Abstract
Background
Daytime napping may improve cognitive function in older adults. However, the association can be complicated by specific features of napping and the older adult’s health. This systematic review aims to synthesize the current literature on napping and cognition in older adults and provide recommendations for future research and daytime sleep practice in older adults.
Methods
Systematic searches for relative research published between January 1995 and October 2022 were conducted at PubMed, MEDLINE, PsycINFO, and Google Scholar using keywords individually and in multiple combinations. Manual searches were performed to identify additional studies. All included studies were critically appraised by 2 authors.
Results
Thirty-five studies, including 23 observational and 12 intervention studies, were reviewed. Findings from observational studies suggest a possible inverted U-shaped association between napping duration and cognitive function: short and moderate duration of naps benefited cognitive health in older adults compared with both non-napping and long or extended napping. Findings from intervention studies suggest one session of afternoon napping might improve psychomotor function and working memory, although with some inconsistency. The effect of multiple nap sessions on cognition was inconclusive due to a limited number of studies.
Conclusion
More rigorous research studies are needed to investigate what causes different patterns of daytime napping, the associations between these distinct patterns and cognitive function, and to determine whether interventions targeting napping patterns can improve cognition in older adults. In addition, future research needs to comprehensively assess daytime napping using a combination of measures such as sleep diary and actigraphy.
Keywords: Cognition, Daytime sleep, Memory
The prevalence of daytime napping in older adults varies across the world, ranging between 20% and 60% (1). It is more common in older adults from regions where napping is culturally regarded as a healthy behavior, such as countries in Asia. For example, about 55%–60% of Chinese older adults regularly take afternoon naps, as reported in national representative surveys (2–4). Naps are likely more common among older adults than among younger people for a range of reasons, including changes in physical and social activity, as well as in physical, mental, and cognitive health along with aging (5). Older adults may have a less structured schedule due to retirement and more free time during the day, making a nap more feasible. Furthermore, older adults may take medications that cause daytime sleepiness, or even take a nap to make up for impaired evening sleep (6).
There is a growing interest in understanding the link between daytime napping behavior and cognitive health in older adults. Daytime napping may improve cognitive performance by compensating for inadequate sleep at night, countering daytime sleepiness and fatigue, and boosting energy levels in older adults. However, the association can be complicated by the features of napping (eg, duration, frequency, timing, intentionality) and the individual older adult’s physical and cognitive health (6). Although a growing body of research investigates the associations between daytime napping behavior and cognitive function and the effect of nap interventions on cognitive performance, no systematic review has been published to examine the evidence regarding the associations of daytime napping with cognitive health in older adults. Therefore, the purpose of this systematic review is to critically analyze and synthesize the literature on daytime napping and cognition to provide recommendations for future research and daytime sleep practices in older adults.
Method
Search Strategy and Data Extraction
This systematic review was conducted according to the recommendations from the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis (7) and the Transparent Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8). Two authors performed systematic and manual searches to identify studies that examined the associations between napping and cognition in older adults. Systematic searches were conducted using the following electronic databases: PubMed, MEDLINE, PsycINFO, and Google Scholar and were limited to articles written in English and published between January 1995 and October 2022. Search terms included: (nap OR napping OR napper OR daytime sleep OR post-lunch sleep OR post-prandial sleep OR siesta) AND (cognition OR cognitive function OR cognitive performance OR executive function OR executive ability OR attention OR orientation OR reaction OR reaction time OR reaction speed OR memory OR visual-spatial OR language OR verbal OR calculation) AND (older OR elder OR aged OR aging OR seniors). Manual searches were performed through reference lists of relevant articles and review papers to identify additional studies.
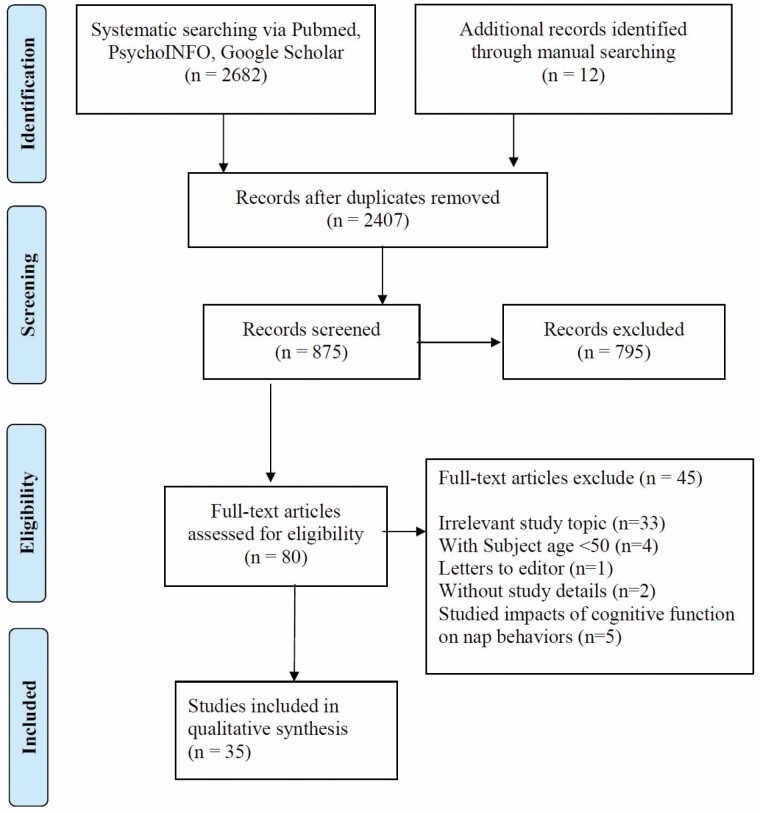
The initial screening took place by 2 authors independently reading the titles and abstracts of 875 articles; 80 articles were eligible for full-text screening. Eligible full-text articles were independently reviewed by 2 authors with discrepancies resolved by a third reviewer. After full-text review, we excluded 45 studies; 35 articles were in the final sample (Figure 1).
Figure 1.
PRISMA flow chart for study selection.
Studies meeting the following criteria were included: (a) participants aged 50 years and above; (b) examined the association between nap characteristics and cognitive function; (c) included at least one assessment of nap characteristics: habits, intention, frequency, duration, or timing; (d) included at least one assessment of cognitive function/performance/status; and (e) interventional or observational study. Studies investigating whether napping characteristics predicted the incidence or severity of AD and dementia were included. Studies published as case report, abstracts, or letters to the editor without study details were excluded.
Data were extracted independently by 2 authors, and differences were resolved by discussion with the third assessor. Data extraction included the surname of the first author, year of publication, country of origin, study design (randomized controlled trial [RCT], within-subjects experiment, cross-sectional, longitudinal, and case–control study), name of the cohort studied, baseline year and follow-up time (if applicable), sample size, age at baseline (mean ± standard deviation, or range), gender, study participant characteristics, napping measurement, napping characteristics, cognition measurement, cognition domains, potential confounding/interaction/modifier variables controlled for in the study design and statistical analysis, and quality appraisal results. We could not conduct a meta-analysis to examine the association between nap duration and cognitive functions as planned due to the high heterogeneity of napping duration groups and cognitive domains assessed among studies.
Study Quality Assessment
To assess the study quality, all included studies were critically appraised independently by 2 authors using the standard critical appraisal instrument from JBI Manual for Evidence Synthesis (7). We used the Critical Appraisal Checklist for randomized controlled trials, quasi-experimental studies, cohort studies, analytical cross-sectional studies, and case–control studies (7). Each study was evaluated for internal validity, external validity, bias, confounding, causal plausibility, and statistical inferences using the appropriate appraisal checklist (7). For each item on the checklist, reviewers selected either: Yes (meets criteria); No (does not meet criteria); Unclear; or Not Applicable. We used a predetermined cut-off score to assesses 4 quality levels: Level-A: all “Yes”; Level-B: one or two items were rated with “No” or “Unclear”; Level-C: 3 or 4 items were rated with “No” or “Unclear”; Level-D: more than 4 items were rated with “No” or “Unclear.” Differences in assessment between the assessors were resolved by discussions between the 2 assessors and a third assessor.
Results
A total of 35 studies met all the inclusion criteria (Figure 1). Of these, there were 23 observational studies and 12 intervention studies. We synthesized findings from observational studies (Supplementary Tables 1 and 2) and intervention studies (Supplementary Table 3) separately. Most studies reported results for males and females; Tamaki et al. (9) did not report the study participants’ sex/gender, Blackwell et al. (10) only included women, and Leng et al. (11) only included men. Studies were included from 10 countries: 12 studies from the United States, 10 from China (including 1 from Hong Kong and 1 from Taiwan), 4 from Japan, 2 from Australia, 2 from Canada, and 1 each from Greece, Germany, Israel, Saudi Arabia, and the United Kingdom.
Overview of Study Quality
Of the 35 included studies, 30 studies were at the level-B quality, and 5 were at level C. Most of the case–control, cross-sectional, and longitudinal cohort studies were graded as Level B due to the lack of a valid napping measure (eg, polysomnography [PSG]). Three cross-sectional studies presented unclear descriptions of identified confounders or what strategies were used to deal with confounding variables and were therefore graded as Level C (12–14). Five of the 6 RCT studies were graded as Level B because participants were not blinded to the intervention, and there was no clear description of whether outcome assessors were blinded to treatment assignments. One RCT study was graded as Level B because there was no clear description of whether the randomization resulted in similar study groups at baseline (15). Four of the 6 non-randomized experimental studies were graded as Level B due to the within-subject design and lack of a control group. Two studies were graded as Level C because there was no clear description of participant information or statistical analysis (9,16).
Findings from Observational Studies
The association between nap duration and cognitive function was examined in 23 observational studies, including 13 cross-sectional, 8 longitudinal cohort studies (two of which also included cross-sectional analyses), and 2 case–control studies. Most observational studies used data from epidemiologic studies or national cohort studies, including the China Health and Retirement Longitudinal Study (CHARLS) (3,4,17–19) Taiwan National Health Interview Survey (20), Study of Osteoporotic Fractures, Osteoporotic Fractures in Men Study (MrOS) (11), U.S. National Health and Aging Trends Study (NHATS) (21,22), Australia Cognitive Function and Ageing Study (CFAS) (23), U.K. Lothian Birth Cohort 1936 (LBC1936) (24), and Greece Cretan Aging Cohort (25).
Napping assessment
In most observational studies (18 out of 23), napping was assessed subjectively using the participants’ self-report (including survey questions or sleep diary) (n = 16), informant report (n = 1) (26), or direct observation of participants (n = 1) (12). The definitions of napping varied in these studies. Most studies defined napping based on participant or informant’s answers to general questions on napping (example questions: Do you usually take a nap during the day? How long did you take a nap in general?) without inquiring about napping frequency or intentionality (ie, whether or not naps were planned). Some studies defined napping according to weekly napping frequency. For example, studies defined nappers as those who reported napping on 3 or more days a week (26) or those who napped at least one day a week (21,22). Most studies assessed napping throughout the daytime; other studies assessed post-lunch/afternoon napping only (3,4,13,17,18,27) or observed napping between 9 am and 3 pm (12). Only one study assessed the intentionality of naps by asking, “In general, were these naps planned, or did you fall asleep without meaning to?” (21).
Five studies assessed napping objectively using actigraphy and reported average daytime napping duration over 3–14 days (10,11,25,28,29). In addition to nap duration, one study reported the number of naps per day and the time of first nap (pre-17:00 hours vs post-17:00 hours) (28).
Nap duration
Nap duration was categorized using various cut points in these studies. In general, participants were categorized as non-nappers or those who napped for less than a given number of minutes (eg, <5, <60). Cut points commonly used were 30, 45, 60, 90, and 120 minutes. In this review, we defined short napping as ≤ 30 minutes, short-moderate napping as <45 or < 60 minutes, moderate napping as 30–60 minutes or 30–90 minutes, long napping as >60 minutes, and extended napping as >90 minutes or≥ 120 minutes.
Napping Duration by Groups
Short napping (≤30 minutes) versus non/long/extended napping (n = 10 studies)
In general, results were consistent and showed that short napping was associated with better cognitive outcomes, compared with non, long, or extended napping. Three of the 4 cross-sectional studies found that short napping was associated with better overall cognitive function (17), episodic memory (21), or less likely to have cognitive impairment (21,27) compared with non-napping (17,27) and long napping (21,27). Five of the 6 longitudinal studies found that, compared to non-napping or long or extended napping, short napping at baseline was associated with less cognitive decline after 2 years (3), within 8 years (19), a decreased risk of subsequent cognitive decline or impairment in 5-year (30) or 12-year follow-up (11), decreased risk of AD between 5 and 10 years (26)
Short-moderate napping (<45 or < 60 minutes) versus non napping (n = 6 studies)
Two studies found short-moderate napping was cross-sectionally associated with better cognitive function (4) or associated with less risk of developing cognitive impairment in 2 and 10 years (23). Four studies reported no cross-sectional association between short-moderate napping and cognition, or cognitive impairment compared with non-napping (14,20,22,31).
Moderate napping (30–60 minutes or 30–90 minutes) versus non/short/long/extended napping (n = 9 studies)
Comparing moderate napping with non-napping, 3 of the 4 cross-sectional studies found that moderate napping was associated with better global cognitive function (17), better attention and episodic memory (17,21), and better spatial ability (21), less risk of having cognitive impairment (27). Three of the 5 longitudinal studies found that moderate napping was associated with less decline in overall cognition, episodic memory, attention (3), a better trajectory of cognition in older women (18), or decreased risk of AD (26) between 5 and 10 years than non-napping (3,18,26). When compared with long/extended nappers, studies found moderate nappers were associated with better cognitive performance (17) or less decline (3). The results on moderate napping versus short napping were inconsistent in the 3 cross-sectional studies. Two studies found that moderate napping was associated with better cognitive performance than short napping in Chinese older adults (17) and U.S. older adults without dementia (21). Conversely, Lin et al. found that moderate nappers were more likely to have cognitive impairment compared to short nappers (27).
Long (>60 minutes)/ extended naps (>90 minutes or ≥ 120 minutes) versus non-napping/ nap <120 minutes (n = 10 studies)
Nine studies examined the association between long or extended napping and cognitive function compared with non-napping. Seven studies, including 2 cross-sectional (4,20) and 5 longitudinal studies (18,19,23,26,30), found no significant difference in cognitive function, risk of developing subsequent cognitive impairment or trajectory of cognitive function between long or extended versus non-nappers. Only two studies found that extended nappers were cross-sectionally (31) or longitudinally (2 years) (3) associated with worse cognitive performance. Another study found extended napping (≥120 minutes as measured using actigraph) was cross-sectionally associated with cognitive impairment in older women, compared to napping <120 minutes (10)
Presence of napping regardless of duration versus non-napping (n = 4 studies)
The findings were inconsistent. Two studies found that napping was associated with better overall cognitive function, orientation, language, and working memory cross-sectionally in healthy Chinese older adults (13) and decreased risk of subsequent cognitive impairment in 2 and 10 years in a sample of Australian older adults who were mostly (84%) cognitively intact (23). Conversely, one study reported nappers had a higher rate of mild cognitive impairment (28) and one study reported that napping was associated with worse performance on autobiographic memory, working memory, and episodic memory in people with dementia (25).
Continuous napping duration (n = 5 studies)
Consistently, longer napping duration was associated with worse performance in verbal memory, processing speed, and verbal fluency (28), more decline in visuospatial reasoning and processing speed over 6 years (24), and higher risk of cognitive impairment (11,25). One study found that decreased napping duration after hospital discharge, compared to pre-discharge, was associated with improvement in cognitive function at 6-month follow-up of hospitalized older adults (29). Of note, all these studies except one (24) used actigraphy to measure nap durations.
Potential moderators of nap duration and cognitive function
Asada et al. found that long napping (>60 minutes) increased the risk of developing Alzheimer’s disease in 5–10 years in APOE e4 carriers but not in non-APOE e4 carriers (26). Fang et al. (20) found a significant interactive effect between chronotype and napping duration. Long napping was associated with better global cognitive performance than non-napping in morning-type older adults but not in the evening‐ or intermediate‐type older adults, or in the overall sample. In addition, long napping was associated with worse immediate recall performance among intentional nappers but not among unintentional nappers (21).
Daily/weekly napping frequency
Only one study examined daily napping frequency and found that a greater number of naps per day, as measured by actigraphy, was associated with poorer verbal fluency and executive function (28). Two studies examined weekly napping frequency and cognition: one found an inverted U-shape association between weekly napping frequency and global cognition, with better cognition in people napping 1–2 days/week than in those with less or more frequent napping (32). Owusu et al. (21) reported that napping on most days was associated with poorer self-reported memory than non-napping.
Time of day
One study found that older adults who took the first nap before 17:00 hours had significantly lower scores on processing speed and executive function than those who had their first nap post-17:00 hours (28).
Unintentional napping versus intentional versus non-napping
Unintentional napping versus intentional versus non-napping was examined in only one study. The study found that unintentional nappers had worse episodic memory and self-rated memory than non-nappers and intentional nappers (21).
Findings from Intervention Studies
Among the 12 intervention studies—all of which were conducted in healthy older adults—6 were RCTs and 6 were within-subject cross-over studies. Ten of these studies tested the effect of one session of napping on cognitive performance on the same day or the next day after the nap. Two studies tested the impact of repeated nap sessions over 17 days or 4 weeks on cognitive performance (33,34). Various cognitive performance measures were used to assess motor/psychomotor functions, verbal memory, alertness/vigilance, and brain activation (see details in Supplementary Table 3).
Napping assessment
All 12 studies that objectively assessed napping used PSG) or EEG; 2 studies used in-lab PSG/EEG in combination with at-home actigraphy or sleep diary (33,34).
Effect of one afternoon nap on cognitive performance
Overview of study interventions.
—Of the 10 one-session afternoon nap studies, 9 were conducted in the laboratory environment and one in participants’ homes (35). In all studies, participants performed cognitive tasks before nap, right after nap, or after overnight sleep. Nine studies provided older adults with a nap opportunity of a certain duration (eg, 20 minutes, 45 minutes, 60 minutes, 90 minutes, or 2 hours) in the early afternoon (started between 12 pm and 2 pm). Two studies gave participants the opportunity to nap at 1 pm and awakened them 30 minutes after sleep onset (9,16).
Motor memory (n = 3 studies).
—Two studies found a 90-minute nap opportunity (on average, participants who napped 57 minutes or 59 minutes) improved motor speed (36) and motor memory consolidation (a composite measure of motor speed and accuracy) (37) in healthy older adults across the same day as the nap and the next day after overnight sleep, compared to a sedentary no-nap group. Furthermore, King et al. (37) found that the enhanced motor memory by napping in the same- and next-day retests was reflected by greater activation in memory-related structures, including the putamen and medial temporal lobe (hippocampus and para-hippocampus). However, the study by Bachhaus et al. (38) failed to observe any benefit of an afternoon nap (10–20 minutes nap or 50–80 minutes nap) on motor memory consolidation in healthy older adults.
Psychomotor function (n = 4 studies).
—Since 3 of the 4 studies found better psychomotor performance (executive processing, detection time, and accuracy) immediately after the nap (9,16,39) and throughout the next day compared to the sedentary no-nap condition (39), one session of afternoon napping may improve psychomotor function. Furthermore, one study found that nap duration and the amount of stage 3 sleep were positively associated with reaction time and processing speed (39); however, another study suggested that neither nap opportunities of 20 or 60 minutes improved reaction time in healthy older adults (40).
Verbal episodic memory (n = 3 studies).
—The 3 studies consistently reported no improvement in verbal memory consolidation by napping (up to 2-hour nap opportunity), compared with sedentary or no nap condition (35,38,41). In addition, neither nap condition nor nap architecture (eg, Slow-wave sleep [SWS]) were associated with hippocampal activation during the verbal memory tests after nap (35).
Executive function including working memory, attention, and logic reasoning (n = 2 studies).
—Improvement in working memory following an afternoon nap (20 minutes, 60 minutes, or 2 hours nap opportunity) was found in both studies (15,40). The effect of an afternoon nap on attention and logical reasoning was examined in one study that found improvement in attention and reasoning on the next day following the nap compared with sedentary no nap control (39). Further, characteristics of the nap were associated with executive function. Nap duration, sleep efficiency, and stage 2 sleep during the nap were positively associated with increased accuracy in logical reasoning following the nap (39); and slow oscillation (SO, 0.5–1 Hz) power and greater coupling between SO and sigma (12–15 Hz) during the nap were associated with better working memory performance (15).
Effect of multiple sessions of nap intervention on cognitive performance.
Two studies tested the effect of regular afternoon naps (multi-session: repeatedly over several days) at home on cognitive performance. The durations of the interventions were daily for 17 days (34) and at least 5 days per week for 4 weeks (33). Monk et al. (34) found that a 90-minute nap opportunity in the early afternoon daily for 17 days (14 at home and 3 in lab) did not improve self-rated alertness/vigilance and psychomotor function in healthy older adults but did reduce objective evening sleepiness compared with no nap conditions. Campbell et al. (33) found both short (a 45-minute nap opportunity) and long nap condition (a 2-hour nap opportunity) enhanced logical reasoning and attention (baseline < mid-study < post-study) but had no impact on psychomotor function. No significant differences in cognitive performance were observed between the 2 nap conditions.
Discussion
This systematic review is the first to examine the current body of literature on the associations between daytime sleep (ie, napping) practice and cognitive outcomes in older adults. Most of the included observational studies used population-based samples and focused only on whether participants napped or nap duration. In general, findings from these studies suggest a possible inverted U-shaped association between nap duration and cognitive function. Short naps (≤30 minutes) or moderate-duration naps (30–90 minutes) might be beneficial for older adults’ cognitive function compared with not napping or long to extended-duration napping (>90 minutes); there were no significant differences between non-napping and long-to-extended-duration napping in association with cognitive health in older adults. More studies are needed to elucidate the associations between nap frequency, timing, and intention (planning) of napping on cognitive performance and changes over time, given that these associations were assessed in only 3 of the 23 observational studies. Findings from the 12 intervention studies in healthy older adults suggested that one session of afternoon nap might benefit psychomotor function and working memory. Findings on the effect of a single afternoon nap on verbal and motor memory and the effect of multiple nap sessions on cognitive function were inconclusive due to the limited number of studies reporting inconsistent findings.
The exact mechanisms of how daytime napping impact cognitive health are unclear. Napping may function similar to nocturnal sleep for cognitive function by facilitating synaptic plasticity, procedural learning processes, and memory consolidation (42–46). Insufficient sleep or sleep loss in older adults not only affects memory consolidation, but also results in decreased attention and vigilance, which are essential for performance in many other cognitive domains, such as psychomotor function and executive function (46–48). Therefore, daytime napping may compensate for inadequate nighttime sleep and improve cognitive function. Our findings support that daytime napping may provide cognitive benefits in older adults with observational studies that suggest that napping routinely for a short or moderate duration (<90 minutes) benefits cognitive health (improved attention, episodic memory, spatial ability, and overall cognitive performance), and intervention studies show that one session of nap in the afternoon improves psychomotor function, attention, and working memory but not long-term memory (episodic memory); and that multi-session afternoon naps may improve attention and reasoning. The effect of multi-session nap interventions on long-term memory (eg, episodic memory) has not yet been studied in current literature.
However, findings also suggest that not all naps were associated with better cognition in older adults, as long or extended napping was consistently associated with poorer cognitive health in the reviewed observational studies. The effect of napping on cognitive function largely depends on features of the nap such as length, timing, frequency, and purpose/intention of the nap.
Daytime napping in the afternoon may be a part of many older adults’ daytime routine; however, older adults with poor health may frequently doze off, and unintentionally nap for a longer time during the day to combat the excessive daytime sleepiness and fatigue caused by comorbidity and medications. In this sense, frequent or extended napping could also be a symptom from existing chronic conditions (1,6). Frequent unintentional naps or longer naps contribute to sedentary behaviors and decreased social engagement, which are known detrimental factors for cognitive health in older adults (49–51). The daily frequency and intention of naps are essential to determine the impact of the nap on cognitive function, but were assessed in only one of the reviewed studies, which found that unintentional nappers had poorer memory performance than intentional nappers, and that long naps were associated with worse immediate recall performance only in intentional nappers, not in unintentional nappers (21). The moderating effect of the intention to nap on the association between nap duration and memory requires further testing in future studies. Also, most of the observational studies focused on nap duration during the day or afternoon only, and all intervention studies provided napping opportunities in the afternoon. Thus, it is unclear from current evidence whether the impact of napping on cognition is moderated by circadian rhythms.
Issues with Napping Assessment
Napping can be measured subjectively via self-report or sleep diary or objectively via actigraphy or PSG/EEG. There is no consensus on which measurement strategy is most valid or a gold standard measure for daytime sleep. Each of these measures has benefits and drawbacks for measuring daytime sleep.
Self-reported nap questions can introduce recall bias, especially for those studies that include older adults with cognitive impairment. However, self-report questions are the most practical and feasible for measuring napping in large-scale or population-based studies. Most observational studies in the present review assessed nap using only self-reports. One main issue with self-reported nap is the lack of clarity and consistency of definitions of habitual napping in current literature, which is a threat to the reliability and validity of the self-reported napping. There was no clear definition of habitual nap provided in these studies. For example, participants in most of these studies were asked about how long they took a nap “in general” or “regularly” or “usually.” There were no detailed explanations of these terms to guide the respondents to answer these questions, and they could respond based on their interpretation of “in general,” “regularly,” or “usually.” In addition, the definition of “napper” or “habitual napper” varied among studies using weekly napping frequency, as some defined “habitual napper” as those who reported napping for 3 or more days a week (26) and others considered those who napped at least one day a week as “nappers” (21,22).
Actigraphy is an objective measure of sleep that has been commonly used to measure sleep in older adults. It provides continuous 24-hour sleep/wake assessment via rest and activity over a prespecified duration of time. One issue with actigraphy is that it measures sleep by monitoring body movement and may score inactivity while awake as sleep and, therefore, overestimate sleep time. Actigraphy may be more problematic as a daytime sleep measure in older adults, a population with a high prevalence of daytime inactivity and sedentary behaviors (1,5). In our review, 5 observational studies only used actigraphy to measure daytime napping and might have overestimated napping duration. Using detailed self-report daytime sleep information (eg, sleep diary) to guide the actigraphy nap scoring may provide a more concise napping assessment. For example, the daytime sleep diary can be used during actigraphy nap scoring to determine whether a period the actigraph detected inactivity is an actual nap or just a period of low activity. However, a detailed and validated protocol for this scoring method needs to be developed and tested in future napping research.
PSG or EEG is used as the gold standard for sleep assessment to quantify sleep time, duration, and stages (52). They are commonly used for short-term sleep monitoring, such as assessing sleep overnight or during a defined period. It is uncommon to use PSG/EEG for continuous sleep monitoring for several days due to the complexity of the procedure, user discomfort, and high cost. All intervention studies in our review used EEG/PSG to monitor nap duration during the assigned nap session in the laboratory environment. However, in-lab PSG is not in a natural sleeping environment and may not be a practical measure for assessing habitual or routine napping in the natural environment.
Consumer-grade wearable devices have been increasingly used over the last few years to track physical activity, fitness, and other health information and facilitate health behaviors. Some of these devices also provide sleep monitoring. These devices and associated technologies may offer opportunities for older adults to self-monitor daytime napping, detect unhealthy napping behavior in real time, and receive just-in-time interventions, including napping/sedentary behavior notifications or reminders of scheduled naps or physical activity. Although it is not yet clear whether these wearable interventions would improve cognitive health, this is an intriguing possibility. Moreover, investigator access to data from consumer-grade wearables could provide valuable opportunities to study population-based napping in large samples, once the algorithms for nap/sleep detections of these devices are validated.
COVID-19 Pandemic and Its Potential Impacts on Napping Behaviors in Older Adults
The COVID-19 pandemic and its related circumstances (social distancing, isolation, home confinement, loneliness, etc.) have led to significant lifestyle changes in the older adult population. Recent studies report pandemic-related decreases in physical and social activity, increase in sedentary behaviors, and change in sleep pattern (eg, poor sleep quality, reduced or extended nocturnal sleep duration, and increased insomnia symptoms, etc.) in older adults (53–57). Although we did not find any studies of pandemic-related daytime napping behavior change in older adults, the pandemic and its related lifestyle changes may have altered individuals’ napping habits. For example, pandemic-related reductions in physical activity or worsening of nocturnal sleep may have led to an increase in the number of naps or nap duration. These potential changes in napping behavior may further impact nocturnal sleep and cognitive health in older adults during and after the pandemic. Future studies are needed investigating these possibilities.
Implications for Future Research
This systematic review provides several implications for future studies. First, future observational studies should use a self-reported napping measure in conjunction with actigraphy to ensure a more thorough measure of daytime napping, where the self-reported daily napping helps score actigraphy and provides details of napping habits. In addition, the timing, frequency, and intentionality/planning of naps should be incorporated into self-report napping measures to enable a more comprehensive analysis of the effects of napping on cognitive health. Also, research studies are needed to investigate what causes different patterns of daytime napping in older adults, which could help identify intervention targets to alter those patterns. Further, future observational and interventional research should examine the intersection of various napping features and cognition (eg, possible moderating roles of circadian rhythms and intentionality), and whether chronic disease burden moderates the association between napping and cognitive health in older adults. Finally, further rigorous intervention research is needed to assess the short-term effect of a single nap and the long-term impact of nap habits on cognitive health in older populations.
Limitations and Strengths of the Systematic Review
This systematic review presents an in-depth review of the current literature on napping and its relationship with cognition with some limitations. As discussed above, nap features other than duration are largely missing in the current literature; there is no consensus on what is a valid, reliable, and practical approach to measure napping in older adults. Various cutoff for nap durations were used in studies which made the comparisons of findings among studies difficult and less precise. The interpretation of findings from intervention studies in our review is limited by the small sample size (eg, n < 30) and napping in an unnatural environment (ie, sleep lab) in most of the studies. In addition, it is unclear whether a nap opportunity at a different time of day or at a natural environment would differentially affect cognitive performance, and if this would vary by participant chronotype. Given these limitations in the literature, we were unable to conduct a meta-analysis and synthesizing the literature was challenging.
Our review does, however, have many strengths. To our knowledge, this is the first and only review that comprehensively examined the current literature on the association between daytime napping and cognitive function, and the effect of nap interventions on cognitive performance in older adults. We minimized our inclusion criteria to maximize study inclusions and to best understand the full breadth of the extant literature. Further, we critically appraised and synthesized both observational and intervention studies from various countries.
Conclusion
The results of this systematic review suggest napping for a short or moderate duration might be beneficial for older adults’ cognitive function compared with both non-napping and long napping. A single afternoon nap might benefit specific aspects of cognition, such as psychomotor function and working memory. The limitations of the current literature suggest critical needs and directions for future research. First, additional research is necessary to understand what causes different patterns of daytime napping in older adults and elucidate the associations between frequency, timing, and intention of napping on cognitive outcomes. Second, sleep diary and actigraph need to be used together to assess daytime napping in older adults. Third, more rigorously designed intervention studies are needed to assess the effects of napping habits on cognitive health. Finally, future observational and interventional research needs to examine the intersection of specific napping characteristics with cognition and determine whether disease burden moderates the association between napping and cognitive health in older adults.
Supplementary Material
Contributor Information
Junxin Li, Johns Hopkins School of Nursing, Baltimore, Maryland, USA.
Miranda V McPhillips, University of Pennsylvania School of Nursing, Philadelphia, Pennsylvania, USA.
Zhongyue Deng, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Fangfang Fan, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA.
Adam Spira, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA; Department of Psychiatry and Behavioral Sciences, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Johns Hopkins Center on Aging and Health, Baltimore, Maryland, USA.
Funding
This work was supported by the National Institutes of Nursing Research (R00NR016484; K23NR018487) and the National Institute on Aging (1R01AG050507).
Conflict of Interest
A.S. received payment for serving as a consultant for Merck and received honoraria from Springer Nature Switzerland AG for guest editing special issues of Current Sleep Medicine Reports. The other authors declare no conflict of interest.
Author Contributions
All authors (J.L., M.V.P., Z.D., F.F., and A.S.) made substantial conceptual contributions and helped with data acquisition, synthesis, and manuscript writing. All authors provided final approval for the version of manuscript submitted.
References
- 1. Zhang Z, Xiao X, Ma W, Li J. Napping in older adults: a review of current literature. Curr Sleep Med Rep. 2020;6(3):129–135. doi: 10.1007/s40675-020-00183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou J, Kessler AS, Su D. Association between daytime napping and chronic diseases in China. Am J Health Behav. 2016;40(2):182–193. doi: 10.5993/AJHB.40.2.3 [DOI] [PubMed] [Google Scholar]
- 3. Li J, Chang YP, Riegel B, et al. Intermediate, but not extended, afternoon naps may preserve cognition in Chinese older adults. J Gerontol A Biol Sci Med Sci. 2018;73(3):360–366. doi: 10.1093/gerona/glx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xin C, Zhang B, Fang S, Zhou J. Daytime napping and successful aging among older adults in China: a cross-sectional study. BMC Geriatr. 2020;20(1):1–12. doi: 10.1186/s12877-019-1408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Vitiello MV, Gooneratne NS. Sleep in normal aging. Sleep Med Clin. 2018;13(1):1–11. doi: 10.1016/j.jsmc.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Gooneratne NS. Sleep and health in older adults. In: Grandner MA, ed. Sleep and Health. 1st ed. Cambridge, Massachusetts: Academic Press; 2019:31. doi: 10.1016/B978-0-12-815373-4.00004-6 [DOI] [Google Scholar]
- 7. Aromataris EMZE, Munn Z. JBI manual for evidence synthesis. JBI. 2020: Adelaide, Australia. 10.46658/JBIMES-20-01 [DOI] [Google Scholar]
- 8. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamaki M, Shirota A, Tanaka H, Hayashi M, Hori T. Effects of a daytime nap in the aged. Psychiatry Clin Neurosci. 1999;53(2):273–275. doi: 10.1046/j.1440-1819.1999.00548.x [DOI] [PubMed] [Google Scholar]
- 10. Blackwell T, Yaffe K, Ancoli-Israel S, et al. ; Study of Osteoporotic Fractures Group. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405 [DOI] [PubMed] [Google Scholar]
- 11. Leng Y, Redline S, Stone KL, Ancoli-Israel S, Yaffe K. Objective napping, cognitive decline, and risk of cognitive impairment in older men. Alzheimers Dement. 2019;15(8):1039–1047. doi: 10.1016/j.jalz.2019.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhn D, Edelman P, Fulton BR. Daytime sleep and the threat to well-being of persons with dementia. Dementia (14713012). 2005;4(2):233–247. doi: 10.1177/1471301205051094 [DOI] [Google Scholar]
- 13. Cai H, Su N, Li W, Li X, Xiao S, Sun L. Relationship between afternoon napping and cognitive function in the ageing Chinese population. General Psychiatr. 2021;34(1):e100361. doi: 10.1136/gpsych-2020-100361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qian YX, Ma QH, Sun HP, Xu Y, Pan CW. Combined effect of three common lifestyle factors on cognitive impairment among older Chinese adults: a community‐based, cross‐sectional survey. Psychogeriatr. 2020;20(6):844–849. doi: 10.1111/psyg.12604 [DOI] [PubMed] [Google Scholar]
- 15. Sattari N, Whitehurst LN, Ahmadi M, Mednick SC. Does working memory improvement benefit from sleep in older adults? Neurobiol Sleep Circadian Rhythms. 2019;6:53–61. doi: 10.1016/j.nbscr.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamaki MS, Hayashi M, Hori T. Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online. 2000;3(3):131–139. [PubMed] [Google Scholar]
- 17. Li J, Cacchione PZ, Hodgson N, et al. Afternoon napping and cognition in Chinese older adults: findings from the China Health and Retirement Longitudinal Study baseline assessment. J Am Geriatr Soc. 2017;65(2):373–380. doi: 10.1111/jgs.14368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sha T, Cheng W, Yan Y. Prospective association between sleep-related factors and the trajectories of cognitive performance in the elderly Chinese population across a 5-year period cohort study. PLoS One. 2019;14(9):e0222192. doi: 10.1371/journal.pone.0222192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li C, Yan Y. Afternoon napping durations in Chinese population over 60 years old: longitudinal associations with cognitive performance. Front Public Health. 2022;10:911498. doi: 10.3389/fpubh.2022.911498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fang SC, Huang CJ, Wu YL, Wu PY, Tsai PS. Effects of napping on cognitive function modulation in elderly adults with a morning chronotype: a nationwide survey. J Sleep Res. 2019;28(5):e12724. doi: 10.1111/jsr.12724 [DOI] [PubMed] [Google Scholar]
- 21. Owusu JT, Wennberg AM, Holingue CB, Tzuang M, Abeson KD, Spira AP. Napping characteristics and cognitive performance in older adults. Int J Geriatr Psychiatry. 2019;34(1):87–96. doi: 10.1002/gps.4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagherian K, Rose K. Long work hours, prolonged daytime naps, and decreased cognitive performance in older adults. Chronobiol Int. 2020;37(9-10):1304–1311. doi: 10.1080/07420528.2020.1811296 [DOI] [PubMed] [Google Scholar]
- 23. Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi: 10.1016/j.sleep.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 24. Cox SR, Ritchie SJ, Allerhand M, et al. Sleep and cognitive aging in the eighth decade of life. Sleep. 2019;42(4):zsz019. doi: 10.1093/sleep/zsz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Basta M, Koutentaki E, Vgontzas A, et al. Objective daytime napping is associated with disease severity and inflammation in patients with mild to moderate dementia. J Alzheimers Dis. 2020;74(3):803–815. doi: 10.3233/jad-190483 [DOI] [PubMed] [Google Scholar]
- 26. Asada T, Motonaga T, Yamagata Z, Uno M, Takahashi K. Associations between retrospectively recalled napping behavior and later development of Alzheimer’s disease: association with APOE genotypes. Sleep. 2000;23(5):629–634. doi: 10.1093/SLEEP/23.5.1E [DOI] [PubMed] [Google Scholar]
- 27. Lin J-F, Li F-D, Chen X-G, et al. Association of postlunch napping duration and night-time sleep duration with cognitive impairment in Chinese elderly: a cross-sectional study. BMJ Open. 2018;8(12):e023188e023188. doi: 10.1136/bmjopen-2018-023188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cross N, Terpening Z, Rogers NL, et al. Napping in older people “at risk” of dementia: relationships with depression, cognition, medical burden and sleep quality. J Sleep Res. 2015;24(5):494–502. doi: 10.1111/jsr.12313 [DOI] [PubMed] [Google Scholar]
- 29. Dzierzewski JM, Fung CH, Jouldjian S, Alessi CA, Irwin MR, Martin JL. Decrease in daytime sleeping is associated with improvement in cognition after hospital discharge in older adults. J Am Geriatr Soc. 2014;62(1):47–53. doi: 10.1111/jgs.12622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitamura K, Watanabe Y, Nakamura K, et al. Short daytime napping reduces the risk of cognitive decline in community-dwelling older adults: a 5-year longitudinal study. BMC Geriatr. 2021;21(1):474. doi: 10.1186/s12877-021-02418-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alqurashi YD, AlHarkan K, Aldhawyan A, et al. Association between nap duration and cognitive functions among saudi older adults. Front Neurosci. 2022;16:917987. doi: 10.3389/fnins.2022.917987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Auyeung TW, Lee JS, Leung J, et al. Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily napping, and prolonged sleep duration--a cross-sectional study in 2,947 community-dwelling older adults. Age (Dordrecht, Netherlands). 2013;35(2):479–486. doi: 10.1007/s11357-011-9366-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campbell SS, Stanchina MD, Schlang JR, Murphy PJ. Effects of a month-long napping regimen in older individuals. J Am Geriatr Soc. 2011;59(2):224–232. doi: 10.1111/j.1532-5415.2010.03264.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001;24(6):680–687. doi: 10.1093/sleep/24.6.680 [DOI] [PubMed] [Google Scholar]
- 35. Baran B, Mantua J, Spencer RM. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016;28(6):792–802. doi: 10.1162/jocn_a_00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Korman M, Dagan Y, Karni A. Nap it or leave it in the elderly: a nap after practice relaxes age-related limitations in procedural memory consolidation. Neurosci Lett. 2015;606:173–176. doi: 10.1016/j.neulet.2015.08.051 [DOI] [PubMed] [Google Scholar]
- 37. King BR, Saucier P, Albouy G, et al. Cerebral activation during initial motor learning forecasts subsequent sleep-facilitated memory consolidation in older adults. Cereb Cortex. 2017;27(2):1588–1601. doi: 10.1093/cercor/bhv347 [DOI] [PubMed] [Google Scholar]
- 38. Backhaus W, Braass H, Renne T, Gerloff C, Hummel FC. Motor performance is not enhanced by daytime naps in older adults. Front Aging Neurosci. 2016;8:125. doi: 10.3389/fnagi.2016.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005;53(1):48–53. doi: 10.1111/j.1532-5415.2005.53009.x [DOI] [PubMed] [Google Scholar]
- 40. Milner CE, Cote KA. A dose-response investigation of the benefits of napping in healthy young, middle-aged and older adults. Sleep Biol Rhythms. 2008;6(1):2–15. doi: 10.1111/j.1479-8425.2007.00328.x [DOI] [Google Scholar]
- 41. Scullin MK, Fairley J, Decker MJ, Bliwise DL. The effects of an afternoon nap on episodic memory in young and older adults. Sleep. 2017;40(5):zsx035. doi: 10.1093/sleep/zsx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Batterink LJ, Westerberg CE, Paller KA. Vocabulary learning benefits from REM after slow-wave sleep. Neurobiol Learn Mem. 2017;144:102–113. doi: 10.1016/j.nlm.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28(1):105–114. doi: 10.1037/a0028830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behav Sleep Med. 2008;6(1):32–54. doi: 10.1080/15402000701796080 [DOI] [PubMed] [Google Scholar]
- 45. Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- 46. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- 47. Dzierzewski JM, Dautovich N, Ravyts S. Sleep and cognition in older adults. Sleep Med Clin 2018;13(1):93–106. doi: 10.1016/j.jsmc.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Teras T, Rovio S, Spira AP, et al. Associations of accelerometer-based sleep duration and self-reported sleep difficulties with cognitive function in late mid-life: the Finnish Retirement and Aging Study. Sleep Med. 2019;68:42–49. doi: 10.1016/j.sleep.2019.08.024 [DOI] [PubMed] [Google Scholar]
- 49. Alsubaie SF, Alkathiry AA, Abdelbasset WK, Nambi G. The physical activity type most related to cognitive function and quality of life. Biomed Res Int. 2020;2020:8856284. doi: 10.1155/2020/8856284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okamoto S, Kobayashi E. Social isolation and cognitive functioning: a quasi-experimental approach. J Gerontol B Psychol Sci Soc Sci. 2021;76(7):1441–1451. doi: 10.1093/geronb/gbaa226 [DOI] [PubMed] [Google Scholar]
- 51. Owusu JT, Ramsey CM, Tzuang M, Kaufmann CN, Parisi JM, Spira AP. Napping characteristics and restricted participation in valued activities among older adults. J Gerontol A Biol Sci Med Sci. 2018;73(3):367–373. doi: 10.1093/gerona/glx166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grote L, Zou D. Chapter 167 - Pulse wave analysis during sleep. In: Kryger M, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine (Sixth Edition). Elsevier; 2017:1624–1632.e1624.
- 53. Browne RA, Macêdo GA, Cabral LL, et al. Initial impact of the COVID-19 pandemic on physical activity and sedentary behavior in hypertensive older adults: an accelerometer-based analysis. Exp Gerontol. 2020;142:111121. doi: 10.1016/j.exger.2020.111121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. 2021;7(1):e000960. doi: 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomaz SA, Coffee P, Ryde GC, et al. Loneliness, wellbeing, and social activity in scottish older adults resulting from social distancing during the COVID-19 pandemic. Int J Environ Res Public Health. 2021;18(9):4517. doi: 10.3390/ijerph18094517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pires GN, Ishikura IA, Xavier SD, et al. Sleep in older adults and its possible relations with COVID-19. Front Aging Neurosci. 2021:235. doi: 10.3389/fnagi.2021.647875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lebrasseur A, Fortin-Bédard N, Lettre J, et al. Impact of the COVID-19 pandemic on older adults: rapid review. JMIR aging. 2021;4(2):e26474. doi: 10.2196/26474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.