Abstract
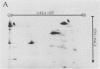
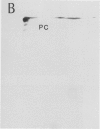
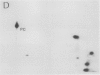
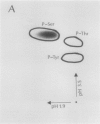
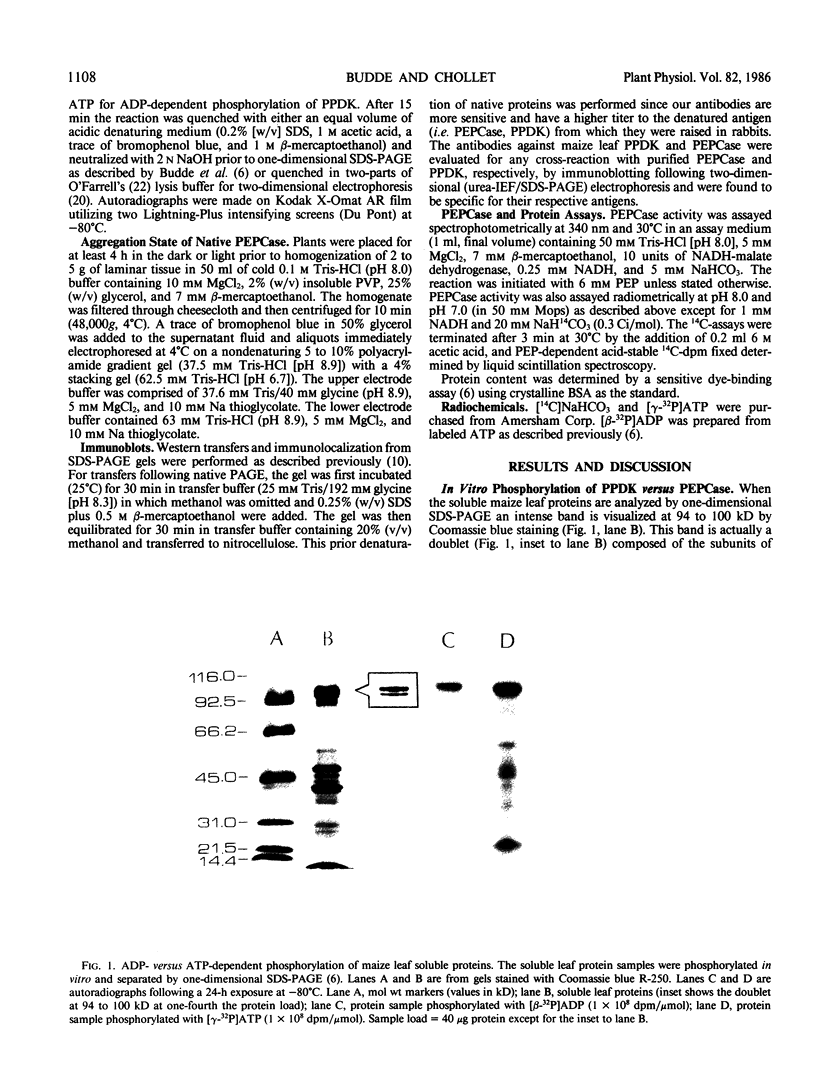
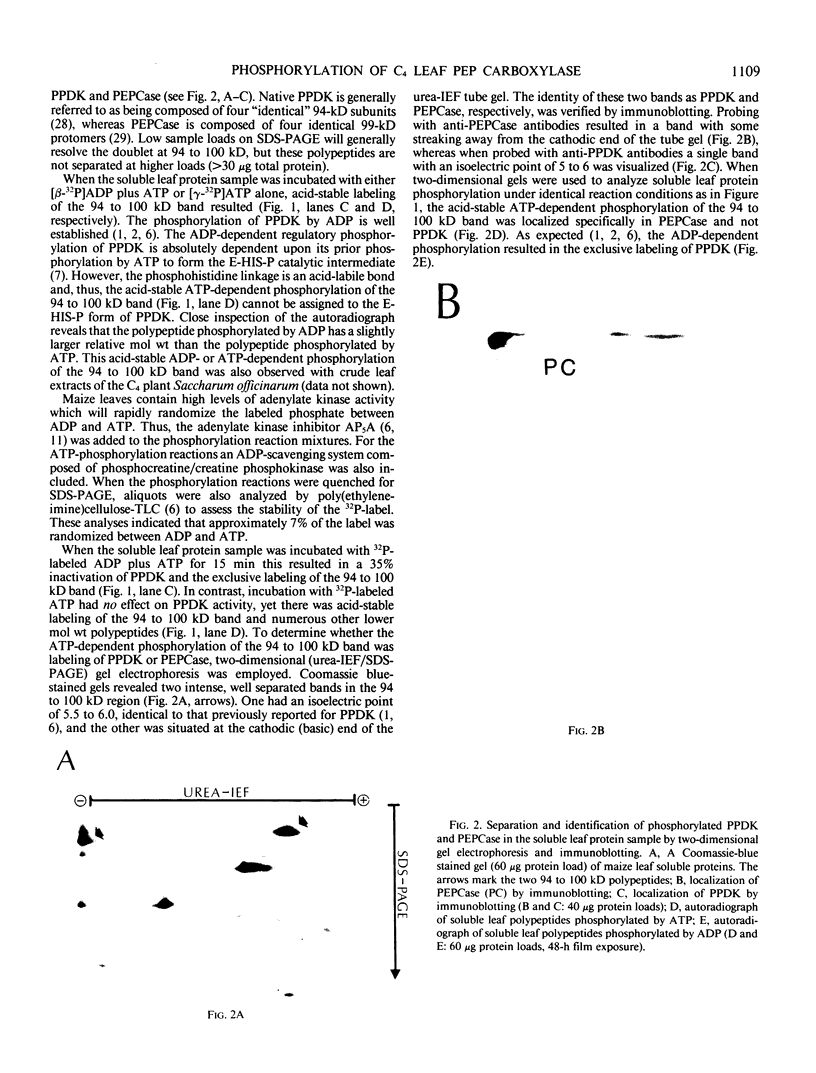
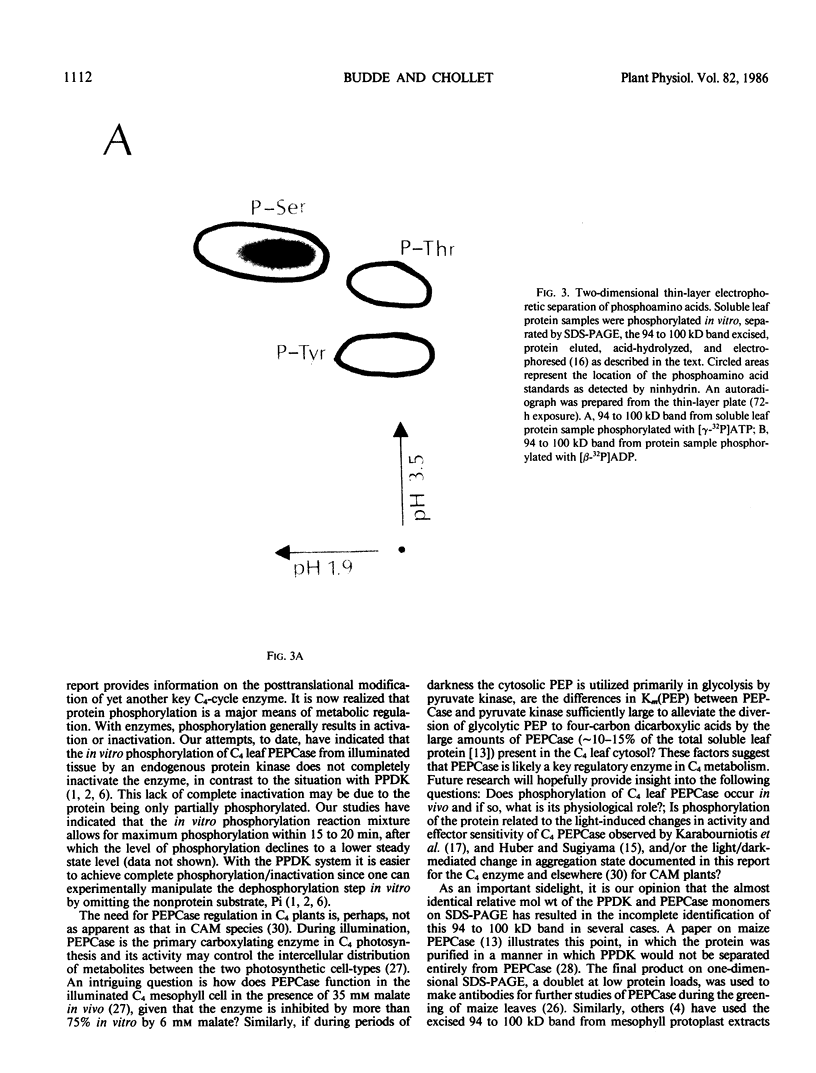
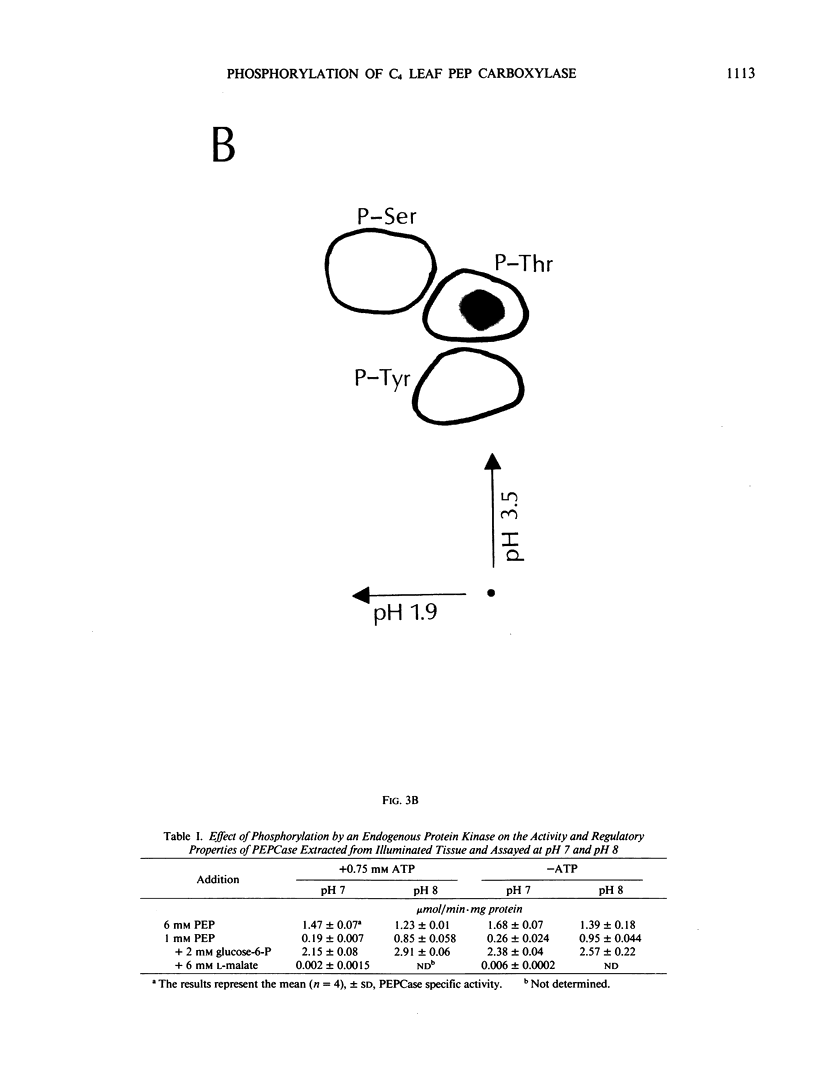
Autoradiography of total soluble maize (Zea mays) leaf proteins incubated with 32P-labeled adenylates and separated by denaturing electrophoresis revealed that many polypeptides were phosphorylated in vitro by endogenous protein kinase(s). The most intense band was at 94 to 100 kilodaltons and was observed when using either [γ-32P]ATP or [β-32P]ADP as the phosphate donor. This band was comprised of the subunits of both pyruvate, Pi dikinase (PPDK) and phosphoenolpyruvate carboxylase (PEPCase). PPDK activity was previously shown to be dark/light-regulated via a novel ADP-dependent phosphorylation/Pi-dependent dephosphorylation of a threonyl residue. The identity of the acid-stable 94 to 100 kilodalton band phosphorylated by ATP was established unequivocally as PEPCase by two-dimensional gel electrophoresis and immunoblotting. The phosphorylated amino acid was a serine residue, as determined by two-dimensional thin-layer electrophoresis. While the in vitro phosphorylation of PEPCase from illuminated maize leaves by an endogenous protein kinase resulted in a partial inactivation (∼25%) of the enzyme when assayed at pH 7 and subsaturating levels of PEP, effector modulation by l-malate and glucose-6-phosphate was relatively unaffected. Changes in the aggregation state of maize PEPCase (homotetrameric native structure) were studied by nondenaturing electrophoresis and immunoblotting. Enzyme from leaves of illuminated plants dissociated upon dilution, whereas the protein from darkened tissue did not dissociate, thus indicating a physical difference between the enzyme from light- versus dark-adapted maize plants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton A. R., Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: inactivation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and activation by phosphorolysis. Arch Biochem Biophys. 1984 May 1;230(2):492–503. doi: 10.1016/0003-9861(84)90429-6. [DOI] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of pyruvate, Pi dikinase by ADP-dependent phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):53–60. doi: 10.1016/0006-291x(83)90967-1. [DOI] [PubMed] [Google Scholar]
- Brulfert J., Vidal J., Le Marechal P., Gadal P., Queiroz O., Kluge M., Kruger I. Phosphorylation-dephosphorylation process as a probable mechanism for the diurnal regulatory changes of phosphoenolpyruvate carboxylase in CAM plants. Biochem Biophys Res Commun. 1986 Apr 14;136(1):151–159. doi: 10.1016/0006-291x(86)90889-2. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Holbrook G. P., Chollet R. Studies on the dark/light regulation of maize leaf pyruvate, orthophosphate dikinase by reversible phosphorylation. Arch Biochem Biophys. 1985 Oct;242(1):283–290. doi: 10.1016/0003-9861(85)90503-x. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Regulation of C4 photosynthesis: catalytic phosphorylation as a prerequisite for ADP-mediated inactivation of pyruvate,Pi dikinase. Biochem Biophys Res Commun. 1984 Jan 13;118(1):65–72. doi: 10.1016/0006-291x(84)91068-4. [DOI] [PubMed] [Google Scholar]
- Camp P. J., Randall D. D. Purification and Characterization of the Pea Chloroplast Pyruvate Dehydrogenase Complex : A Source of Acetyl-CoA and NADH for Fatty Acid Biosynthesis. Plant Physiol. 1985 Mar;77(3):571–577. doi: 10.1104/pp.77.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S. M., Budde R. J., Chollet R. Partial purification and characterization of pyruvate, orthophosphate dikinase from Rhodospirillum rubrum. J Bacteriol. 1986 Feb;165(2):483–488. doi: 10.1128/jb.165.2.483-488.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhau P., Fröhlich T., Goody R. S., Isakov M., Schirmer R. H. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur J Biochem. 1975 Sep 1;57(1):197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Foyer C. H. Stromal protein phosphorylation in spinach (Spinacia oleracea) chloroplasts. Biochem J. 1985 Oct 1;231(1):97–103. doi: 10.1042/bj2310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hague D. R., Sims T. L. Evidence for Light-stimulated Synthesis of Phosphoenolpyruvate Carboxylase in Leaves of Maize. Plant Physiol. 1980 Sep;66(3):505–509. doi: 10.1104/pp.66.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiken J. F., Lawrence J. C., Jr Rat skeletal muscle glycogen synthase: phosphorylation of the purified enzyme by cAMP-dependent and -independent protein kinases. Arch Biochem Biophys. 1985 Jan;236(1):59–71. doi: 10.1016/0003-9861(85)90606-x. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Detecting Photoactivation of Phosphoenolpyruvate Carboxylase in C(4) Plants : An Effect of pH. Plant Physiol. 1985 Feb;77(2):300–302. doi: 10.1104/pp.77.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. P., Randall D. D. Plant pyruvate dehydrogenase complex: inactivation and reactivation by phosphorylation and dephosphorylation. Arch Biochem Biophys. 1980 Apr 1;200(2):461–466. doi: 10.1016/0003-9861(80)90377-x. [DOI] [PubMed] [Google Scholar]
- Sims T. L., Hague D. R. Light-stimulated increase of translatable mRNA for phosphoenolpyruvate carboxylase in leaves of maize. J Biol Chem. 1981 Aug 25;256(16):8252–8255. [PubMed] [Google Scholar]
- Sugiyama T. Purification, molecular, and catalytic properties of pyruvate phosphate dikinase from the maize leaf. Biochemistry. 1973 Jul 17;12(15):2862–2868. doi: 10.1021/bi00739a014. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]