Abstract
Background
Social isolation is prevalent and associated with dementia, yet the directionality and mechanisms are less understood. This study examined the association between social isolation and cognitive functioning and explored the mediating role of sleep disturbance on the social isolation–cognition relationship.
Methods
Data from 5 753 dementia-free Americans aged ≥50 of 2006 (T1), 2010 (T2), and 2014 (T3) waves of the Health and Retirement Study. Social isolation was measured by the Steptoe Social Isolation Index. Cognitive functioning was measured by the Telephone Interview of Cognitive Status. Sleep disturbance was measured with the modified Jenkins Sleep Scale. We used cross-lagged panel models to determine the associations between social isolation, sleep disturbance, and cognitive functioning.
Results
Social isolation is significantly associated with subsequent cognitive functioning (T1 to T2: β = −0.055, standard error [SE] = 0.014, p < .001; T2 to T3: β = −0.044, SE = 0.016, p < .001). Lower cognitive functioning is significantly associated with greater subsequent social isolation (T1 to T2: β = −0.101, SE = 0.020, p < .001; T2 to T3: β = −0.058, SE = .011, p < .001). Sleep disturbance at T2 partially mediated the effect of social isolation (T1) on cognitive functioning (T3), accounting for 6.2% of the total effect (β = −0.003, SE = 0.001, p < .01).
Conclusions
Social isolation may deteriorate cognitive functioning and vice versa. The association between social isolation and cognition is partially explained by sleep disturbance.
Keywords: Cognition, Cognitive aging, Psychosocial, Sleep, Social relationship
Dementia is a neurodegenerative disease characterized by a progressive decline in cognitive functioning that severely affects daily life activities and social functioning. In 2021, over 6.2 million older adults in the United States were living with Alzheimer’s disease, and the total cost for all individuals with Alzheimer’s disease and related dementias (ADRD) is $355 billion (1). As the world population aged 65 years and older continues to grow in size, so will the number and percentage of individuals with ADRD. It is estimated that a total of 153 million people will be living with ADRD by 2050 (2). However, current dementia-modifying medications have only limited efficacy (3). Risk factors and pathways for diminished cognitive functioning need to be identified so that interventions to prevent and/or manage dementia can be further developed.
Being socially connected is a fundamental drive and core human need (4). Social isolation is defined as having few social relationships or infrequent social contact with others, while loneliness is identified as the mismatch between actual and desired social relationships. Social isolation and loneliness are distinct constructs that differentially relate to health outcomes such as psychological well-being, physical and cognitive decline, dementia, and mortality (5–13). Individuals with frequent social contact with others may feel lonely; conversely, socially isolated individuals can be satisfied with their social relationships. Several studies have shown that social isolation increases the risks for cognitive decline, cognitive impairment, and ADRD (9,10,14,15). It is estimated that 24% of older adults (estimated 11.0 million) in the United States are socially isolated (16). The Lancet Commission on dementia prevention, intervention, and care estimated that social isolation is associated with a 4% reduction in dementia prevalence if addressed in later life (17).
The stress-buffering theory (18) has been proposed to explain the association between social isolation and diminished cognitive functioning. This theory posits that social relationships are beneficial in mitigating the negative impacts of stressful situations (18). Stress is well-recognized to be associated with cognitive decline due to neuronal structural changes in the hippocampus (19). Social relationships may prevent or modify responses to stressful life events that are damaging to cognitive health. Other than the psychological distress mechanism, it is also speculated that social isolation affects cognitive functioning through health-related behavioral changes and physiological dysregulation. For example, social isolation may promote unhealthy lifestyles such as smoking and physical inactivity (20), as well as upregulate neuroinflammatory responses and increase oxidative stress (21). Although these mechanisms/pathways have been proposed, few empirical studies have tested these hypotheses. The mechanism by which social isolation impacts cognitive functioning is still not fully understood (21).
Social isolation could also be a consequence of diminished cognitive functioning. For example, individuals with cognitive impairment may have difficulties with their engagement in social and leisure activities and communication with family, relatives, and friends (22). Negative feelings brought by dementia or cognitive impairment, such as shame and embarrassment, will lead to social withdrawal (23). In addition, synapse loss and neurodegeneration in the entorhinal cortex, a part of the affiliation network that is disrupted by Alzheimer’s disease, could lead to social and emotional withdrawal from other individuals, aggravating social isolation (24). However, existing studies usually focus on unidirectional associations between social isolation and cognitive functioning. There is a need to disentangle the temporal nature of the isolation–cognition relationship.
Poor sleep quality is one of the most common symptoms in older adults. It is estimated that insomnia symptoms increase with advancing age, with prevalence rates of approximately 50% in older adults (25). Social isolation is identified as a risk factor for poor sleep quality (26,27). For example, using nationally representative data from National Social Life, Health and Aging Project, Benson and colleagues (26) found that social isolation was associated with actigraphy measures of disrupted sleep. Additionally, a 6-year prospective study conducted in Taiwan found a significant association between social isolation and sleep disturbance (27). Sufficient, high-quality sleep is essential for brain health. According to the restorative theory, sleep enables the body to replenish and repair cellular components needed for psychological functions that get exhausted during an awake day (28). Sleep disturbance might affect cognitive function through several pathways, including growing amyloid-β (a trigger and marker of the progression of Alzheimer’s disease), neuroinflammation, and modifications in specific neurotransmitter systems (29,30). Additionally, individuals with clinically diagnosed insomnia and long-term hypnotics use have been twice as likely to develop dementia than those without insomnia (31). These lines of evidence suggest that sleep disturbance may be a risk factor for cognitive impairment (30) and mediate the isolation–cognition relationship.
Given the crucial role that social connection plays in overall psychosocial adjustment across the life span, it is imperative to examine the pathways by which social isolation relates to diminished cognitive functioning. However, little is known about the complex associations between sleep, social isolation, and cognitive functioning. The conceptual model outlined by Hawkley and Cacioppo (4) suggests that through maladaptive hypervigilance, the poor social relationship may influence cognitive functioning by affecting the physiological repair and maintenance process (eg, sleep). As such, sleep disturbance may constitute a possible pathway through which social isolation impacts cognitive functioning. Nevertheless, the nature and directionality of the associations between social isolation, sleep, and cognitive functioning have not been clarified because most relevant research has been based on cross-sectional data or one-time assessments of social isolation (32), and very few studies have explored their reciprocal relationship and mechanisms underlying such relationships.
We aim to address these gaps in the literature by examining the directionality of the longitudinal association between social isolation and cognitive functioning as well as the potential mediating effect of sleep disturbance among adults aged 50 years and older in the United States. We hypothesize that (i) there is a bidirectional negative association between social isolation and cognitive functioning and (ii) sleep disturbance mediates the association between social isolation and cognitive functioning.
Method
Data Set and Working Sample
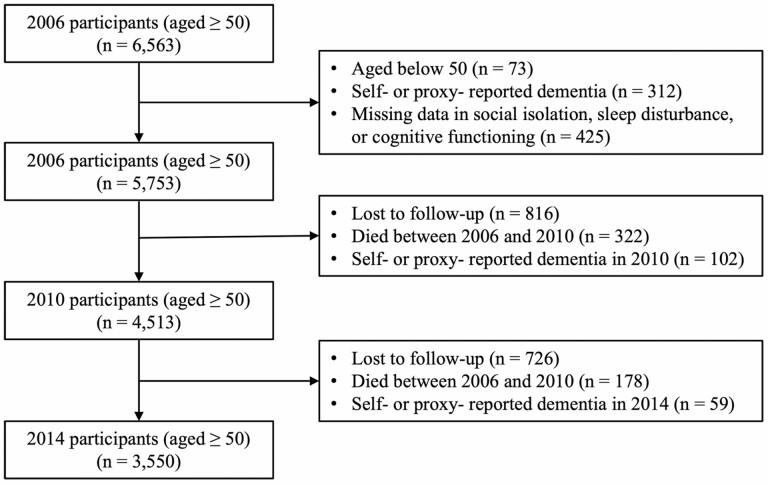
Data came from the 2006 (T1), 2010 (T2), and 2014 (T3) waves of the Health and Retirement Study (HRS), a nationally representative biennial panel study of noninstitutionalized adults aged 50 years and older. The HRS collects information on health characteristics, well-being, family, employment, and assets. The study oversampled Black and Hispanics and provided sampling weights to account for the differential probabilities of selection and nonresponse in the study (33). More details about the study design, sampling, and measures of the HRS are available elsewhere (34). Since 2006, the psychosocial and lifestyle questionnaire (PLQ) has been administered to a random half of the core panel participants. This results in 50% of the sample completing the survey on a rotating basis every 4 years. The PLQ is a self-administered questionnaire that includes questions about lifestyle, psychological well-being (eg, stress, loneliness), and social relationships. We merged the 2006 (T1), 2010 (T2), and 2014 (T3) waves of PLQ modules with the RAND HRS longitudinal data file and generated the working data set. Given the longitudinal nature of the analysis, we excluded the subsample recruited at T2/T3 from the working data set for these analyses. Participants with self- or proxy-reported having diagnosed dementia in any of the 3 waves were excluded from the analysis. The analytic sample for this study included 5 753 participants at T1, 4 615 participants at T2 (19.8% attrition rate), and 3 711 participants at T3 (19.6% attrition rate). The sample selection flowchart is shown in Figure 1. The HRS has received approval from the University of Michigan Institutional Review Board, and all participants provided informed consent.
Figure 1.
Flowchart of study sample inclusion and follow-up.
Measures
Social isolation
There is no golden standard for measuring social isolation (5). However, the consensus in the field is that scales should include multiple items and measure interpersonal relationships with individuals, groups, and communities (5,35). We used a 5-item Steptoe Social Isolation Index meeting these criteria. This index was first validated in the English Longitudinal Study of Aging (6) and has been adapted to the HRS (36). We assigned the social isolation score to each participant based on whether they (i) were unmarried/living alone; (ii) had less than monthly contact with children (all contact including face-to-face, by telephone, or writing/e-mail); (iii) had less than monthly contact with other family members; (iv) had less than monthly contact with friends; and (v) did not participate monthly in any groups, clubs, or other social organizations. For items 2, 3, and 4, participants with no children, family members, or friends were coded “1” (positive response) for less social contact. This index yielded scores from 0 to 5, with a higher score indicating a higher level of social isolation.
Sleep disturbance
Sleep disturbance was measured using a modified version of the Jenkins Sleep Scale (37) and included 4 items that assessed participants’ frequency of reporting: (i) difficulty falling asleep; (ii) difficulty staying asleep; (iii) waking up too early during the night; and (iv) feeling rested when waking up in the morning. Responses include “1 = rarely or never,” “ 2 = sometimes,” and “3 = most of the time.” We reverse-coded the last item and summed the score of the 4 items (38). The total score ranged from 3 to 12, with a higher score indicating a higher level of sleep disturbance. The overall measure of sleep disturbance was internally consistent at all 3 waves of the survey (Cronbach’s α = 0.75 [T1], 0.72 [T2], and 0.75 [T3], respectively).
Cognitive functioning
HRS assessed cognitive functioning via the modified version of the Telephone Interview for Cognitive Status (TICS). TICS is a 27-item global cognition scale, which includes immediate and delayed recall of 10 words from a word list randomly assigned for each participant (0–20 points), backward counting (0–2 points), and serial-7 subtraction (0–5 points) (39). The scores ranged from 0 to 27, with a higher score indicating better cognitive functioning in episodic memory, attention, and working memory domains (39).
Covariates
We selected several covariates based on their associations with social relationships and cognitive decline (9,10,15). The covariates included age (years), sex (0 = men, 1 = women), race/ethnicity (0 = non-Hispanic White, 1 = non-Hispanic Black, 2 = Hispanics, and 3 = others), education levels (0 = less than college education, 1 = some college or associate degree, 2 = college degree or above), annual household income (0 = ≤$49,999, 1 = $50,000–$99,999, 2 = $100,000–$199,999, 3 = ≥$200,000), smoking (0 = no, 1 = former, and 2 = current), loneliness, depressive symptoms, and the number of comorbidities. Loneliness was assessed with a modified 3-item version of the UCLA Loneliness Scale (40). Specifically, participants were asked, “How much of the time do you feel (i) lack companionship, (ii) left out, and (iii) isolated from others? Participants rated each item on a scale of “1 = often,” “2 = some of the time,” and “3 = hardly ever or never.” Items were reversely coded, with a higher value indicating a higher level of loneliness (total score ranged from 3 to 9) (40). The Cronbach’s α was 0.88 at T1, 0.86 at T2, and 0.90 at T3, respectively. Depressive symptoms were measured by the Center for Epidemiologic Studies Depression scale (CESD-8). We removed the items on unrested sleep and feeling lonely to avoid potential overlap. The depressive symptoms measurement in this study was based on how frequently in the past week the respondent felt: (i) depressed, (ii) everything was an effort, (iii) happy, (iv) life was enjoyable, (v) sad, and (vi) unable to get going. We reverse-coded the items on “happy” and “life was enjoyable”; a higher score indicates a higher level of depressive symptoms. Comorbidities were measured using the sum score of 7 self-reported physician-diagnosed chronic conditions: type 2 diabetes, stroke, hypertension, heart disease, chronic lung disease, arthritis, and cancer.
Statistical Analysis
We summarized the characteristics of our participants across T1, T2, and T3. We used frequencies/proportions for categorical variables and means ± standard deviations for normally distributed variables. Pearson correlation analysis examined the collinearity between social isolation, sleep disturbance, and cognitive functioning in 3 waves. Estimates were weighted to adjust for differential probabilities of selection and nonresponse of HRS participants.
Structural equation modeling (SEM) was used to test the study’s hypotheses. The cross-lagged panel model (CLPM), a form of SEM, is widely used for describing reciprocal relationships, or directional influences, between variables over time. Researchers use CLPM to examine how well different variables associate future iterations of each other, helping to make more substantial causal claims by establishing temporal precedence (41). A CLPM approach was used to examine the interrelationship between social isolation and cognitive functioning at T1, T2, and T3. In CLPM, change in each variable over time is modeled using the stability coefficients between time-adjacent measures of each variable (eg, T1 social isolation is associated with T2 social isolation, and T2 social isolation, in turn, is associated with T3 social isolation), and the cross-lagged associations between social isolation, sleep disturbance, and cognitive functioning are captured by the cross-lagged effects between two variables (eg, T1 social isolation is associated with T2 sleep disturbance, and T2 sleep disturbance is associated with T3 cognitive functioning).
The first set of unadjusted models examined linkages of social isolation, sleep disturbance, and cognitive functioning without controlling for covariates. Fully-adjusted models tested those same associations by adding the complete set of covariates described above. In the fully-adjusted model, demographics that include age, sex, race/ethnicity, and education were treated as time-invariant variables, whereas all other covariates were time-varying variables. Although we calculated the chi-square (χ 2) statistic, we did not rely on it to assess model fit because it is sensitive to sample size (42). Instead, we used standard fit indices, including the root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker-Lewis Index (TLI) (43). An RMSEA of <0.06, CFI of >0.90, and TLI of >0.90 indicated a good model fit (43).
Full information maximum likelihood (FIML) estimation was used to handle missing data in the models. FIML is a single-step maximum likelihood approach for missing data that has been widely used in SEM (44). For the attrition analysis, inverse-probability weighting was used (45), with analysis limited to those participating in all waves. Total attrition over the 2006–2014 period amounted to 38.29%. Multivariable logistic regression was first used to fit predictive models for any attrition. Covariates included participants’ socio-demographic and health characteristics: age, sex, race/ethnicity, education, income, smoking, loneliness, depressive symptoms, and the number of comorbidities. Based on predicted probabilities from these analyses, stabilized inverse-probability-of-attrition weights (IPAWs) were created (45). Models were then weighted by the cumulative product of the IPAWs using the sampling weight from the HRS 2006 psychosocial leave-behind questionnaire. Standard errors were adjusted for sample stratification (sampling strata) and clustering (individuals within primary sampling units). Descriptive analysis and the construction of weights were conducted with the Stata MP version 17.0 (StataCorp LLC, College Station, TX), and Mplus version 8.0 was used for all other analyses.
Results
Descriptive Statistics
Table 1 contains sample characteristics for all 3 waves. The mean age of the participants was 65.1 (standard deviation [SD] = 9.5) at T1. More than half of the participants were women, and 51.3% had at least a college education. About 81.4% self-identified as non-Hispanic White, 10.3% as non-Hispanic Black, 6.7% as Hispanic, and 1.6% as other. On a scale ranging from 0 to 5, the mean social isolation score was 1.9 (SD = 1.0) at T1. The score did not significantly change in the following 3 waves (F = 2.324, p = .511). The average sleep disturbance score was 6.5 (range 3–12) at baseline, indicating a moderate level of sleep disturbance (46). A slight increase in loneliness levels was observed in the subsequent waves (T2 mean score = 6.6, T3 mean score = 6.7). The mean cognitive functioning score at T1 was 16.4 and declined slightly at T2 (mean = 15.9) and T3 (mean = 15.7) but indicated normal cognitive functioning (39). Multicollinearity in linear models was checked using variance inflation factor (VIF) (VIF > 4 indicates multicollinearity) (47). The VIFs of all variables ranged from 1.01 to 2.12, indicating no multicollinearity issue was identified. The results in Table 2 indicate that social isolation was correlated with higher sleep disturbance and poorer cognitive functioning at all time points.
Table 1.
Sample Characteristics of Participants from Health and Retirement Study (HRS, n = 5 753)
| Time | |||
|---|---|---|---|
| Variables | 2006 (n = 5 753) | 2010 (n = 4 513) | 2014 (n = 3 550) |
| Age (in years), M ± SD | 65.1 ± 9.5 | 63.7 ± 8.4 | 62.7 ± 7.8 |
| Sex (women), % | 57.8 | 57.6 | 59.5 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 81.4 | 84.6 | 83.7 |
| Non-Hispanic Black | 10.3 | 8.6 | 8.8 |
| Hispanic | 6.7 | 5.2 | 5.9 |
| Non-Hispanic others | 1.6 | 1.6 | 1.6 |
| Education (school years), % | |||
| Less than college education | 13.9 | 10.6 | 10.0 |
| Some college or associate degree | 34.8 | 35.0 | 33.6 |
| College degree or above | 51.3 | 54.4 | 56.4 |
| Annual household income ($), % | |||
| ≤49,999 | 49.7 | 50.4 | 48.0 |
| 50,000–99,999 | 27.7 | 28.4 | 30.1 |
| 100,000–200,000 | 16.6 | 15.3 | 15.6 |
| >200,000 | 6.0 | 5.9 | 6.3 |
| Smoking, % | |||
| No | 43.8 | 44.4 | 45.9 |
| Former | 43.2 | 45.0 | 44.8 |
| Current | 14.0 | 10.6 | 9.3 |
| Loneliness*, M ± SD | 4.4 ± 1.6 | 4.3 ± 1.5 | 4.3 ± 1.5 |
| CESD*,†, M ± SD | 0.9 ± 1.4 | 0.8 ± 1.3 | 0.8 ± 1.3 |
| Number of chronic conditions, M ± SD | 2.1 ± 0.5 | 1.9 ± 0.6 | 2.0 ± 0.6 |
| Social isolation*, M ± SD | 1.9 ± 1.0 | 1.9 ± 1.0 | 1.9 ± 1.1 |
| Sleep disturbance*, M ± SD | 6.5 ± 2.1 | 6.6 ± 2.0 | 6.7 ± 2.1 |
| Cognitive functioning‡, M ± SD | 16.4 ± 3.8 | 15.9 ± 4.0 | 15.7 ± 4.1 |
Notes: CESD = Center for Epidemiological Studies Depression scale; M ± SD = mean ± standard deviation. Estimates were weighted to adjust for differential probabilities of selection and nonresponse of HRS participants.
*Higher scores indicate higher levels of loneliness, depressive symptoms, social isolation, and sleep disturbance.
†Based on the CESD-8 scale that excluded the loneliness and sleep items.
‡A higher score indicates better cognitive functioning.
Table 2.
Pearson Correlation Matrix of Baseline Social Isolation, Sleep Disturbance, and Cognitive Functioning
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Social isolation in 2006 | --- | ||||||||
| 2. Social isolation in 2010 | 0.48*** | --- | |||||||
| 3. Social isolation in 2014 | 0.44*** | 0.49*** | --- | ||||||
| 4. Sleep disturbance in 2006 | 0.05*** | 0.04** | 0.07** | --- | |||||
| 5. Sleep disturbance in 2010 | 0.08*** | 0.05** | 0.07** | 0.50*** | --- | ||||
| 6. Sleep disturbance in 2014 | 0.07*** | 0.04** | 0.06** | 0.46*** | 0.50*** | --- | |||
| 7. Cognitive functioning in 2006 | −0.14*** | −0.17*** | −0.14*** | −0.04** | −0.04* | −0.07*** | --- | ||
| 8. Cognitive functioning in 2010 | −0.13*** | −0.20*** | −0.16*** | −0.04** | −0.06* | −0.08*** | 0.45*** | --- | |
| 9. Cognitive functioning in 2014 | −0.10*** | −0.18*** | −0.17*** | −0.05** | −0.07*** | −0.07*** | 0.47*** | 0.48*** | --- |
Note:
*p < .05,
** p < .01,
*** p < .001.
Bidirectional Association Between Social Isolation and Cognitive Functioning
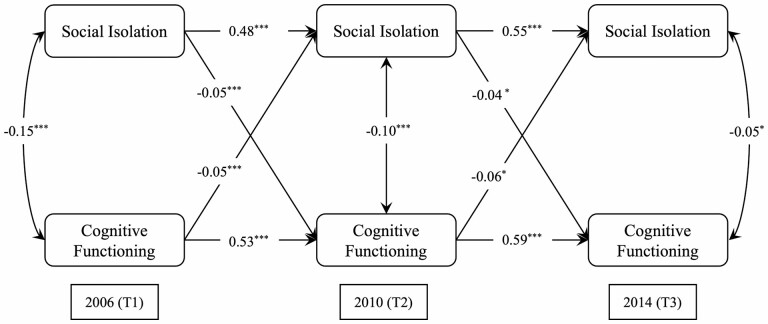
The unadjusted CLPM on the association between social isolation and cognitive functioning with standardized path estimates is presented in Supplementary Figure S1 and Supplementary Table S1. The statistically significant cross-lagged paths illustrate the longitudinal bidirectional association between social isolation and cognitive functioning. In the fully-adjusted model that controlled for all covariates (see Figure 2), social isolation still had a significant bidirectional negative association with cognitive functioning: social isolation is associated with poor cognitive functioning in the next wave (T1 to T2: β = −0.045, standard error [SE] = 0.014, p < .001; T2 to T3: β = −0.037, SE = 0.013, p < .05), and a lower level of cognitive functioning is associated with higher social isolation in the next wave (T1 to T2: β = −0.051, SE = 0.011, p < .01; T2 to T3: β = −0.060, SE = 0.025, p < .05). This result supports our first hypothesis, namely that social isolation is associated with poorer cognitive functioning, and lower cognitive functioning is associated with more social isolation. The RMSEA was 0.048 (≤0.06 is reflective of good fit); the CFI was 0.934, and the TLI was 0.905 (≥0.90 is reflective of good fit). Overall, these indices are consistent with a good model fit. Supplementary Table S2 summarizes all standardized path estimates and model fit indices.
Figure 2.
Cross-lagged panel model for social isolation with cognitive functioning. Note: All path coefficients were standardized, and all models controlled for age, sex, race/ethnicity, education, income, smoking, loneliness, depressive symptoms, and the number of comorbidities. Estimates were weighted to adjust for differential probabilities of selection and nonresponse of Health and Retirement Study participants. Longitudinal attrition was handled through inverse-probability-of-attrition weights. Single-headed arrows represented regression paths. Double-headed arrows represented correlations. The solid lines indicate paths statistically significant at p < .05. Dashed lines are nonsignificant. Model fit: CFI = 0.934, TLI = 0.905, RMSEA = 0.048 (90% CI, 0.036–0.060), Chi2 (4) = 410.211. *p < .05, **p < .01, ***p < .001. CFI = comparative fit index; RMSEA = root mean square error of approximation; TLI = Tucker-Lewis Index.
The Mediation Effect of Sleep Disturbance
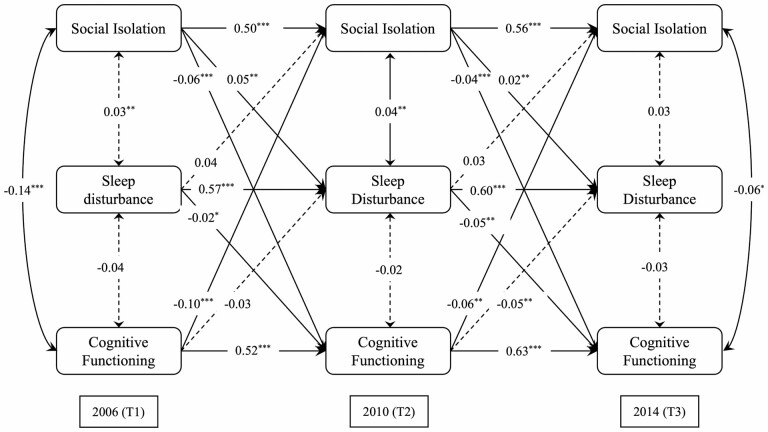
The results of the unadjusted CLPM that examined the mediating effect of sleep disturbance are presented in Supplementary Figure S2 and Supplementary Table S3. In the fully-adjusted model, path a (from social isolation at T1 to sleep disturbance at T2) and path b (from sleep disturbance at T2 to cognitive functioning at T3) were significant and divergent in direction (β = 0.054, SE = 0.016, p < .01; β = −0.052, SE = 0.017, p < .01, respectively). This result supports the second hypothesis that sleep disturbance mediates the relationship between social isolation and cognitive functioning.
Bias-corrected bootstrapping with 5,000 resamples (48) was used to determine the indirect effect of social isolation at T1 on cognitive functioning at T3 through sleep disturbance at T2. The indirect effect was significant (β = −0.003, 95% CI [−0.004, −0.002], p < .01): social isolation at T1 is associated with greater sleep disturbance at T2, which in turn associated with worse cognitive functioning at T3 (Figure 3). The effect size of the longitudinal mediation was calculated using MacKinnon’s formula for obtaining the mediated percentage, which is the indirect effect divided by the total effect (49). On average, the indirect effect accounted for 6.2% of the total effect demonstrated. The effect size fell in the low range according to Cohen’s guidelines (50), suggesting that the mediation captured relatively small covariation over time.
Figure 3.
Cross-lagged panel model for social isolation, sleep disturbance, and cognitive functioning. Notes: All path coefficients were standardized, and all models controlled for age, sex, race/ethnicity, education, income, smoking, loneliness, depressive symptoms, and the number of comorbidities. Estimates were weighted to adjust for differential probabilities of selection and nonresponse of Health and Retirement Study participants. Longitudinal attrition was handled through inverse-probability-of-attrition weights. Single-headed arrows represented regression paths. Double-headed arrows represented correlations. Solid line: p < .05. Dashed lines are nonsignificant. Model fit: CFI = 0.903, TLI = 0.827, RMSEA = 0.057 (90% CI, 0.044–0.070), Chi2 (69) = 1,726.885. *p < .05, **p < .01, ***p < .001. CFI = comparative fit index; RMSEA = root mean square error of approximation; TLI = Tucker-Lewis Index.
Notably, the indirect effects of prior cognitive functioning on social isolation via sleep disturbance were insignificant across the 3 time intervals (T1 to T2: β = −0.029, SE = 0.018, p = .125; T2 to T3: β = 0.026, SE = 0.023, p = .363). For the model fit indices, the RMSEA was 0.057, the CFI was 0.903, and the TLI was 0.827. Overall, these indices are consistent with the adequate model fit. Supplementary Table S4 summarizes all standardized path estimates and model fit indices.
Discussion
This is one of the first large-scale 8-year panel studies in the U.S. population that examined the bidirectional association between social isolation and cognitive functioning. Our findings strengthen existing evidence that social isolation has an adverse impact on cognitive functioning but also indicate that diminished cognitive functioning exacerbates social isolation. The indirect effect of social isolation on cognition through sleep disturbance is small, but the statistically significant supports a pathway through which social isolation affects cognitive functioning via its effect on sleep disturbance.
The bidirectional social isolation–cognition association over time remained significant after accounting for sociodemographic characteristics, lifestyle, loneliness, depressive symptoms, and comorbidities, indicating that these covariates do not fully explain the bidirectional associations. The significant direct effects of social isolation on cognitive functioning, and vice-versa the direct effects of worse cognitive functioning on social isolation, are in accordance with previous research (9,10). Similarly, the significant direct effect of worse cognitive functioning on social isolation is consistent with previous studies reporting that diminished cognitive functioning affects various aspects of social disconnection (22,23,51). A longitudinal study found that lower baseline cognitive performance was associated with a decline in social engagement independent of the β-amyloid level in community-dwelling older Americans (51). The finding extends previous studies by demonstrating that less social contact with others may also result from cognitive impairment.
Sleep disturbance has been associated with daytime impairments, such as physical and intellectual fatigue, anxiety and depression disorders, and cognitive and/or memory problems (30,52). The significant association between social isolation and sleep disturbance found in our study can be explained from an evolutionary perspective (53). A safe environment during sleep had been needed to protect life from danger throughout human history, and this need has traditionally been met by co-sleeping. Socially isolated individuals may not benefit from this process since being disconnected from others does not provide a safe environment needed for salubrious sleep (4). Sleep disturbance in socially isolated individuals is driven by elevated feelings of vulnerability and implicit vigilance for social threats (53). Previous studies further demonstrate that poor social relationships have been associated with sleep fragmentation (54) and poor sleep efficiency (time asleep/time in bed) (55). As noted earlier, the stress-buffering theory suggests that exposure to stressful life events precedes and increases the risk for various health problems. Sleep offers physiological restoration to counter the physiological effects of stressful situations and, therefore, may prevent or modify responses to situations damaging to cognitive health (4). This model has been supported by previous studies revealing that sleep quality mediates the effects of depressive symptoms and low-level social capital on cognitive functioning in older adults (56,57).
It is important to note that we only tested one potential mechanism and found the mediating effect of sleep disturbance on the unidirectional effect of social isolation on cognitive functioning. Although statistically significant, sleep disturbance only explained a small portion (6.2%) of the direct effect of social isolation on cognitive functioning. The small effect size could be due to the 4-year lag across 3 waves: large effect sizes would be unlikely even if sleep disturbance were a major mechanism underlying the isolation–cognition relationship because of the assessment of these variables over the years (38). It is worth noting that cognitive functioning was not associated with subsequent waves of sleep disturbance in this study. Cross-sectional data suggest an association between individual cognition and sleep disturbance (30), which indicates that individuals with cognitive impairment or dementia have sleep disturbance at the same time. However, the evidence does not provide causal implications for the association between cognitive functioning and sleep disturbance. Moreover, we have excluded participants who were self- or proxy-reported having dementia during the study period. Previous research suggests that lower cognitive performance does not necessarily cause insomnia symptoms using data from UK Biobank (58).
Notably, the pathway that went from sleep disturbance (T1) to cognitive functioning (T2) and then to social isolation (T3) was also significant. In our study, sleep disturbance was linked to worse subsequent cognitive functioning, which subsequently leads to more social isolation. This finding accords with research showing that sleep disturbance is associated with a higher risk of cognitive impairment (59), all of which are associated with social withdrawal and social disengagement among older adults (22,23). We are mindful that the process of a bidirectional isolation–cognition relationship might involve a “mediator chain” that includes many biological, psychosocial, and clinical markers. The mediating effect of sleep disturbance identified in the present study may be only one of the many steps between social isolation and cognitive functioning. Further research is necessary to assess other potential mediators to develop a comprehensive understanding of how social isolation shapes cognitive health.
Both social isolation and dementia are major global public health challenges (2,60). Given their reciprocal relationships, multidisciplinary interventions that attempt to alleviate social isolation and maintain or delay the progression of cognitive decline, or their associated risk factors, may be helpful for the cognitive health of middle-aged and older adults. Targeting these pathways could interrupt the possible vicious cycle between social isolation and cognitive decline. Sleep disturbance could be a treatment target to mitigate the potential effect of social isolation on cognitive decline, given promising evidence in light of the efficacy of behavioral sleep medicine interventions for late-life insomnia (61). Interventions targeted at reducing social isolation may help address underlying causes of sleep disturbance and cognitive decline. More studies are needed to reveal the underlying pathways between isolation and cognition. Public health initiatives could reduce sleep disturbance by facilitating social network integration and participation in community activities, thereby protecting against cognitive decline.
Several limitations should be noted in this study. First, although this study’s longitudinal design and use of cross-lagged panel models allow for the careful examination of the direction of effects, causal inference cannot be made due to unavoidable omitted-variable bias. Second, the measurement of sleep disturbance is a composite of self-reported sleep items rather than objective sleep measures (eg, actigraphy, polysomnography). Although self-reported measurement may be affected by measurement error and recall bias, evidence suggests that self-reported sleep parameters are reliable and are closely in accordance with objective measures of sleep assessed using actigraphy (62). Furthermore, the measure of sleep disturbance used in this study was unable to differentiate the subtypes of sleep disturbance (eg, insomnia, sleep-disordered breathing, and other sleep problems). Future research is needed to replicate the analysis using objective sleep measurements such as actigraphy or polysomnography. Finally, the HRS sample comprises primarily non-Hispanic Whites (81.4% at baseline) and relatively well-educated individuals. Our findings might not be generalizable to racial/ethnic underrepresented adults and those with a disadvantaged socioeconomic status.
Conclusion
In summary, 8-year cross-lagged longitudinal data from the HRS suggest a bidirectional association between social isolation and diminished cognitive functioning, with sleep disturbance partially mediates the effect of social isolation on cognitive functioning. Both social isolation and dementia are major global public health challenges. Public health initiatives could help address the issue of sleep disturbance by promoting social connection and social engagement. These measures could help improve or maintain cognitive health among middle-aged and older adults in the United States.
Supplementary Material
Acknowledgments
The Health and Retirement Study is sponsored by the National Institute on Aging (grant number U01AG009740) and is conducted by the University of Michigan. The authors thank all participants and staff for their contribution to this study.
Contributor Information
Xiang Qi, Rory Meyers College of Nursing, New York University, New York City, New York, USA.
Yaolin Pei, Rory Meyers College of Nursing, New York University, New York City, New York, USA.
Susan K Malone, Rory Meyers College of Nursing, New York University, New York City, New York, USA.
Bei Wu, Rory Meyers College of Nursing, New York University, New York City, New York, USA.
Funding
This study is partially supported by National Institute on Aging (P30AG059304) and National Institute on Minority Health and Health Disparities (P50MD017356).
Conflict of Interest
None declared.
Author Contributions
X.Q.: Conceptualization, methodology, formal analysis, investigation, software, writing—original draft, writing—review and editing. Y.P.: Methodology, data curation, formal analysis, validation, writing—original draft, writing—review and editing. S.K.M.: Methodology, writing—original draft, writing—review and editing, supervision. B.W.: Conceptualization, methodology, validation, investigation, data curation, writing—original draft, writing—review and editing, supervision, funding acquisition.
Data Availability
The Health and Retirement Study (HRS) data sets are publicly available at the University of Michigan Institute for Social Research. Researchers may obtain the data sets after sending a data user agreement to the HRS team (https://hrs.isr.umich.edu/data-products).
References
- 1. Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. 2021. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
- 2. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seibert M, Mühlbauer V, Holbrook J, et al. Efficacy and safety of pharmacotherapy for Alzheimer’s disease and for behavioural and psychological symptoms of dementia in older patients with moderate and severe functional impairments: a systematic review of controlled trials. Alzheimers Res Ther. 2021;13(1):131. doi: 10.1186/s13195-021-00867-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hawkley LC, Cacioppo JT. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann Behav Med. 2010;40(2):218–227. doi: 10.1007/s12160-010-9210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donovan NJ, Blazer D. Social isolation and loneliness in older adults: review and commentary of a national academies report. Am J Geriatr Psychiatry. 2020;28(12):1233–1244. doi: 10.1016/j.jagp.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci USA. 2013;110(15):5797–5801. doi: 10.1073/pnas.1219686110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shankar A, Rafnsson SB, Steptoe A. Longitudinal associations between social connections and subjective wellbeing in the English Longitudinal Study of Ageing. Psychology & Health. 2015;30(6):686–698. doi: 10.1080/08870446.2014.979823 [DOI] [PubMed] [Google Scholar]
- 8. Qi X, Pei Y, Wang K, Han S, Wu B. Social isolation, loneliness and accelerated tooth loss among Chinese older adults: a longitudinal study. Community Dent Oral Epidemiol. 2022:cdoe.12727. doi: 10.1111/cdoe.12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu B, Steptoe A, Chen Y, Jia X. Social isolation, rather than loneliness, is associated with cognitive decline in older adults: the China Health and Retirement Longitudinal Study. Psychol Med. 2021; 51( 14): 2414– 2421. doi: 10.1017/S0033291720001014 [DOI] [PubMed] [Google Scholar]
- 10. Shen C, Rolls ET, Cheng W, et al. Associations of social isolation and loneliness with later dementia. Neurology. 2022; 99( 2): e164– e175. doi: 10.1212/WNL.0000000000200583 [DOI] [PubMed] [Google Scholar]
- 11. Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64(2):234. doi: 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- 12. Yin J, Lassale C, Steptoe A, Cadar D. Exploring the bidirectional associations between loneliness and cognitive functioning over 10 years: the English Longitudinal Study of Ageing. Int J Epidemiol. 2019;48(6):1937–1948. doi: 10.1093/ije/dyz085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qi X, Zhang W, Wang K, Pei Y, Wu B. Social isolation and psychological well-being among older Chinese Americans: Does resilience mediate the association? Int J Geriatr Psychiatry. 2022;37(8):gps.5791. doi: 10.1002/gps.5791 [DOI] [PubMed] [Google Scholar]
- 14. Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: a systematic review and meta-analysis. J Alzheimers Dis. 2019;70(s1):S119–S144. doi: 10.3233/JAD-180501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joyce J, Ryan J, Owen A, et al. Social isolation, social support, and loneliness and their relationship with cognitive health and dementia. Int J Geriatr Psychiatry. 2022;37(1):gps.5644. doi: 10.1002/gps.5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cudjoe TKM, Roth DL, Szanton SL, Wolff JL, Boyd CM, Thorpe RJ. The epidemiology of social isolation: National Health and Aging Trends Study. J Gerontol B Psychol Sci Soc Sci. 2020;75(1):107–113. doi: 10.1093/geronb/gby037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32(1):149–162. doi: 10.3233/rnn-139008 [DOI] [PubMed] [Google Scholar]
- 19. McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30(4):377–385. doi: 10.1037/a0022826 [DOI] [PubMed] [Google Scholar]
- 21. Drinkwater E, Davies C, Spires-Jones TL. Potential neurobiological links between social isolation and Alzheimer’s disease risk. Eur J Neurosci. 2022;56(9):5397–5412. doi: 10.1111/ejn.15373 [DOI] [PubMed] [Google Scholar]
- 22. Amano T, Morrow-Howell N, Park S. Patterns of social engagement among older adults with mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci. 2020;75(7):1361–1371. doi: 10.1093/geronb/gbz051 [DOI] [PubMed] [Google Scholar]
- 23. Rewerska-Juśko M, Rejdak K. Social stigma of people with dementia. J Alzheimers Dis. 2020;78(4):1339–1343. doi: 10.3233/jad-201004 [DOI] [PubMed] [Google Scholar]
- 24. Porcelli S, Van Der Wee N, van der Werff S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. 2019;97:10–33. doi: 10.1016/j.neubiorev.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 25. Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benson JA, McSorley VE, Hawkley LC, Lauderdale DS. Associations of loneliness and social isolation with actigraph and self-reported sleep quality in a national sample of older adults. Sleep. 2021;44(1):zsaa140. doi: 10.1093/sleep/zsaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu B, Steptoe A, Niu K, Ku PW, Chen LJ. Prospective associations of social isolation and loneliness with poor sleep quality in older adults. Qual Life Res. 2018;27(3):683–691. doi: 10.1007/s11136-017-1752-9 [DOI] [PubMed] [Google Scholar]
- 28. Tubbs AS, Dollish HK, Fernandez F, Grandner MA. The basics of sleep physiology and behavior. In: Grandner MA, ed. Sleep and Health. Cambridge, Massachusetts: Elsevier; 2019:3–10. doi: 10.1016/B978-0-12-815373-4.00001-0 [DOI] [Google Scholar]
- 29. Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543. doi: 10.1001/jamaneurol.2013.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–1028. doi: 10.1016/s1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- 31. Chen PL, Lee WJ, Sun WZ, Oyang YJ, Fuh JL. Risk of dementia in patients with insomnia and long-term use of hypnotics: a population-based retrospective cohort study. PLoS ONE. 2012;7(11):e49113. doi: 10.1371/journal.pone.0049113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Academies of Sciences, Engineering, and Medicine [ed.]. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, DC: National Academies Press; 2020. [PubMed] [Google Scholar]
- 33. Sonnega A, Weir DR. The Health and Retirement Study: a public data resource for research on aging. Open Health Data. 2014;2(1):e7. doi: 10.5334/ohd.am [DOI] [Google Scholar]
- 34. Ryan L, Fisher G, Smith J. [Health and Retirement Study Leave Behind Questionnaire]*. HRS Psychosocial and Lifestyle Questionnaire 2006-2016. 2018. https://hrs.isr.umich.edu/sites/default/files/biblio/HRS%202006-2016%20SAQ%20Documentation_07.06.17_0.pdf [Google Scholar]
- 35. Zavaleta D, Samuel K, Mills CT. Measures of social isolation. Soc Indic Res. 2017;131(1):367–391. doi: 10.1007/s11205-016-1252-2 [DOI] [Google Scholar]
- 36. Crowe CL, Domingue BW, Graf GH, Keyes KM, Kwon D, Belsky DW. Associations of loneliness and social isolation with health span and life span in the U.S. Health and Retirement Study. Newman AB, ed. J Gerontol Ser A. 2021; 76( 11): 1997– 2006. doi: 10.1093/gerona/glab128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2 [DOI] [PubMed] [Google Scholar]
- 38. Griffin SC, Mladen SN, Williams AB, et al. Sleep disturbance mediates the association between loneliness and health in older Americans. Int J Behav Med. 2021;28(1):64–72. doi: 10.1007/s12529-020-09897-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B(Supplement 1):i162–i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. 2004;26(6):655–672. doi: 10.1177/0164027504268574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Newsom JT. Longitudinal Structural Equation Modeling: A Comprehensive Introduction. New York: Routledge, Taylor & Francis Group; 2015. [Google Scholar]
- 42. Wang J, Wang X.. Structural Equation Modeling: Applications Using Mplus. Second edition. Hoboken, NJ: Wiley; 2020. [Google Scholar]
- 43. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- 44. Allison PD. Missing data techniques for structural equation modeling. J Abnorm Psychol. 2003;112(4):545–557. doi: 10.1037/0021-843x.112.4.545 [DOI] [PubMed] [Google Scholar]
- 45. Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–128. doi: 10.1097/ede.0b013e318230e861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim ES, Hershner SD, Strecher VJ. Purpose in life and incidence of sleep disturbances. J Behav Med. 2015;38(3):590–597. doi: 10.1007/s10865-015-9635-4 [DOI] [PubMed] [Google Scholar]
- 47. O’brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity. 2007;41(5):673–690. doi: 10.1007/s11135-006-9018-6 [DOI] [Google Scholar]
- 48. Cole DA, Maxwell SE. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112(4):558–577. doi: 10.1037/0021-843x.112.4.558 [DOI] [PubMed] [Google Scholar]
- 49. MacKinnon DP. Introduction to Statistical Mediation Analysis. 1st ed. New York: Routledge; 2012. doi: 10.4324/9780203809556 [DOI] [Google Scholar]
- 50. Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. doi: 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- 51. Biddle KD, d’Oleire Uquillas F, Jacobs HIL, et al. Social engagement and amyloid-β-related cognitive decline in cognitively normal older adults. Am J Geriatr Psychiatry. 2019;27(11):1247–1256. doi: 10.1016/j.jagp.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165(1):35. doi: 10.1001/archinte.165.1.35 [DOI] [PubMed] [Google Scholar]
- 53. Cacioppo JT, Cacioppo S. Loneliness in the modern age: an evolutionary theory of loneliness (ETL). In: Olson JM, ed. Advances in Experimental Social Psychology. Cambridge, Massachusetts: Elsevier; 2018:127–197. doi: 10.1016/bs.aesp.2018.03.003 [DOI] [Google Scholar]
- 54. Kurina LM, Knutson KL, Hawkley LC, Cacioppo JT, Lauderdale DS, Ober C. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34(11):1519–1526. doi: 10.5665/sleep.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Friedman EM, Hayney MS, Love GD, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proc Natl Acad Sci USA. 2005;102(51):18757–18762. doi: 10.1073/pnas.0509281102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guo H, Zhang Y, Wang Z, Shen H. Sleep quality partially mediate the relationship between depressive symptoms and cognitive function in older Chinese: a longitudinal study across 10 years. Psychol Res Behav Manag. 2022;15: 785–799. doi: 10.2147/PRBM.S353987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang L, Li J, Wang Z, et al. Social capital and cognitive decline: Does sleep duration mediate the association? PLOS ONE. 2021;16(5):e0252208. doi: 10.1371/journal.pone.0252208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kyle SD, Sexton CE, Feige B, et al. Sleep and cognitive performance: cross-sectional associations in the UK Biobank. Sleep Med. 2017;38:85–91. doi: 10.1016/j.sleep.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wennberg A, Wu M, Rosenberg P, Spira A. Sleep disturbance, cognitive decline, and dementia: a review. Semin Neurol. 2017;37(04):395–406. doi: 10.1055/s-0037-1604351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu B. Social isolation and loneliness among older adults in the context of COVID-19: a global challenge. Global Health Research and Policy. 2020;5(1):27. doi: 10.1186/s41256-020-00154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991991. doi: 10.1001/jama.281.11.991 [DOI] [PubMed] [Google Scholar]
- 62. Biddle DJ, Robillard R, Hermens DF, Hickie IB, Glozier N. Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Health. 2015;1(3):214–220. doi: 10.1016/j.sleh.2015.07.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Health and Retirement Study (HRS) data sets are publicly available at the University of Michigan Institute for Social Research. Researchers may obtain the data sets after sending a data user agreement to the HRS team (https://hrs.isr.umich.edu/data-products).