Key Points
Question
Which type of exercise is most effective in reducing insulin resistance markers, and what is the dose-response association between exercise dose and these markers in children and adolescents with overweight and obesity?
Findings
In this systematic review and network meta-analysis of 55 studies with a total of 3051 children and adolescents, high-intensity interval training alone or combined with resistance training exerted the greatest reduction in insulin resistance markers. In addition, the minimum exercise dosage required to yield clinically meaningful improvements in fasting insulin and homeostatic model assessment for insulin resistance (HOMA-IR) was approximately 900 to 1200 metabolic equivalent of task minutes per week; however, the certainty of evidence varied from low to moderate.
Meaning
These findings suggest that youths with excess weight who engage in a minimum of two to three 60-minute sessions of moderate to vigorous activity per week, preferably through high-intensity interval training alone or combined with resistance training, achieve substantial improvements in fasting insulin and HOMA-IR.
This systematic review and network meta-analysis compares the effectiveness of exercise training modalities in reducing insulin resistance markers and the dose-response association between exercise dose and these markers in children and adolescents with excess weight.
Abstract
Importance
Although benefits have been reported for most exercise modalities, the most effective exercise approaches for reducing insulin resistance in children and adolescents with excess weight and the optimal exercise dose remain unknown.
Objective
To compare exercise training modalities and their association with changes in insulin resistance markers among children and adolescents with excess weight and to establish the optimal exercise dose.
Data Sources
For this systematic review and network meta-analysis, 6 electronic databases (PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Scopus, Web of Science, and CINAHL) were searched for studies from inception to April 1, 2023.
Study Selection
Randomized clinical trials (ie, randomized controlled trials and randomized trials without a control group) were included if they reported outcomes associated with aerobic training, resistance training, high-intensity interval training (HIIT), or a combination of these interventions.
Data Extraction and Synthesis
Data extraction for this systematic review was conducted following a network meta-analysis extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline. Effect sizes were calculated as the mean difference (MD) with 95% CI using random-effects inverse-variance models with the Hartung-Knapp-Sidik-Jonkman method. The hierarchy of competing interventions was defined using the surface under the cumulative ranking curve. The Cochrane Risk-of-Bias tool, version 2 (RoB2), was used to independently assess the risk of bias of the included studies. The certainty of evidence in consistent networks was assessed using the Grading of Recommendation, Assessment, Development and Evaluation approach. The study protocol was prospectively registered with PROSPERO. Data analyses were conducted between May and June 2023.
Main Outcomes and Measures
The primary outcomes were fasting glucose, insulin, and homeostatic model assessment for insulin resistance (HOMA-IR).
Results
This analysis included 55 studies with a total of 3051 children and adolescents (mean [SD] age, 13.5 [2.3] years; 1537 girls [50.4%] and 1514 boys [49.6%]). Exercise was associated with reductions in fasting insulin (MD, −4.38 μU/mL [95% CI, −5.94 to −2.82 μU/mL]) and HOMA-IR (MD, –0.87 [95% CI, –1.20 to –0.53]). A nonlinear association in both markers was observed, with a required minimal exercise dosage of approximately 900 to 1200 metabolic equivalent of task minutes per week, especially in children and adolescents with insulin resistance at baseline. Combination HIIT and resistance training and concurrent training were the most effective approaches for reducing insulin resistance markers. On average, the certainty of evidence varied from low to moderate.
Conclusions and Relevance
These findings underscore the role of exercise interventions in enhancing insulin resistance markers among children and adolescents with overweight and obesity. It is advisable to include resistance exercises alongside aerobic and HIIT programs for a minimum of two to three 60-minute sessions per week.
Introduction
In both developed and developing countries, childhood obesity remains a major public health challenge in the 21st century.1,2 The exact causes of childhood obesity are unclear but are believed to involve genetics, physical inactivity, unhealthy eating habits, and psychological factors.3 In children and adolescents, obesity-related insulin resistance and impaired glucose metabolism can increase the risk of developing type 2 diabetes and other metabolic disorders in adolescence and beyond.4,5
It is crucial to promote healthy habits to prevent and manage childhood obesity,6 as this can help reduce the risk of developing the above-mentioned metabolic disorders.7 Although diet and physical activity are typically the primary approaches for treating children and adolescents with obesity,8 research has shown that increasing physical activity alone, rather than restricting energy intake, can be effective in improving several health outcomes, such as body composition,9 cardiometabolic parameters,10,11 and cardiorespiratory fitness.10,11 Traditional meta-analysis methods synthesize important overarching questions, but they generally do not include all study information and often ignore or are unable to account for important treatment heterogeneity in design and delivery characteristics.12 In terms of regulation of glucose metabolism, for example, traditional meta-analysis methods cannot answer important questions about exercise variable dosage (eg, exercise intensity, exercise duration, and type of exercise)13,14,15,16 because they compare only 2 treatments at a time and do not allow full analysis of trials investigating multiple treatment groups within studies. Therefore, although benefits have been reported for most exercise modalities,11 which exercise approaches are most effective for preventing and reducing insulin resistance in children and adolescents with excess weight, as well as the optimal exercise dose, remains unknown. Network meta-analysis (NMA) methods have the potential to better identify the best approach for exercise interventions to improve insulin resistance in children and adolescents with excess weight.17
The aim of this study was 3-fold: (1) to determine the association between different exercise modalities and insulin resistance markers (eg, fasting glucose, fasting insulin, and homeostatic model assessment for insulin resistance [HOMA-IR]), (2) to establish which exercise type (eg, aerobic, resistance, high-intensity interval training [HIIT], or their combination) is most effective in reducing insulin resistance markers, and (3) to examine the dose-response association between exercise dose and insulin resistance markers in children and adolescents with excess weight.
Methods
Study Protocol and Registration
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (PRISMA-NMA).18 The study protocol was prospectively registered with PROSPERO (CRD42023413048).
Eligibility Criteria
Studies were included if they reported the following: (1) a study population composed of children and adolescents (aged 5-18 years) with excess weight (ie, overweight and obesity), as defined by the authors; (2) a study design that either compared 2 or more exercise interventions (categorized as aerobic or endurance, resistance or strength training, concurrent training [combination aerobic and resistance training, regardless of intensity], or HIIT [alternating short bursts of intense physical activity with brief periods of rest or lower-intensity exercise] or their combination) or compared an exercise intervention group with a comparative control group (eg, no treatment or usual care, wait-list control, or education) (ie, a randomized clinical trial [RCT]); (3) supervised exercise interventions with a duration of 4 weeks or more; and (4) a primary outcome of interest of fasting glucose, fasting insulin, and/or homeostatic model assessment (HOMA-IR). Other insulin-resistance parameters, such as quantitative insulin-sensitivity check index, 2-hour glucose, 2-hour insulin, acute insulin response, disposition index, or glycated hemoglobin, were considered as secondary outcomes. Studies that included either a dietary intervention or drug therapy in combination with exercise or participants presenting with other medical conditions (eg, diabetes) were excluded.
Information Sources and Search
The PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Scopus, Web of Science, and CINAHL electronic databases were searched for studies from inception to April 1, 2023. Searches were limited to youths aged 5 to 18 years. Cross-referencing was also performed by examining the reference lists of articles that met the inclusion criteria. A librarian was consulted to supervise the quality of the search. All search strategies are detailed in eMethods 1 in Supplement 1.
Study Selection and Data Collection Process
Two authors (A.G.-H. and Y.E.) carried out the entire process of selecting literature and extracting data independently. If there were any differences in opinions, they were resolved by discussion with a third researcher (J.F.L.-G.).
The variables coded in this study were grouped into 4 major categories: (1) study characteristics, such as the author, journal, and year of publication; (2) participant characteristics, such as age, sex, and body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]); (3) intervention characteristics, such as intervention type, length, frequency, dose (intensity and duration), and compliance; and (4) data for primary and secondary outcomes, including sample sizes and baseline and postexercise means (SDs). If needed, data from figures were extracted using WebPlotDigitizer, version 4.5.19 Interagreement was assessed with the Cohen κ statistic.
Risk of Bias in Individual Studies
Two reviewers (A.G.-H. and Y.E.) used the Cochrane Risk-of-Bias tool, version 2 (RoB2), to independently assess the risk of bias of the included studies.20 Each study and every domain was judged as “low risk of bias,” “some concerns,” or “high risk of bias.” Any disagreements in quality ratings were solved by discussion. If consensus could not be reached, a third member of the review team (J.F.L.-G.) was consulted.
Statistical Analysis
Stata software, version 17.0 (StataCorp), was used to conduct both the traditional and dose-response meta-analysis. In addition, we employed the Confidence in Network Meta-Analysis (CINeMA) web application to perform the NMA.21 Publication bias was assessed using the Luis Furuya-Kanamori (LFK) index.22 Further details are presented in eMethods 2 in Supplement 1.
We assessed the certainty of evidence in consistent networks using the Grading of Recommendation, Assessment, Development and Evaluation approach (GRADE).23 We considered 4 levels of certainty, ranging from very low to high (indicating a high likelihood of clinically meaningful differences between the true and estimated effect sizes, or a high level of confidence in the similarity between the true and estimated effect sizes, respectively). The assessment considered the risk of bias within comparisons, publication bias, indirectness, imprecision, heterogeneity, and incoherence.
P < .05 (2 tailed) was considered statistically significant. Data analysis was conducted between May and June 2023.
Results
Study Selection
Of the 1495 articles screened after duplicates were removed, 55 met our inclusion criteria (eTable 1 in Supplement 1).24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75 Figure 1 illustrates the identification, screening, and inclusion process. The Cohen κ statistic for interrater reliability was 0.84 (95% CI, 0.72 to 0.96). The list of excluded studies is presented in eMethods 3 in Supplement 1.
Figure 1. Study Flow Diagram.
Study Characteristics
The studies included in this meta-analysis involved a total of 3051 children and adolescents with overweight or obesity (mean [SD] age, 13.5 [2.3] years; 1537 girls [50.4%] and 1514 boys [49.6%]). Baseline characteristics of participants are presented in eTable 1 in Supplement 1. Most studies were RCTs,24,25,26,27,28,30,31,34,35,36,37,40,41,42,43,44,45,46,47,48,49,50,51,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,71,75,76,77,78 but 8 were RCTs with no control group.29,32,33,38,39,57,70,74 Although most studies included both boys and girls in their samples, 11 studies included only boys42,47,48,54,57,61,62,67,72,74,75 and 9 included only girls.25,26,34,49,51,53,64,68,73 The definition of overweight and obesity was mostly based on age and sex-specific BMI cut points. The types of exercise programs analyzed included aerobic, resistance, concurrent training, HIIT, and combination HIIT/resistance training (eTable 1 in Supplement 1).
Risk of Bias Within Studies
The results of the risk of bias analyses are shown in eTable 2 in Supplement 1. All studies were deemed to be at low risk of bias for the “randomization process” domain because we only included randomized studies. Inversely, the “deviations from intended interventions” domain was rated to be at high risk of bias in all studies due to the impossibility of blinding participants and individuals delivering the intervention to group assignment in the exercise interventions. For the rest of the items, most studies were considered to have some concerns regarding risk of bias.
Characteristics of Treatments (Traditional Meta-Analysis)
Compared with the control intervention, physical exercise favored a reduction in fasting insulin (mean difference [MD], −4.38 μU/mL [95% CI, −5.94 to −2.82 μU/mL]; P < .001; I2 = 77.8%) and HOMA-IR (MD, –0.87 [95% CI, –1.20 to –0.53]; P < .001; I2 = 68.2%) (eFigures 1 and 2 in Supplement 1), but not in fasting glucose (MD, −0.28 mg/dL [95% CI, −0.58 to 0.02 mg/dL]; P = .07; I2 = 62.7), 2-hour oral glucose tolerance (MD, −0.57 mg/dL [95% CI, −2.15 to 1.02 mg/dL]; P = .66; I2 = 0%), or glycated hemoglobin (MD, −0.04% [95% CI, −0.13% to 0.06%]; P = .41; I2 = 4.5%) (eFigures 3-5 in Supplement 1). The effect size of HOMA-IR increased when we analyzed participants with levels of 3.16 or greater at baseline (MD, −1.36 [95% CI, −1.88 to −0.84]; P < .001; I2 = 80.1%). A small-study effect size was observed for fasting glucose (LFK = −7.88) and 2-hour oral glucose tolerance (LFK = −6.92), but not for fasting insulin (LFK = −1.07) and HOMA-IR (LFK = 0.45).
In sensitivity analyses, we observed no notable modifications in the results for fasting insulin and HOMA-IR (eFigures 6 and 7 in Supplement 1) after removing 1 study at a time. However, the study of Kelly et al76 significantly affected the overall results for 2-hour glucose tolerance (MD, −1.94 mg/dL [95% CI, −3.74 to −0.15 mg/dL]; P = .04) (eFigure 8 in Supplement 1). Similar results were observed for fasting glucose, in which studies by Davis et al77 (MD, −0.35 mg/dL [95% CI, −0.69 to −0.01 mg/dL]; P = .045) and Farpour-Lambert et al78 (MD, −0.34 mg/dL [95% CI, −0.67 to −0.01 mg/dL]; P = .043) significantly affected the overall results (eFigure 9 in Supplement 1).
Dose-Response Meta-Analysis
Figure 2 presents the nonlinear dose-response associations of exercise dose with fasting insulin and HOMA-IR. For fasting insulin, a minimal exercise dosage of approximately 900 metabolic equivalent of task minutes (MET-min) per week was required to achieve the identified MD of −4.38 μU/mL for fasting insulin, with a flattening effect at around 1500 MET-min/wk (−0.09 μU/mL [95% CI, −0.12 to −0.05 μU/mL] for every 100 MET-min; P < .001 for nonlinearity).
Figure 2. Dose-Response Association Between Exercise Dose and Differences in Fasting Insulin and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) Among Children and Adolescents With Excess Weight.
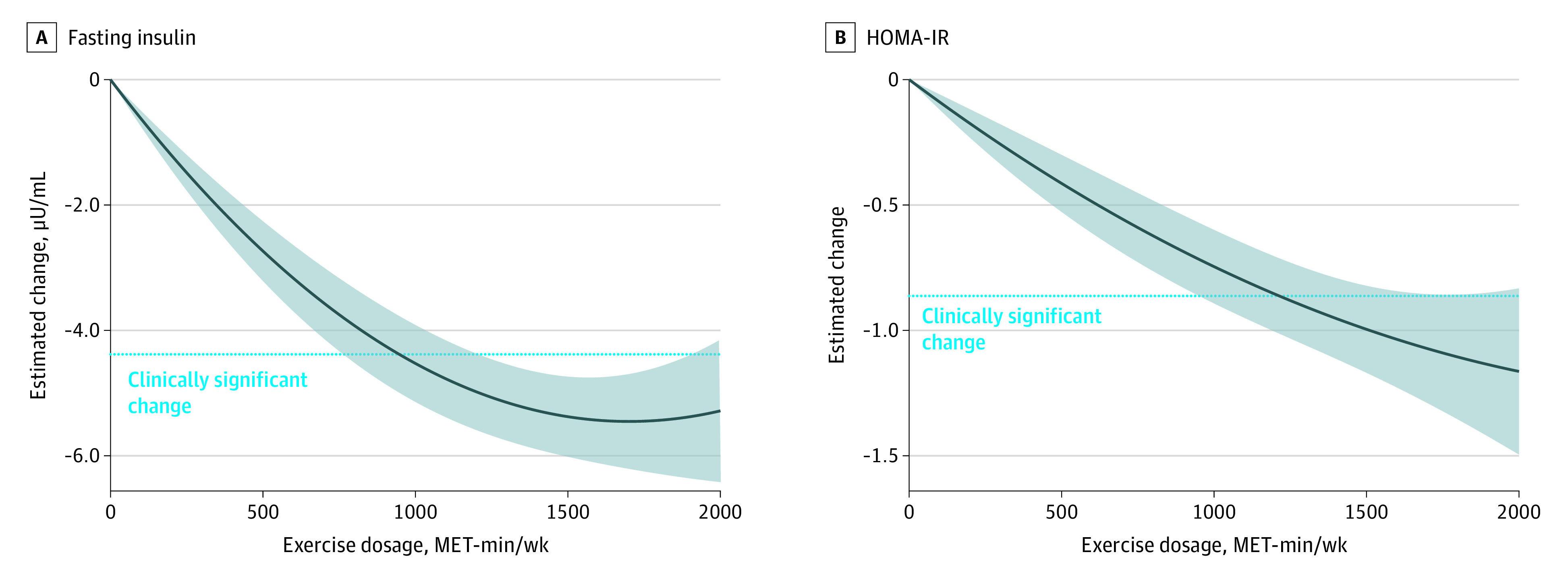
The horizontal dotted line represents the observed change in each parameter in the traditional meta-analysis. Shaded areas represent 95% CIs. MET-min indicates metabolic equivalent of task minutes.
Similarly, for HOMA-IR, a minimal exercise dosage of approximately 1200 MET-min/wk was required to achieve the identified MD of −0.87 (−0.09 [95% CI, −0.12 to −0.06] for every 100 MET-min; P < .001 nonlinearity). Higher doses continued to contribute to marker improvements. After studies that included children and adolescents with a HOMA-IR of 3.16 or greater were selected,79 this dose response significantly increased (−0.16 [95% CI, −0.21 to −0.10] for every 100 MET-min; P < .001 for nonlinearity) (eFigure 10 in Supplement 1).
Finally, no association was observed between exercise dose and fasting glucose (−0.13 mg/dL [95% CI, −0.28 to 0.02 mg/dL] for every 100 MET-min; P = .09 for nonlinearity) (eFigure 11 in Supplement 1).
Network Meta-Analysis
Comparisons of associations between different types of exercise and fasting glucose, insulin, and HOMA-IR levels are presented in Figure 3. The NMA estimates (Table) suggested that aerobic training, concurrent training, and combination HIIT/resistance training substantially decreased fasting glucose levels compared with the control intervention. Specifically, the MD was −1.43 mg/dL (95% CI, −2.70 to −0.71 mg/dL) for aerobic training, −2.81 mg/dL (95% CI, −4.80 to −0.93 mg/dL) for concurrent training, and −5.01 mg/dL (95% CI, −8.78 to −1.37 mg/dL) for HIIT/resistance training.
Figure 3. Network Plot of Available Comparisons Between Different Exercise Interventions on Fasting Glucose, Fasting Insulin, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) Among Children and Adolescents With Excess Weight.
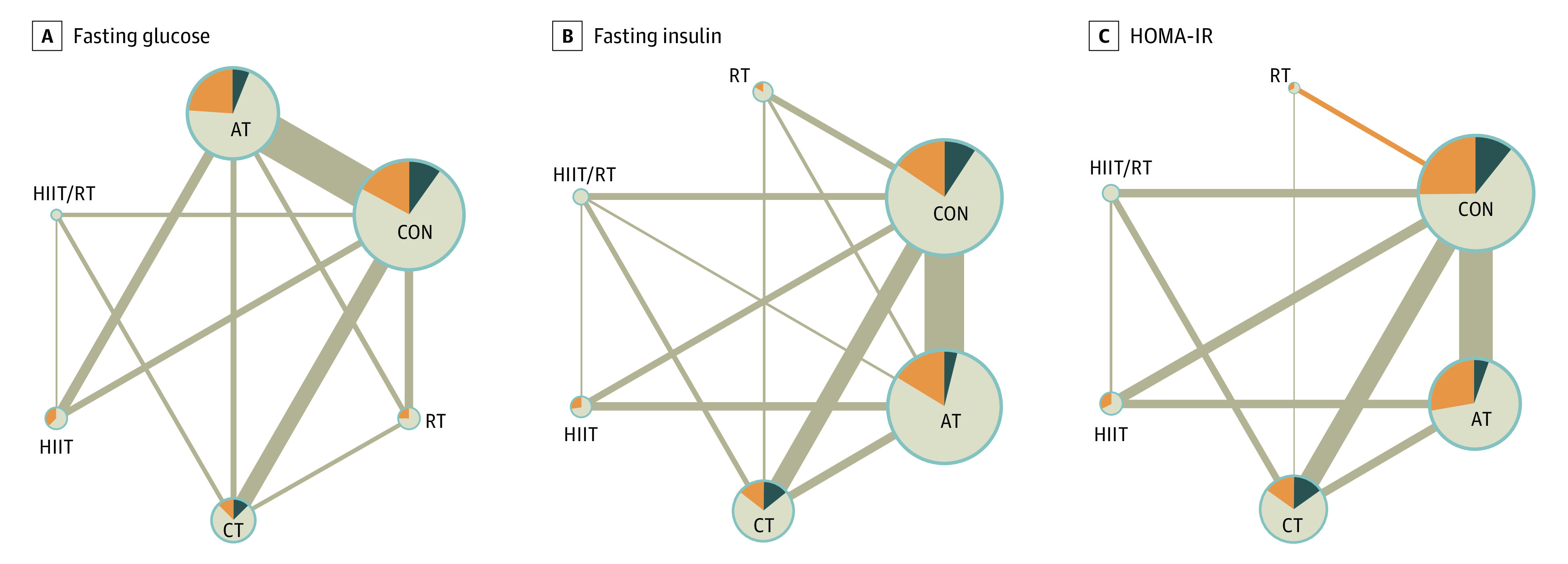
A, Fasting glucose. B, Fasting insulin. C, HOMA-IR. The size of the nodes is proportional to the number of participants randomized to each intervention, and nodes are colored according to the proportion of studies with low (dark blue), moderate (light brown), and high (orange) indirectness. The width of the edges corresponds to the number of studies directly comparing the 2 interventions. Edges are colored according to average Risk of Bias 2 status on each comparison, where dark blue refers to low risk, light brown to some concerns, and orange to high risk of bias. Lines represent indirect comparisons. AT indicates aerobic training; CON, control; CT, concurrent training; HIIT, high-intensity interval training; HIIT/RT, combination HIIT and RT; RT, resistance training.
Table. Network Meta-Analyses of the Association Between Exercise and Insulin Resistance Markers in Children and Adolescents With Excess Weighta.
| Marker | Aerobic | Resistance | Concurrent | HIIT | HIIT/resistance |
| Fasting glucose (mg/dL) | |||||
| Resistance | −0.55 (−2.86 to 1.77) | ||||
| Concurrent | −1.26 (−3.48 to 0.77) | 0.71 (−1.94 to 3.68) | |||
| HIIT | 0.16 (−2.16 to 2.64) | −0.72 (−3.92 to 2.46) | 1.42 (−1.57 to 4.48) | ||
| HIIT/resistance | −3.54 (−7.37 to 0.25) | 2.99 (−1.21 to 7.13) | −2.28 (−6.06 to 1.77) | −3.70 (−7.84 to 0.51) | |
| Control | −1.49 (−2.73 to −0.39)b | −2.04 (−4.30 to 0.21) | −2.75 (−4.95 to −0.79)b | −1.32 (−3.71 to 0.96) | −5.02 (−8.71 to −1.34)b |
| Fasting insulin (μU/mL) | |||||
| Resistance | −0.58 (−2.98 to 1.61) | ||||
| Concurrent | −1.28 (−3.47 to 0.72) | 0.70 (−2.03 to 3.28) | |||
| HIIT | 0.29 (−2.04 to 2.53) | −0.86 (−3.90 to 2.21) | 1.56 (−1.28 to 4.59) | ||
| HIIT/resistance | −3.64 (−7.28 to −0.02)b | 3.06 (−1.08 to 7.13) | −2.37 (−5.97 to 1.46) | −3.93 (−7.72 to 0.15) | |
| Control | −1.48 (−2.72 to −0.32)b | −2.05 (−4.37 to 0.13) | −2.75 (−4.90 to −0.79)b | −1.19 (−3.58 to −1.09) | −5.12 (−8.83 to −1.54)b |
| HOMA-IR | |||||
| Resistance | 0.18 (−1.20 to 1.51) | ||||
| Concurrent | −0.33 (−0.96 to 0.28) | 0.50 (−0.87 to 1.84) | |||
| HIIT | −0.19 (−0.93 to 0.54) | 0.37 (−1.12 to 1.78) | 0.14 (−0.74 to 0.99) | ||
| HIIT/resistance | −0.51 (−1.42 to 0.46) | 0.69 (−0.89 to 2.23) | −0.18 (−1.06 to 0.80) | −0.32 (−1.34 to 0.72) | |
| Control | −0.71 (−1.20 to −0.24)b | −0.53 (−1.84 to 0.72) | −1.03 (−1.57 to −0.52)b | −0.90 (−1.62 to −0.22)b | −1.22 (−2.04 to −0.36)b |
Abbreviations: HIIT, high-intensity interval training; HIIT/resistance, combination HIIT and resistance training; HOMA-IR, homeostasis model assessment of insulin resistance; MD, mean difference.
Data are expressed as the MD (95% CI). Comparisons should be read from left to right. Values correspond to differences in MDs (95% CIs) between column and row; for positive values, the exercise protocol indicated in the column is favored (eg, aerobic training had a mean fasting glucose loss of 0.55 mg/dL compared with resistance training).
P < .05.
Similarly, the NMA estimates suggested that participants in the aerobic training, concurrent training, and HIIT/resistance training groups had substantially decreased fasting insulin levels compared with the control group. The MD was −1.42 μU/mL (95% CI, −2.64 to −0.34 μU/mL) for aerobic training, −2.70 μU/mL (95% CI, −4.74 to −0.82 μU/mL) for concurrent training, and −4.98 μU/mL (95% CI, −8.85 to −1.28 μU/mL) for HIIT/resistance training.
Furthermore, the NMA estimates suggested that aerobic training, concurrent training, HIIT, and HIIT/resistance training substantially diminished HOMA-IR levels compared with the control intervention. The MD was −0.70 (95% CI, −1.17 to −0.26) for aerobic training, −0.87 (95% CI, −1.57 to −0.19) for HIIT, −1.03 (95% CI, −1.58 to −0.56) for concurrent training, and −1.20 (95% CI, −2.09 to −0.41) for HIIT/resistance training.
Intervention Ranking
For fasting glucose levels, combination HIIT/resistance training had the highest surface under cumulative ranking (SUCRA) (93.9%), followed by concurrent training (70.9%). In terms of fasting insulin, HIIT/resistance training had the highest SUCRA (94.7%), followed by concurrent training (72.0%). For HOMA-IR, HIIT/resistance training had the highest SUCRA (95.1%), followed by concurrent training (69.4%) (eFigure 12 in Supplement 1).
Certainty of NMA Evidence
eFigure 13 in Supplement 1 presents the results provided by the CINeMA approach for the NMA. Most comparisons had “some concerns” for the domains within-study bias and reporting bias domains. The incoherence, heterogeneity, and imprecision domains were also assessed as having “some concerns” for most comparisons, which affects confidence in the results. We considered a meaningful clinical difference in fasting glucose, insulin, and HOMA-IR to be −0.28 mg/dL, −4.38 μU/mL, and −0.87, respectively, based on MDs observed in traditional meta-analyses. Overall, only 2 of 15 comparisons for fasting glucose, 5 of 15 for fasting insulin, and 4 of 15 (for HOMA-IR) were considered to have a high confidence rating.
Discussion
The results of this study suggest that exercise was associated with reductions in fasting insulin and HOMA-IR in children and adolescents with overweight and obesity. A notable finding is the nonlinear association of exercise dose with fasting insulin and HOMA-IR, with a minimal dosage requirement of approximately 900 to 1200 MET-min/wk, equivalent to two to three 60-minute sessions of moderate to vigorous activity per week. Furthermore, higher exercise doses contributed to continued improvement in the HOMA-IR marker. Combination HIIT/resistance training and concurrent training were ranked as the best exercise interventions for reducing all insulin resistance markers.
Overall, our results are consistent with previous meta-analyses in this population,13,14 which reported slightly lower effect sizes in fasting insulin and HOMA-IR. These findings are highly relevant because impaired insulin sensitivity, which may appear prior to glucose dysregulation in youths,80 is a major component of obesity and comorbid disease, such as type 2 diabetes and cardiovascular disease. In contrast, our findings related to fasting glucose differ from those reported by García-Hermoso et al,13 who observed a reduction in fasting glucose in children and adolescents with obesity. Studies that analyzed 2-hour glucose concentration after glucose tolerance testing, a more robust and valid assessment to determine insulin sensitivity,81 showed inconsistent results, which are reflected in our meta-analysis.
Another relevant finding is the nonlinear association of exercise dose with fasting insulin and HOMA-IR, with a minimal exercise dose requirement of approximately 900 to 1200 MET-min/wk, equivalent to at least two to three 60-minute sessions of moderate to vigorous activity per week. This finding is consistent with results reported by Davis et al,24 who investigated the association between different doses of aerobic exercise (with no dietary restrictions) and oral glucose tolerance in children with overweight and obesity. Their exercise program was administered 5 days per week, with sessions lasting either 20 minutes (low dose) or 40 minutes (high dose). After 3 months, the researchers noted a trend toward a dose-response association, as reductions in the insulin area under curve were greater in both the high-dose and low-dose exercise groups (adjusted MD, −3.56 and −2.96 μU/mL) compared with the control group. Therefore, these findings suggest that even a modest increase in physical activity per week can yield notable improvements in insulin sensitivity. Therefore, incorporating exercise interventions into the management of overweight and obesity in children and adolescents is a promising strategy for improving insulin resistance and preventing the development of cardiometabolic disease.
Our NMA highlights combination HIIT/resistance training and concurrent training as the most efficient exercise protocols for reducing insulin resistance in this population.16 Performing HIIT, with or without resistance training, induces metabolic stress on the muscles, leading to increased glucose uptake.82 Consequently, insulin sensitivity is improved in this population.25 High-intensity interval training has been demonstrated to enhance insulin sensitivity and glycemic control in several populations, including those with and without cardiometabolic disease.82 Therefore, these findings highlight the importance of incorporating muscle strength training into HIIT. For instance, Racil et al26 observed substantial reductions in blood glucose and insulin concentrations in girls with obesity who participated in 12 weeks of HIIT combined with plyometric exercises.
Our finding that concurrent training is the second most efficient exercise protocol for reducing insulin resistance in children and adolescents with overweight or obesity aligns with the study by Kelley et al,10 which ranked these protocols as the best for reducing fat mass and the percentage of fat in a similar population. In addition, García-Hermoso et al15 demonstrated that concurrent exercise is more efficient for increasing lean body mass and adiponectin concentration compared with aerobic training alone.
Several mechanisms could explain our results. First, reducing fat mass can decrease inflammation in adipose tissue, thereby improving insulin sensitivity.83 Second, larger muscle mass requires more glucose uptake to support energy demands84 and implies a greater quantity of mitochondria present, enhancing muscle capacity to oxidize substrates like glucose and free fatty acids.85 Finally, increased adiponectin concentration is associated with increased insulin sensitivity.86
Limitations
This study has several limitations. First, our results were limited by the quality of the included evidence, which was judged to have a low to moderate confidence rating for most treatment comparisons. Second, some treatments (eg, HIIT and combination HIIT/resistance training) had limited data, potentially affecting the statistical power to detect clinically meaningful effect sizes. In addition, unexplained heterogeneity persisted despite exploration of multiple populations, exercise types, and methodological characteristics, impacting the confidence in the available evidence. Third, variation in criteria to define overweight and obesity in the included studies may have influenced our findings. Fourth, the study was focused on fasting glucose, fasting insulin, and/or HOMA-IR as primary outcomes, which may have led to a narrow perspective on the association between exercise and insulin resistance, potentially overlooking other critical secondary outcomes. Finally, the estimation of METs could have resulted in deviation from the actual energy expenditure of physical exercise programs.
Conclusions
Our findings in this systematic review and NMA suggest that incorporating resistance exercises alongside aerobic training and HIIT programs may reduce insulin resistance markers in children and adolescents with overweight or obesity. To achieve clinically meaningful improvements in fasting insulin and HOMA-IR, the minimal dosage of exercise is approximately 900 to 1200 MET-min/wk, which corresponds to two to three 60-minute sessions of moderate to vigorous activity per week. However, the certainty of evidence varied from low to moderate. It will be crucial to further investigate and establish minimum physical activity recommendations that effectively address insulin resistance, prevent metabolic syndrome, and reduce type 2 diabetes risk in this specific population.
eMethods 1. Electronic Search Strategy
eTable 1. Table of Characteristics
eMethods 2. Data Analysis
eMethods 3. Excluded Studies and Reasons for Exclusion
eTable 2. Results of the Cochrane Risk-of-Bias Tool for Randomized Controlled Trials (RoB-2)
eFigure 1. Effect of Exercise Programs on Fasting Insulin in Children and Adolescents With Excess Weight
eFigure 2. Effect of Exercise Programs on Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) in Children and Adolescents With Excess Weight
eFigure 3. Effect of Exercise Programs on Fasting Glucose in Children and Adolescents With Excess Weight
eFigure 4. Effect of Exercise Programs on 2-Hour Oral Glucose Tolerance in Children and Adolescents With Excess Weight
eFigure 5. Effect of Exercise Programs on Glycated Hemoglobin in Children and Adolescents With Excess Weight
eFigure 6. Sensitivity Analyses Once Each Study Was Excluded for Fasting Insulin
eFigure 7. Sensitivity Analyses Once Each Study Was Excluded for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
eFigure 8. Sensitivity Analyses Once Each Study Was Excluded for 2-Hour Oral Glucose Tolerance
eFigure 9. Sensitivity Analyses Once Each Study Was Excluded for Fasting Glucose
eFigure 10. Dose-Response Association Between Metabolic Equivalent of Task Minutes (MET-min) per Week and Differences in Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) in Children and Adolescents With Excess Weight and Insulin Resistance (ie, HOMA-IR≥3.16)
eFigure 11. Dose-Response Association Between Metabolic Equivalent of Task Minutes (MET-min) per Week and Differences in Fasting Glucose in Children and Adolescents With Excess Weight
eFigure 12. Rankogram for Each Type of Physical Exercise
eFigure 13. Network Meta-Analysis Confidence Rating for Glucose, Insulin, and Homeostatic Model Assessment for Insulin Resistance Outcomes
eReferences
Data Sharing Statement
References
- 1.Agha M, Agha R. The rising prevalence of obesity: part A: impact on public health. Int J Surg Oncol (N Y). 2017;2(7):e17-e17. doi: 10.1097/IJ9.0000000000000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nisar N. Childhood obesity: a major public health challenge of 21st century. J Coll Physicians Surg Pak. 2018;28(11):815-816. doi: 10.29271/jcpsp.2018.11.815 [DOI] [PubMed] [Google Scholar]
- 3.Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care. 2015;4(2):187-192. doi: 10.4103/2249-4863.154628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He QX, Zhao L, Tong JS, et al. The impact of obesity epidemic on type 2 diabetes in children and adolescents: A systematic review and meta-analysis. Prim Care Diabetes. 2022;16(6):736-744. doi: 10.1016/j.pcd.2022.09.006 [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362(9388):951-957. doi: 10.1016/S0140-6736(03)14364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampl SE, Hassink SG, Skinner AC, et al. Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. 2023;151(2):e2022060640. doi: 10.1542/peds.2022-060640 [DOI] [PubMed] [Google Scholar]
- 7.Brown T, Summerbell C. Systematic review of school-based interventions that focus on changing dietary intake and physical activity levels to prevent childhood obesity: an update to the obesity guidance produced by the National Institute for Health and Clinical Excellence. Obes Rev. 2009;10(1):110-141. doi: 10.1111/j.1467-789X.2008.00515.x [DOI] [PubMed] [Google Scholar]
- 8.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association childhood obesity research summit: executive summary. Circulation. 2009;119(15):2114-2123. doi: 10.1161/CIRCULATIONAHA.109.192215 [DOI] [PubMed] [Google Scholar]
- 9.Kelley GA, Kelley KS. Exercise and BMI z-score in overweight and obese children and adolescents: protocol for a systematic review and network meta-analysis of randomised trials. BMJ Open. 2016;6(4):e011258-e011258. doi: 10.1136/bmjopen-2016-011258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley GA, Kelley KS, Pate RR. Exercise and adiposity in overweight and obese children and adolescents: a systematic review with network meta-analysis of randomised trials. BMJ Open. 2019;9(11):e031220. doi: 10.1136/bmjopen-2019-031220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Hermoso A, Ramírez-Vélez R, Saavedra JM. Exercise, health outcomes, and pædiatric obesity: a systematic review of meta-analyses. J Sci Med Sport. 2019;22(1):76-84. doi: 10.1016/j.jsams.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 12.Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34(11):1489-1496. doi: 10.1007/s00296-014-2994-2 [DOI] [PubMed] [Google Scholar]
- 13.García-Hermoso A, Saavedra JM, Escalante Y, Sánchez-López M, Martínez-Vizcaíno V. Endocrinology and Adolescence: aerobic exercise reduces insulin resistance markers in obese youth: a meta-analysis of randomized controlled trials. Eur J Endocrinol. 2014;171(4):R163-R171. doi: 10.1530/EJE-14-0291 [DOI] [PubMed] [Google Scholar]
- 14.Marson EC, Delevatti RS, Prado AKG, Netto N, Kruel LFM. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: a systematic review and meta-analysis. Prev Med. 2016;93(93):211-218. doi: 10.1016/j.ypmed.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 15.García-Hermoso A, Ramírez-Vélez R, Ramírez-Campillo R, Peterson MD, Martínez-Vizcaíno V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: a systematic review and meta-analysis. Br J Sports Med. 2018;52(3):161-166. doi: 10.1136/bjsports-2016-096605 [DOI] [PubMed] [Google Scholar]
- 16.García-Hermoso A, Cerrillo-Urbina AJ, Herrera-Valenzuela T, Cristi-Montero C, Saavedra JM, Martínez-Vizcaíno V. Is high-intensity interval training more effective on improving cardiometabolic risk and aerobic capacity than other forms of exercise in overweight and obese youth? A meta-analysis. Obes Rev. 2016;17(6):531-540. doi: 10.1111/obr.12395 [DOI] [PubMed] [Google Scholar]
- 17.Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Undertaking network meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration; 2019:285-320. doi: 10.1002/9781119536604.ch11 [DOI] [Google Scholar]
- 18.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 19.Rohatgi A. WebPlotDigitizer, version 4.5. 2022. Accessed May 23, 2023. https://automeris.io/WebPlotDigitizer/index.html
- 20.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 21.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16(4):195-203. doi: 10.1097/XEB.0000000000000141 [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis CL, Pollock NK, Waller JL, et al. Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. JAMA. 2012;308(11):1103-1112. doi: 10.1001/2012.jama.10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Racil G, Ben Ounis O, Hammouda O, et al. Effects of high vs. moderate exercise intensity during interval training on lipids and adiponectin levels in obese young females. Eur J Appl Physiol. 2013;113(10):2531-2540. doi: 10.1007/s00421-013-2689-5 [DOI] [PubMed] [Google Scholar]
- 26.Racil G, Zouhal H, Elmontassar W, et al. Plyometric exercise combined with high-intensity interval training improves metabolic abnormalities in young obese females more so than interval training alone. Appl Physiol Nutr Metab. 2016;41(1):103-109. doi: 10.1139/apnm-2015-0384 [DOI] [PubMed] [Google Scholar]
- 27.Ben Ounis O, Elloumi M, Makni E, et al. Exercise improves the ApoB/ApoA-I ratio, a marker of the metabolic syndrome in obese children. Acta Paediatr. 2010;99(11):1679-1685. doi: 10.1111/j.1651-2227.2010.01920.x [DOI] [PubMed] [Google Scholar]
- 28.Benson AC, Torode ME, Fiatarone Singh MA. The effect of high-intensity progressive resistance training on adiposity in children: a randomized controlled trial. Int J Obes (Lond). 2008;32(6):1016-1027. doi: 10.1038/ijo.2008.5 [DOI] [PubMed] [Google Scholar]
- 29.Campos RM, de Mello MT, Tock L, et al. Aerobic plus resistance training improves bone metabolism and inflammation in adolescents who are obese. J Strength Cond Res. 2014;28(3):758-766. doi: 10.1519/JSC.0b013e3182a996df [DOI] [PubMed] [Google Scholar]
- 30.Carrel AL, Clark RR, Peterson SE, Nemeth BA, Sullivan J, Allen DB. Improvement of fitness, body composition, and insulin sensitivity in overweight children in a school-based exercise program: a randomized, controlled study. Arch Pediatr Adolesc Med. 2005;159(10):963-968. doi: 10.1001/archpedi.159.10.963 [DOI] [PubMed] [Google Scholar]
- 31.Chae HW, Kwon YN, Rhie YJ, et al. Effects of a structured exercise program on insulin resistance, inflammatory markers and physical fitness in obese Korean children. J Pediatr Endocrinol Metab. 2010;23(10):1065-1072. doi: 10.1515/jpem.2010.168 [DOI] [PubMed] [Google Scholar]
- 32.Corte de Araujo AC, Roschel H, Picanço AR, et al. Similar health benefits of endurance and high-intensity interval training in obese children. PLoS One. 2012;7(8):e42747. doi: 10.1371/journal.pone.0042747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dâmaso AR, da Silveira Campos RM, Caranti DA, et al. Aerobic plus resistance training was more effective in improving the visceral adiposity, metabolic profile and inflammatory markers than aerobic training in obese adolescents. J Sports Sci. 2014;32(15):1435-1445. doi: 10.1080/02640414.2014.900692 [DOI] [PubMed] [Google Scholar]
- 34.Davis JN, Gyllenhammer LE, Vanni AA, et al. Startup circuit training program reduces metabolic risk in Latino adolescents. Med Sci Sports Exerc. 2011;43(11):2195-2203. doi: 10.1249/MSS.0b013e31821f5d4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Lira CT, Dos Santos MA, Gomes PP, et al. Aerobic training performed at ventilatory threshold improves liver enzymes and lipid profile related to non-alcoholic fatty liver disease in adolescents with obesity. Nutr Health. 2017;23(4):281-288. doi: 10.1177/0260106017720350 [DOI] [PubMed] [Google Scholar]
- 36.de Souza F, da Silva LA, Ferreira GS, et al. Karate training improves metabolic health in overweight and obese adolescents: a randomized clinical trial. Pediatr Exerc Sci. 2022;34(2):108-118. doi: 10.1123/pes.2020-0193 [DOI] [PubMed] [Google Scholar]
- 37.Dias KA, Ingul CB, Tjønna AE, et al. Effect of high-intensity interval training on fitness, fat mass and cardiometabolic biomarkers in children with obesity: a randomised controlled trial. Sports Med. 2018;48(3):733-746. doi: 10.1007/s40279-017-0777-0 [DOI] [PubMed] [Google Scholar]
- 38.Farah BQ, Ritti-Dias RM, Balagopal PB, Hill JO, Prado WL. Does exercise intensity affect blood pressure and heart rate in obese adolescents? A 6-month multidisciplinary randomized intervention study. Pediatr Obes. 2014;9(2):111-120. doi: 10.1111/j.2047-6310.2012.00145.x [DOI] [PubMed] [Google Scholar]
- 39.Silva HJ, Andersen LB, Lofrano-Prado MC, et al. Improvements on cardiovascular diseases risk factors in obese adolescents: a randomized exercise intervention study. J Phys Act Health. 2015;12(4):553-560. doi: 10.1123/jpah.2013-0199 [DOI] [PubMed] [Google Scholar]
- 40.Faria WF, Mendonça FR, Santos GC, Kennedy SG, Elias RGM, Stabelini Neto A. Effects of 2 methods of combined training on cardiometabolic risk factors in adolescents: a randomized controlled trial. Pediatr Exerc Sci. 2020;32(4):217-226. doi: 10.1123/pes.2020-0016 [DOI] [PubMed] [Google Scholar]
- 41.Ferguson MA, Gutin B, Le NA, et al. Effects of exercise training and its cessation on components of the insulin resistance syndrome in obese children. Int J Obes Relat Metab Disord. 1999;23(8):889-895. doi: 10.1038/sj.ijo.0800968 [DOI] [PubMed] [Google Scholar]
- 42.Ghorbanian B, Ravassi A, Kordi MR, Hedayati M. The effects of rope training on lymphocyte ABCA1 expression, plasma ApoA-I and HDL-c in boy adolescents. Int J Endocrinol Metab. 2013;11(2):76-81. doi: 10.5812/ijem.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Ruíz K, Correa-Bautista JE, Izquierdo M, et al. Exercise dose on hepatic fat and cardiovascular health in adolescents with excess of adiposity. Pediatr Obes. 2022;17(4):e12869. doi: 10.1111/ijpo.12869 [DOI] [PubMed] [Google Scholar]
- 44.Grace J, Biggs C, Naicker A, Moss S. Effect of physical activity and nutrition education on body mass index, blood pressure and biochemical variables in overweight and obese adolescents. Ann Glob Health. 2021;87(1):9. doi: 10.5334/aogh.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon JY, Han J, Kim HJ, Park MS, Seo DY, Kwak YS. The combined effects of physical exercise training and detraining on adiponectin in overweight and obese children. Integr Med Res. 2013;2(4):145-150. doi: 10.1016/j.imr.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang HS, Gutin B, Barbeau P, et al. Physical training improves insulin resistance syndrome markers in obese adolescents. Med Sci Sports Exerc. 2002;34(12):1920-1927. doi: 10.1097/00005768-200212000-00010 [DOI] [PubMed] [Google Scholar]
- 47.Karacabey K. The effect of exercise on leptin, insulin, cortisol and lipid profiles in obese children. J Int Med Res. 2009;37(5):1472-1478. doi: 10.1177/147323000903700523 [DOI] [PubMed] [Google Scholar]
- 48.Kim ES, Im JA, Kim KC, et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring). 2007;15(12):3023-3030. doi: 10.1038/oby.2007.360 [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Son WM, Headid Iii RJ, Pekas EJ, Noble JM, Park SY. The effects of a 12-week jump rope exercise program on body composition, insulin sensitivity, and academic self-efficacy in obese adolescent girls. J Pediatr Endocrinol Metab. 2020;33(1):129-137. doi: 10.1515/jpem-2019-0327 [DOI] [PubMed] [Google Scholar]
- 50.Koot BGP, van der Baan-Slootweg OH, Vinke S, et al. Intensive lifestyle treatment for non-alcoholic fatty liver disease in children with severe obesity: inpatient versus ambulatory treatment. Int J Obes (Lond). 2016;40(1):51-57. doi: 10.1038/ijo.2015.175 [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab. 2013;305(10):E1222-E1229. doi: 10.1152/ajpendo.00285.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Libman I, Hughan K, et al. Effects of exercise modality on insulin resistance and ectopic fat in adolescents with overweight and obesity: a randomized clinical trial. J Pediatr. 2019;206:91-98.e1. doi: 10.1016/j.jpeds.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KJ, Shin YA, Lee KY, Jun TW, Song W. Aerobic exercise training-induced decrease in plasma visfatin and insulin resistance in obese female adolescents. Int J Sport Nutr Exerc Metab. 2010;20(4):275-281. doi: 10.1123/ijsnem.20.4.275 [DOI] [PubMed] [Google Scholar]
- 54.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012;61(11):2787-2795. doi: 10.2337/db12-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leite N, Tadiotto MC, Corazza PRP, et al. Responsiveness on metabolic syndrome criteria and hepatic parameters after 12 weeks and 24 weeks of multidisciplinary intervention in overweight adolescents. J Endocrinol Invest. 2022;45(4):741-752. doi: 10.1007/s40618-021-01699-x [DOI] [PubMed] [Google Scholar]
- 56.McCormack SE, McCarthy MA, Harrington SG, et al. Effects of exercise and lifestyle modification on fitness, insulin resistance, skeletal muscle oxidative phosphorylation and intramyocellular lipid content in obese children and adolescents. Pediatr Obes. 2014;9(4):281-291. doi: 10.1111/j.2047-6310.2013.00180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng C, Yucheng T, Shu L, Yu Z. Effects of school-based high-intensity interval training on body composition, cardiorespiratory fitness and cardiometabolic markers in adolescent boys with obesity: a randomized controlled trial. BMC Pediatr. 2022;22(1):112. doi: 10.1186/s12887-021-03079-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. J Am Coll Cardiol. 2006;48(9):1865-1870. doi: 10.1016/j.jacc.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 59.Murphy EC, Carson L, Neal W, Baylis C, Donley D, Yeater R. Effects of an exercise intervention using Dance Dance Revolution on endothelial function and other risk factors in overweight children. Int J Pediatr Obes. 2009;4(4):205-214. doi: 10.3109/17477160902846187 [DOI] [PubMed] [Google Scholar]
- 60.Park JH, Miyashita M, Kwon YC, et al. A 12-week after-school physical activity programme improves endothelial cell function in overweight and obese children: a randomised controlled study. BMC Pediatr. 2012;12:111. doi: 10.1186/1471-2431-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramezani A, Gaeini AA, Hosseini M, Mohammadi J, Mohammadi B. Effects of three methods of exercise training on cardiovascular risk factors in obese boys. Iran J Pediatr. 2017;27(5):e7145. doi: 10.5812/ijp.7145 [DOI] [Google Scholar]
- 62.Rasooli SA, Fathi R, Golzar FAK, Baghersalimi M. The effect of circuit resistance training on plasma levels of amino acids, alpha-hydroxybutyrate, mannose, and urinary levels of glycine conjugated adducts in obese adolescent boys. Appl Physiol Nutr Metab. 2021;46(6):561-570. doi: 10.1139/apnm-2020-0171 [DOI] [PubMed] [Google Scholar]
- 63.Rosenbaum M, Nonas C, Weil R, et al. ; Camino Diabetes Prevention Group . School-based intervention acutely improves insulin sensitivity and decreases inflammatory markers and body fatness in junior high school students. J Clin Endocrinol Metab. 2007;92(2):504-508. doi: 10.1210/jc.2006-1516 [DOI] [PubMed] [Google Scholar]
- 64.Salahshoornezhad S, Sohrabi Z, Mani A, et al. Effect of a multi-disciplinary program on anthropometric and biochemical parameters in obese and overweight elementary school girls: A randomized clinical trial. Nutr Metab Cardiovasc Dis. 2022;32(8):1982-1989. doi: 10.1016/j.numecd.2022.04.014 [DOI] [PubMed] [Google Scholar]
- 65.Savoye M, Caprio S, Dziura J, et al. Reversal of early abnormalities in glucose metabolism in obese youth: results of an intensive lifestyle randomized controlled trial. Diabetes Care. 2014;37(2):317-324. doi: 10.2337/dc13-1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo YG, Lim H, Kim Y, et al. The effect of a multidisciplinary lifestyle intervention on obesity status, body composition, physical fitness, and cardiometabolic risk markers in children and adolescents with obesity. Nutrients. 2019;11(1):137. doi: 10.3390/nu11010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shaibi GQ, Cruz ML, Ball GD, et al. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med Sci Sports Exerc. 2006;38(7):1208-1215. doi: 10.1249/01.mss.0000227304.88406.0f [DOI] [PubMed] [Google Scholar]
- 68.Son WM, Sung KD, Bharath LP, Choi KJ, Park SY. Combined exercise training reduces blood pressure, arterial stiffness, and insulin resistance in obese prehypertensive adolescent girls. Clin Exp Hypertens. 2017;39(6):546-552. doi: 10.1080/10641963.2017.1288742 [DOI] [PubMed] [Google Scholar]
- 69.Sun MX, Huang XQ, Yan Y, et al. One-hour after-school exercise ameliorates central adiposity and lipids in overweight Chinese adolescents: a randomized controlled trial. Chin Med J (Engl). 2011;124(3):323-329. [PubMed] [Google Scholar]
- 70.Tjønna AE, Stølen TO, Bye A, et al. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clin Sci (Lond). 2009;116(4):317-326. doi: 10.1042/CS20080249 [DOI] [PubMed] [Google Scholar]
- 71.Vasconcellos F, Seabra A, Cunha F, et al. Health markers in obese adolescents improved by a 12-week recreational soccer program: a randomised controlled trial. J Sports Sci. 2016;34(6):564-575. doi: 10.1080/02640414.2015.1064150 [DOI] [PubMed] [Google Scholar]
- 72.Wong PC, Chia MY, Tsou IY, et al. Effects of a 12-week exercise training programme on aerobic fitness, body composition, blood lipids and C-reactive protein in adolescents with obesity. Ann Acad Med Singap. 2008;37(4):286-293. doi: 10.47102/annals-acadmedsg.V37N4p286 [DOI] [PubMed] [Google Scholar]
- 73.Wong A, Sanchez-Gonzalez MA, Son WM, Kwak YS, Park SY. The effects of a 12-week combined exercise training program on arterial stiffness, vasoactive substances, inflammatory markers, metabolic profile, and body composition in obese adolescent girls. Pediatr Exerc Sci. 2018;30(4):480-486. doi: 10.1123/pes.2017-0198 [DOI] [PubMed] [Google Scholar]
- 74.Yetgin MK, Agopyan A, Küçükler FK, et al. The effects of resistance and aerobic exercises on adiponectin, insulin resistance, lipid profile and body composition in adolescent boys with obesity. Istanbul Medical Journal. 2020;21(3):182-189. doi: 10.4274/imj.galenos.2020.55938 [DOI] [Google Scholar]
- 75.Zehsaz F, Farhangi N, Ghahramani M. The response of circulating omentin-1 concentration to 16-week exercise training in male children with obesity. Phys Sportsmed. 2016;44(4):355-361. doi: 10.1080/00913847.2016.1248223 [DOI] [PubMed] [Google Scholar]
- 76.Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004;145(6):731-736. doi: 10.1016/j.jpeds.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 77.Davis CL, Litwin SE, Pollock NK, et al. Exercise effects on arterial stiffness and heart health in children with excess weight: the SMART RCT. Int J Obes (Lond). 2020;44(5):1152-1163. doi: 10.1038/s41366-019-0482-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farpour-Lambert NJ, Aggoun Y, Marchand LM, Martin XE, Herrmann FR, Beghetti M. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54(25):2396-2406. doi: 10.1016/j.jacc.2009.08.030 [DOI] [PubMed] [Google Scholar]
- 79.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500-e503. doi: 10.1542/peds.2004-1921 [DOI] [PubMed] [Google Scholar]
- 80.Giannini C, Weiss R, Cali A, et al. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes. 2012;61(3):606-614. doi: 10.2337/db11-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Henderson M, Rabasa-Lhoret R, Bastard JP, et al. Measuring insulin sensitivity in youth: how do the different indices compare with the gold-standard method? Diabetes Metab. 2011;37(1):72-78. doi: 10.1016/j.diabet.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 82.Islam H, Gillen JB. Skeletal muscle mechanisms contributing to improved glycemic control following intense interval exercise and training. Sports Med Health Sci. 2023;5(1):20-28. doi: 10.1016/j.smhs.2023.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Larson-Meyer DE, Newcomer BR, Ravussin E, et al. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia. 2011;54(4):869-875. doi: 10.1007/s00125-010-2022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kjaer M, Kiens B, Hargreaves M, Richter EA. Influence of active muscle mass on glucose homeostasis during exercise in humans. J Appl Physiol (1985). 1991;71(2):552-557. doi: 10.1152/jappl.1991.71.2.552 [DOI] [PubMed] [Google Scholar]
- 85.Joseph AM, Hood DA. Relationships between exercise, mitochondrial biogenesis and type 2 diabetes. Med Sport Sci. 2014;60:48-61. doi: 10.1159/000357335 [DOI] [PubMed] [Google Scholar]
- 86.Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care. 2004;27(2):629-630. doi: 10.2337/diacare.27.2.629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Electronic Search Strategy
eTable 1. Table of Characteristics
eMethods 2. Data Analysis
eMethods 3. Excluded Studies and Reasons for Exclusion
eTable 2. Results of the Cochrane Risk-of-Bias Tool for Randomized Controlled Trials (RoB-2)
eFigure 1. Effect of Exercise Programs on Fasting Insulin in Children and Adolescents With Excess Weight
eFigure 2. Effect of Exercise Programs on Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) in Children and Adolescents With Excess Weight
eFigure 3. Effect of Exercise Programs on Fasting Glucose in Children and Adolescents With Excess Weight
eFigure 4. Effect of Exercise Programs on 2-Hour Oral Glucose Tolerance in Children and Adolescents With Excess Weight
eFigure 5. Effect of Exercise Programs on Glycated Hemoglobin in Children and Adolescents With Excess Weight
eFigure 6. Sensitivity Analyses Once Each Study Was Excluded for Fasting Insulin
eFigure 7. Sensitivity Analyses Once Each Study Was Excluded for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
eFigure 8. Sensitivity Analyses Once Each Study Was Excluded for 2-Hour Oral Glucose Tolerance
eFigure 9. Sensitivity Analyses Once Each Study Was Excluded for Fasting Glucose
eFigure 10. Dose-Response Association Between Metabolic Equivalent of Task Minutes (MET-min) per Week and Differences in Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) in Children and Adolescents With Excess Weight and Insulin Resistance (ie, HOMA-IR≥3.16)
eFigure 11. Dose-Response Association Between Metabolic Equivalent of Task Minutes (MET-min) per Week and Differences in Fasting Glucose in Children and Adolescents With Excess Weight
eFigure 12. Rankogram for Each Type of Physical Exercise
eFigure 13. Network Meta-Analysis Confidence Rating for Glucose, Insulin, and Homeostatic Model Assessment for Insulin Resistance Outcomes
eReferences
Data Sharing Statement