Abstract
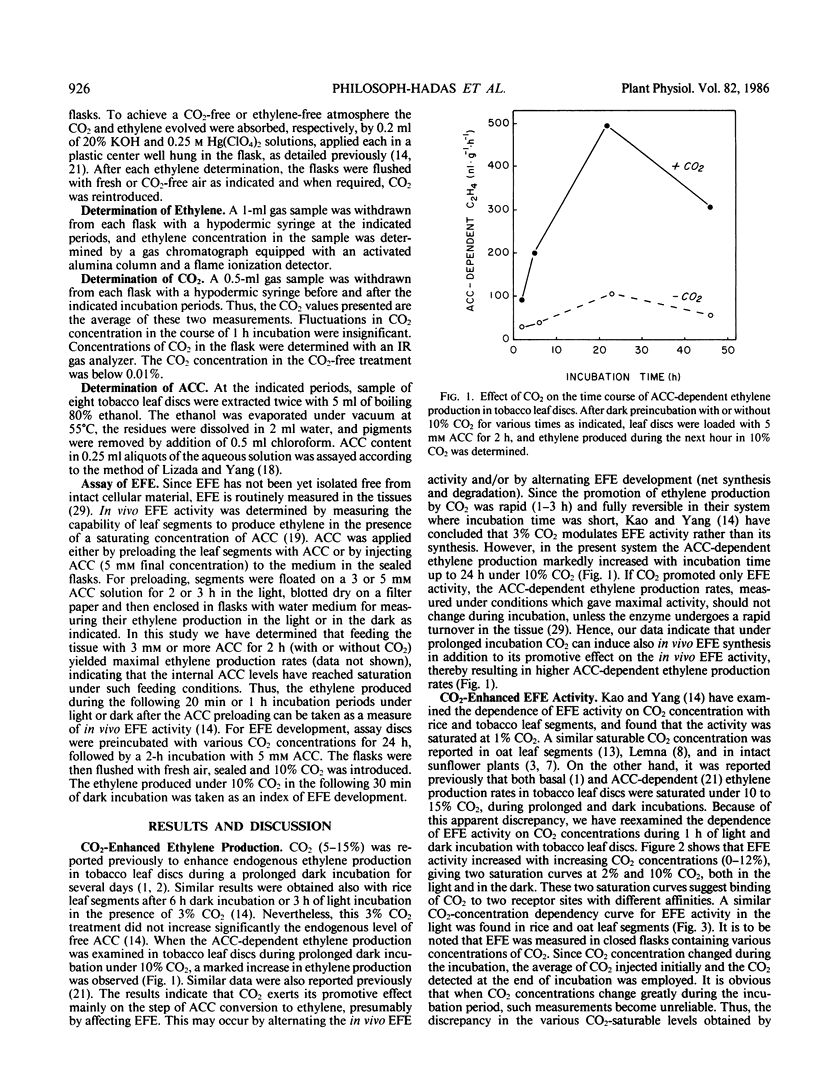
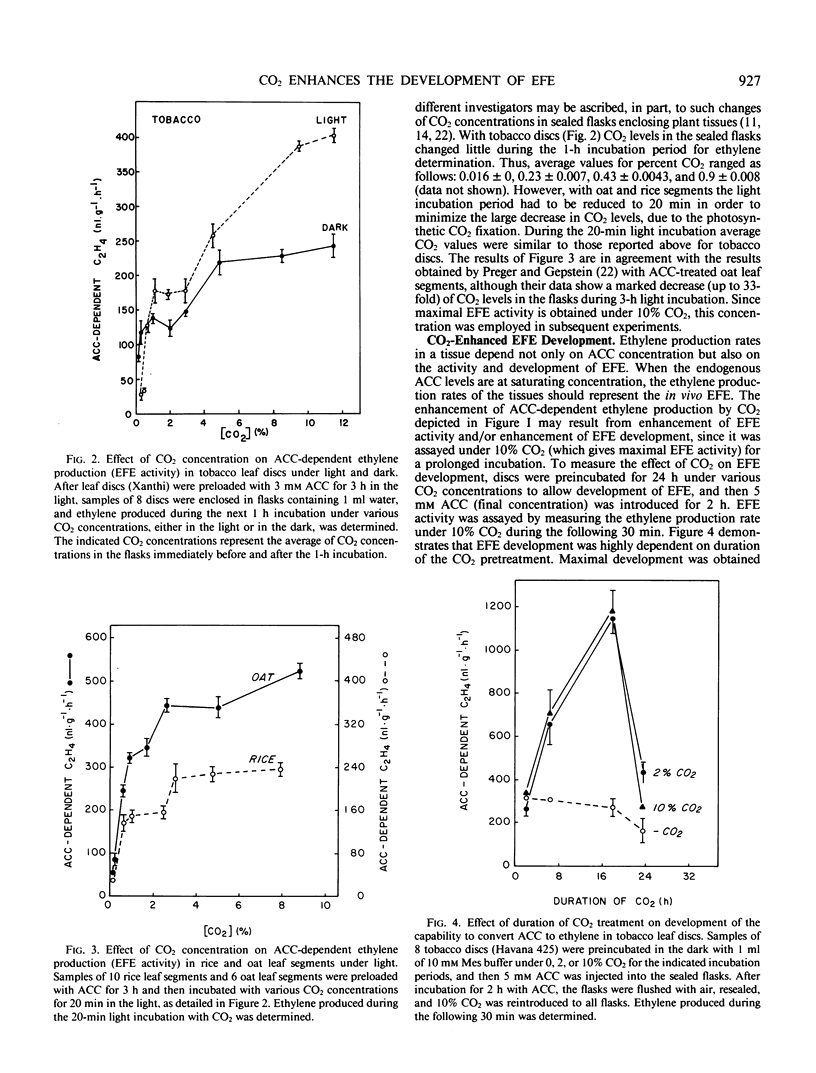
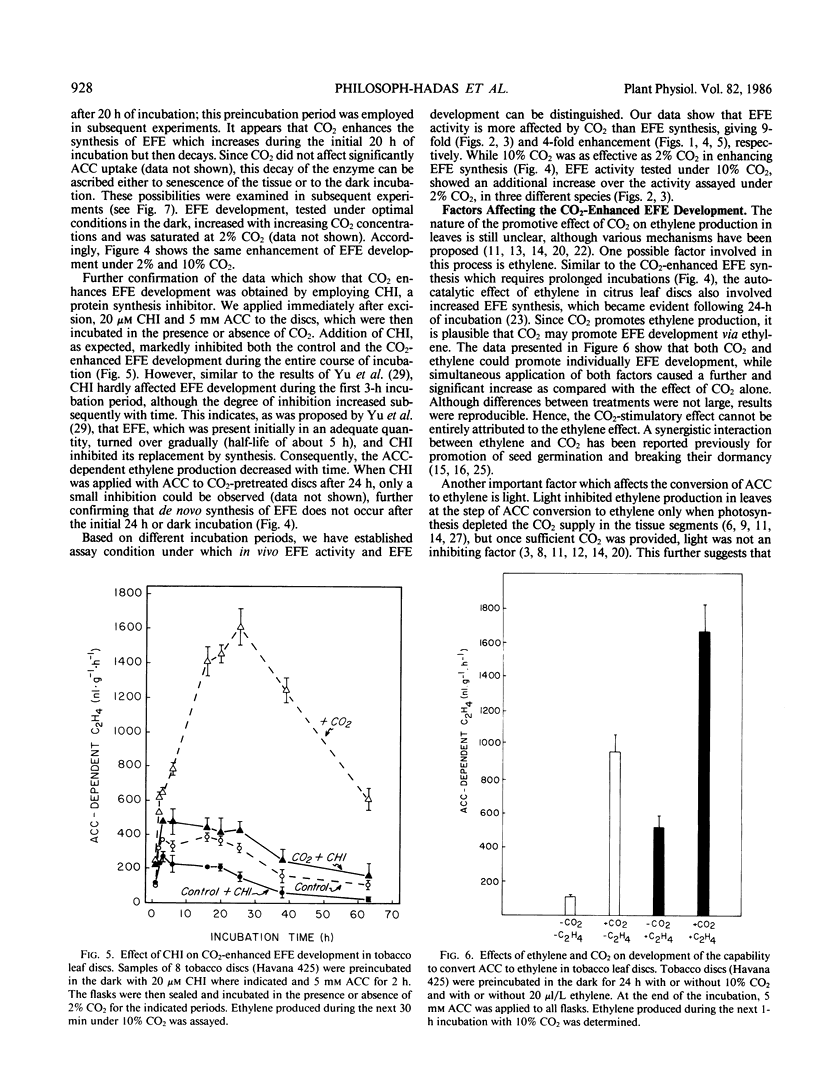
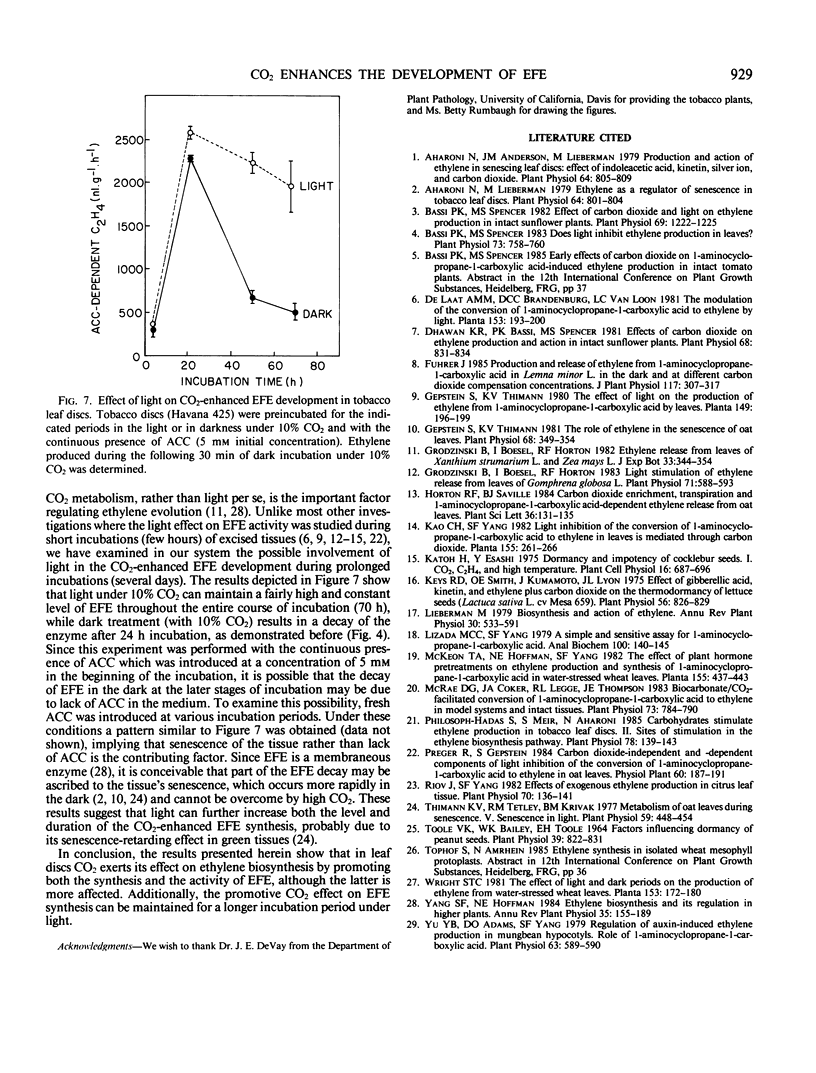
Since CO2 is known to stimulate ethylene production by promoting the conversion of 1-aminocyclopropane-1-carboxylic acid (ACC) to ethylene, the effect of CO2 on the activity and the development of the ethylene forming enzyme (EFE) was studied in tobacco (Nicotiana tabacum L. cv Havana 425 and Xanthi) leaf discs. In addition to previous observations that EFE activity is dependent on CO2 concentration and is saturable with 2% CO2, present data show two saturation curves at 2% and 10% CO2. Promotion of EFE development was dependent also on CO2 concentration (saturated at 2% CO2) and duration (maximum at 24 in the dark), and was abolished by 20 micromolar cycloheximide. Application of exogenous ethylene (20 microliters per liter) or light treatment further increased the CO2-enhanced development of EFE, implying that these two factors can also affect EFE development via interaction with CO2. The results suggest that CO2 exerts its stimulatory effect on the conversion of ACC to ethylene by enhancing not only the activity but also the synthesis of EFE in leaf discs.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. Does light inhibit ethylene production in leaves? Plant Physiol. 1983 Nov;73(3):758–760. doi: 10.1104/pp.73.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. Effect of carbon dioxide and light on ethylene production in intact sunflower plants. Plant Physiol. 1982 May;69(5):1222–1225. doi: 10.1104/pp.69.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan K. R., Bassi P. K., Spencer M. S. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol. 1981 Oct;68(4):831–834. doi: 10.1104/pp.68.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. The role of ethylene in the senescence of oat leaves. Plant Physiol. 1981 Aug;68(2):349–354. doi: 10.1104/pp.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinski B., Boesel I., Horton R. F. Light Stimulation of Ethylene Release from Leaves of Gomphrena globosa L. Plant Physiol. 1983 Mar;71(3):588–593. doi: 10.1104/pp.71.3.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys R. D., Smith O. E., Kumamoto J., Lyon J. L. Effect of Gibberellic Acid, Kinetin, and Ethylene plus Carbon Dioxide on the Thermodormancy of Lettuce Seed (Lactuca sativa L. cv. Mesa 659). Plant Physiol. 1975 Dec;56(6):826–829. doi: 10.1104/pp.56.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- McRae D. G., Coker J. A., Legge R. L., Thompson J. E. Bicarbonate/CO(2)-Facilitated Conversion of 1-Amino-cyclopropane-1-carboxylic Acid to Ethylene in Model Systems and Intact Tissues. Plant Physiol. 1983 Nov;73(3):784–790. doi: 10.1104/pp.73.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph-Hadas S., Meir S., Aharoni N. Carbohydrates Stimulate Ethylene Production in Tobacco Leaf Discs : II. Sites of Stimulation in the Ethylene Biosynthesis Pathway. Plant Physiol. 1985 May;78(1):139–143. doi: 10.1104/pp.78.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Yang S. F. Effects of exogenous ethylene on ethylene production in citrus leaf tissue. Plant Physiol. 1982 Jul;70(1):136–141. doi: 10.1104/pp.70.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. M., Krivak B. M. Metabolism of Oat Leaves during Senescence: V. Senescence in Light. Plant Physiol. 1977 Mar;59(3):448–454. doi: 10.1104/pp.59.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole V. K., Bailey W. K., Toole E. H. Factors Influencing Dormancy of Peanut Seeds. Plant Physiol. 1964 Sep;39(5):822–832. doi: 10.1104/pp.39.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]


