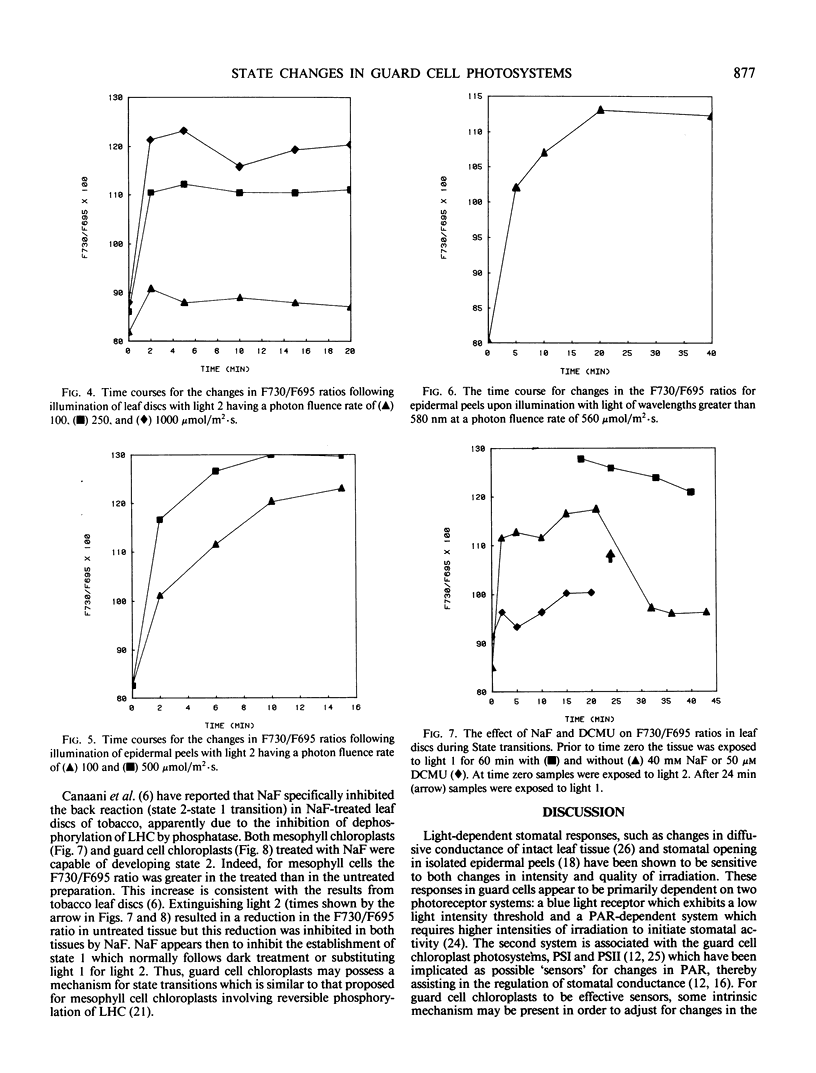
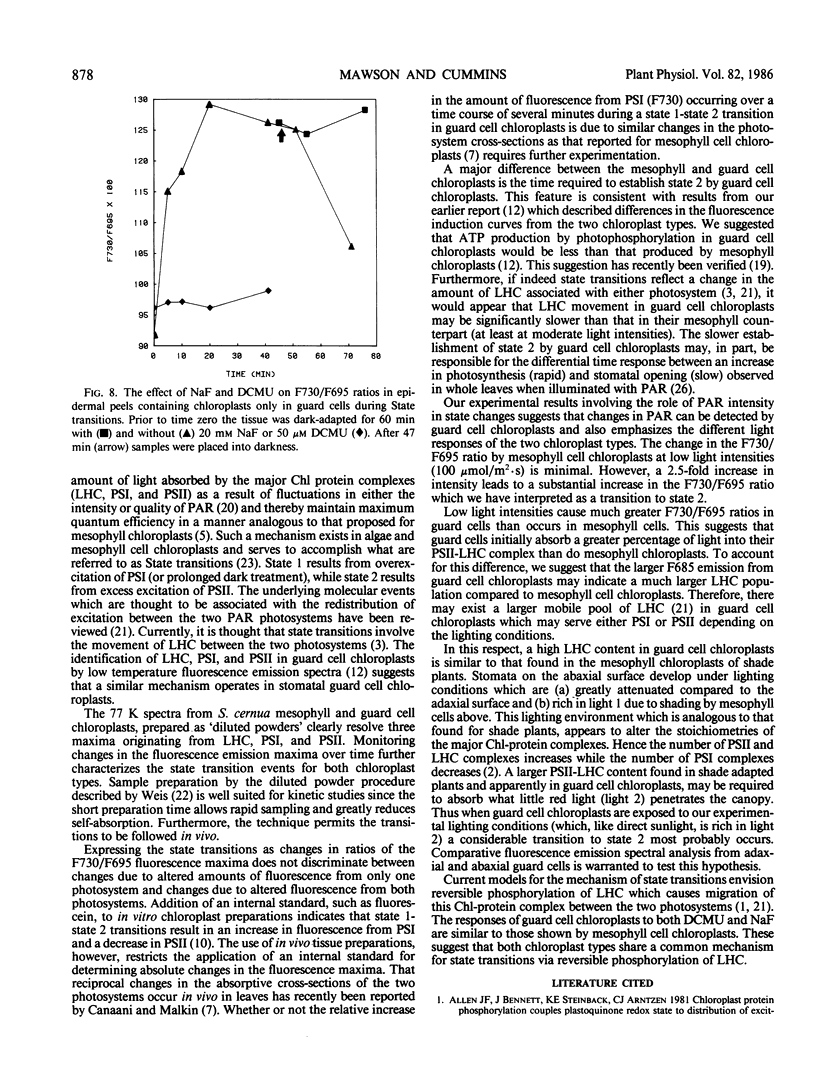
Fluorescence emission spectral peaks at 685, 695 and 730 nanometers (F685, F695, and F730) were recorded 77 K from diluted leaf tissue and epidermal powders prepared from Saxifraga cernua. The time course for state 1 to state 2 transitions was monitored as changes in the ratios of the three emission peaks. During illumination with light 2 (580 nm) the F730/F695 and F730/F685 ratios increased within minutes to establish a condition characteristic of state 2. A major difference between the two chloroplast types was the more rapid establishment of state 2 by mesophyll chloroplasts. An increase in light 2 intensity caused an increase in the magnitude of the F730/F695 ratio for both chloroplast types and, for guard cell chloroplasts, a decrease in the time required to establish the new ratio. The role of reversible phosphorylation of the light-harvesting chlorophyll a/b protein complex in regulating state transitions for both mesophyll and guard cell chloroplasts was assessed using DCMU and sodium fluoride, a specific phosphatase inhibitor. DCMU-treated mesophyll and epidermal tissues failed to show a state 1-state 2 transition. NaF-treated tissues attained state 2 but lacked the ability to revert back to state 1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The role of chlorophyll-protein complexes in the function and structure of chloroplast thylakoids. Mol Cell Biochem. 1982 Aug 6;46(3):161–172. doi: 10.1007/BF00239665. [DOI] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., Steinback K. E., Arntzen C. J. Chloroplast phosphoproteins: regulation of excitation energy transfer by phosphorylation of thylakoid membrane polypeptides. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5253–5257. doi: 10.1073/pnas.77.9.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawson B. T., Franklin A., Filion W. G., Cummins W. R. Comparative Studies of Fluorescence from Mesophyll and Guard Cell Chloroplasts in Saxifraga cernua: Analysis of Fluorescence Kinetics as a Function of Excitation Intensity. Plant Physiol. 1984 Mar;74(3):481–486. doi: 10.1104/pp.74.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969 Feb 25;172(2):242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- Murata N., Sugahara K. Control of excitation transfer in photosynthesis. 3. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):182–192. doi: 10.1016/0005-2728(69)90046-2. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Cyclic and Noncyclic Photophosphorylation in Isolated Guard Cell Chloroplasts from Vicia faba L. Plant Physiol. 1985 Jun;78(2):211–214. doi: 10.1104/pp.78.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis E. Short term acclimation of spinach to high temperatures: effect on chlorophyll fluorescence at 293 and 77 Kelvin in intact leaves. Plant Physiol. 1984 Feb;74(2):402–407. doi: 10.1104/pp.74.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Field C. Photocontrol of the Functional Coupling between Photosynthesis and Stomatal Conductance in the Intact Leaf : Blue Light and Par-Dependent Photosystems in Guard Cells. Plant Physiol. 1982 Aug;70(2):370–375. doi: 10.1104/pp.70.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]


