Abstract
Background
Adverse drug reaction (ADR) related to angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) may negatively affect patients’ treatment outcomes.
Aim
To investigate the impact of ACEIs/ARBs-related ADR consultation on cardiovascular disease (CVD) events and all-cause mortality.
Design and setting
Propensity score-matched cohort study of ACEIs/ARBs between 2004 and 2019 using UK IQVIA medical research data.
Method
ADR consultations were identified using standardised designated codes. Propensity scores were calculated based on comorbidities, concomitant medications, frailty, and polypharmacy. Cox’s proportional hazard regression model was used to compare the outcomes between patients in ADR and non-ADR groups. In the secondary analysis, treatment- pattern changes following the ADR were examined and the subsequent outcomes were compared.
Results
Among 1 471 906 eligible users of ACEIs/ARBs, 13 652 (0.93%) patients had ACEIs/ARBs- related ADR consultation in primary care. Patients with ACEIs/ARBs-related ADR consultation had an increased risk of subsequent CVD events and all- cause mortality in both primary prevention (CVD events: adjusted hazard ratio [aHR] 1.22, 95% confidence interval [CI] = 1.05 to 1.43; all-cause mortality: aHR 1.14, 95% CI = 1.01 to 1.27) and secondary prevention cohorts (CVD events: aHR 1.13, 95% CI = 1.05 to 1.21; all-cause mortality: aHR 1.15, 95% CI = 1.09 to 1.21). Half (50.19%) of patients with ADR continued to use ACEIs/ARBs, and these patients had a reduced risk of mortality (aHR 0.88, 95% CI = 0.82 to 0.95) compared with those who discontinued using ACEIs/ARBs.
Conclusion
This study provides information on the burden of ADR on patients and the health system. The findings call for additional monitoring and treatment strategies for patients affected by ADR to mitigate the risks of adverse clinical outcomes.
Keywords: adverse drug reaction, drug-related side effects and adverse reactions, primary health care
INTRODUCTION
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are two renin- angiotensin- aldosterone system inhibitors (RAASIs) that are among the most frequently prescribed drugs worldwide.1–3 These medications are commonly indicated for a number of conditions, including hypertension, chronic kidney diseases (CKDs), and heart failure (HF).4–9 Treatment with ACEIs/ARBs has been shown to reduce morbidity and mortality.10–12
Previous studies have reported that up to 3.9% of users of ACEIs/ARBs may develop adverse drug reactions (ADRs), including persistent dry cough, hyperkalaemia, dizziness, hypotension, gastrointestinal symptoms, palpitation, excessive urination, and angioedema.13,14 A UK-based study by Tsang et al found that ACEIs were among the most common drug class involved in ADRs in primary care.15 Risk of ACEIs/ARBs-related ADR increased with dual RAASI combinations, history of smoking, progression of CKD stages, hypoaldosteronism, and the use of concomitant medication, such as other antihypertensive drugs, non-steroidal anti- inflammatory drugs (NSAIDs), heparin, and immunosuppressants.16–19
In addition to direct physiological impact, ADRs may have negative consequences on patients’ treatment outcomes.20 Previous studies have shown that up to one-third of patients with hypertension had their treatment reduced and/or interrupted owing to ADRs, which precluded treatment options to achieve their blood pressure target.14,21 Clinical guidelines indicate that, depending on the severity of the reactions and underlying comorbidities, management of ACEIs/ARBs- related ADR may vary between patients, including: altering the dosage regimen; switching between ACEIs/ARBs and/or other drug classes; and ascertaining the necessary monitoring, for example, that of renal function and electrolytes.22,23
How this fits in
| Adverse drug reactions (ADRs) represent a considerable burden for patients and the healthcare system. ADRs related to angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) were among the most frequent ADRs documented in primary care records; however, there is limited information on the impact of ACEI/ARB-related ADRs on patient outcomes and changes to treatment patterns post-ADR in this setting. This study found that ACEI/ARB-related ADR consultations were associated with subsequent major cardiovascular events and all-cause mortality, indicating that the affected patients should be monitored more closely by healthcare professionals to mitigate the risk of adverse clinical outcomes. |
There is limited information on the impact of ACEIs/ARBs-related ADR on patients’ clinical outcomes. Such findings may help to improve understanding of the burden of ADRs, and better inform patient care and monitoring for individuals at high risk of untoward clinical outcomes. The objectives of this study were to examine the impact of ACEIs/ARBs-related ADR consultation on subsequent cardiovascular disease (CVD) events and all-cause mortality, and investigate treatment-pattern changes following these ADRs in UK primary care settings.
METHOD
Data source
This study was conducted using IQVIA Medical Research Data UK that incorporates data from The Health Improvement Network (THIN).24 The data contains de-identified information provided by patients as part of their routine primary care. UK primary care databases have been used previously to investigate ADR-related consultations.15,25–27
Study design
This cohort study included patients who used ACEIs/ARBs between 2004 and 2019. Patients were excluded if they:
had a missing date of birth or sex;
were aged <18 years at the date of first ACEI/ARB prescription;
had an ACEI/ARB-related ADR before 2004;
were registered <1 year before the index date; or
had a history of cancer.
As a history of CVD increases the risk of recurrent CVD events and mortality, the analysis was stratified based on CVD primary prevention and secondary prevention — that is, without and with a history of CVD, respectively. CVD was defined as coronary heart disease (angina and myocardial infarction), cerebrovascular disease (stroke and transient ischaemic attack [TIA]), and peripheral arterial disease. Patients with HF were included in the secondary prevention because of their level of risk being equivalent to that of people with an established CVD.28,29 The study design is presented in Supplementary Figure S1.
Exposed cohort
The exposed cohort comprised patients with an ACEI/ARB-related ADR consultation in primary care. ‘ACEI/ARB- related ADR consultation’ was defined using standardised designated codes, for example, Read code chapter TJ (adverse drug reactions), as previously examined.15,25–27 As this study used designated codes specific to ACEIs/ARBs- related ADR consultation, it was estimated that the ADR consultation was attributed to ACEI/ARB therapy. The index date was defined as the date of the first ACEI/ARB-related ADR consultation (see Supplementary Box S1).
Control cohort
The control cohort comprised users of ACEIs/ARBs, who did not have an ACEI/ARB- related ADR consultation in primary care. To generate a control cohort, an index date was assigned at random to a sample of 30% of unexposed patients — that is, those without an ACEI/ARB-related ADR — by incidence density sampling from the distribution of index dates in the exposed cohort.30 After excluding patients who died or transferred before, or at, the index date, or had been registered for <1 year, or had history of cancer, propensity score matching (1:1) was used to select the control group using the greedy matching algorithm.31 Patients with a history of any cancer were excluded, as cancers negatively affect survival.
Covariates
The covariates measured were:
age;
sex;
interval between the ACEI/ARB initiation date and the index date;
comorbidities: hypertension, CKD, type 1 and type 2 diabetes mellitus, dyslipidaemia, chronic liver disease, chronic obstructive pulmonary disease, and rheumatic disease — recorded at any time before, or on, the index date;
use of concomitant medications: calcium channel blockers (CCBs), diuretics, beta-blockers, statins, antiplatelets/anticoagulants, antidiabetics, nitrates, and NSAIDs — recorded ≤180 days before the index date;
electronic frailty index (eFi), comprising 36 health conditions as developed and validated by Clegg et al;32 and
polypharmacy.
Frailty index categories were: fit, mild, moderate, and severe frailty. Polypharmacy was defined as the use of between five and nine medications; excessive polypharmacy was defined as the use of ≥10 medications.33
Propensity score
The propensity score was defined as the probability of receiving the exposure (ACEI/ARB-related ADR), which was estimated using a logistic regression model based on all covariates at baseline.34 It was used to control for confounding due to non-randomised exposure allocation, by generating a comparable distribution of measured covariates across exposed and control groups. In the matched sample, the balance of covariates was assessed using standardised mean difference (SMD). An SMD of <0.2 indicated a negligible difference in covariates between both groups.35
Outcomes and follow-up period
The primary outcome was the first composite CVD events (myocardial infarction and stroke/TIA), and the secondary outcome was all-cause mortality. The follow-up for each patient commenced from the date of ACEI/ARB-related ADR or the index date until the occurrence of the outcome or any censoring event (patient transferred out, death, study end date), whichever was earlier.
Secondary, subgroup, and sensitivity analysis
In the secondary analysis, treatment-pattern changes within 12 months following the ADR consultation were examined and the subsequent outcomes were compared. The continued ACEI/ARB prescription was defined as any prescription within 12 months after the ADR consultation, as used in a previous study examining continued drug prescription following ADRs.36 Patients who died, transferred, had their last day of follow-up, or CVD events within 1 year after the ADR date were excluded to reduce immortal time bias. The eligible patients were classified as:
continued ACEIs/ARBs — either continuing the current treatment or switching to another ACEI/ARB; or
discontinued ACEIs/ARBs.
The subsequent outcomes were compared between those who continued and discontinued using ACEIs/ARBs following the ADR using stabilised inverse probability of treatment weighting (IPTW), with the propensity score estimated from all covariates (as in the main analysis). The follow-up commenced from 12 months following the ADR until the earliest of the following: outcome of interest, patient transferred out, death, or study end date. A competing risk analysis was performed using Fine–Gray’s subdistribution hazard model.37
Subgroup analyses were performed separately based on different indications for ACEIs/ARBs — that is, hypertension, CKD, and HF. A sensitivity analysis using stabilised IPTW was conducted for the primary analysis. The window period to examine treatment changes in the secondary analysis was adjusted from 12 to 6 months to evaluate robustness.26
As UK clinical guidelines consider ethnicity differences for the selection of antihypertensive drugs, including ACEIs/ARBs, a separate sensitivity analysis was conducted for those patients for whom ethnicity data were available. An additional analysis among those who continued using ACEIs/ARBs in the ADR group versus the control group was conducted to examine whether the continuation of ACEIs/ARBs affected the outcomes.
Statistical analyses
Baseline characteristics were expressed as frequencies and percentages for categorical variables, and as means with standard deviations (SDs) for continuous variables. Cox’s proportional hazard regression model and the Kaplan–Meier method were used to estimate the risk of CVD events and all-cause mortality. The results were presented as adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs). A two- sided P-value <0.05 was considered statistically significant. Analyses were performed using SAS (version 9.4).
RESULTS
Baseline characteristics
During the study period (2004–2019), 1 513 241 users of ACEIs/ARBs were identified; after exclusion, 1 471 906 patients were eligible to be included in the analysis. Of these, 13 652 (0.93%) patients had an ACEI/ARB-related ADR consultation in primary care. The flowchart of the participant selection process is shown in Supplementary Figure S2.
The mean ages were 68.11 years (SD 13.28) and 74.58 years (SD 10.91) for the CVD primary (n = 6196) and secondary (n = 14 238) prevention cohorts, respectively (Table 1). After matching, the SMD of all covariates was <0.2, indicating comparability between the ADR consultation and non-ADR consultation groups in both the primary and secondary prevention cohorts. The baseline characteristics before and after propensity score matching are given in Supplementary Table S1.
Table 1.
Baseline characteristics of the participants
| Characteristics | CVD primary prevention matched cohort, n (%)a | CVD secondary prevention matched cohort, n (%)a | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| With ACEI/ARB-related ADR consultation | Without ACEI/ARB-related ADR consultation | SMDb | With ACEI/ARB-related ADR consultation | Without ACEI/ARB-related ADR consultation | SMDb | |
| Total | 3098 | 3098 | 7119 | 7119 | ||
|
| ||||||
| Mean age, years (±SD) | 68.11 (13.38) | 68.11 (13.18) | −0.0006 | 74.58 (10.96) | 74.59 (10.86) | −0.0008 |
|
| ||||||
| Sex, male | 1210 (39.06) | 1177 (37.99) | 0.0219 | 3712 (52.14) | 3741 (52.55) | −0.0082 |
|
| ||||||
| Mean interval between commencement of ACEI/ARB therapy and index date, years (±SD) | 3.60 (3.80) | 3.60 (2.98) | −0.0003 | 5.04 (4.41) | 4.98 (3.74) | 0.0161 |
|
| ||||||
| Comorbidities | ||||||
| Hypertension | 2728 (88.06) | 2739 (88.41) | −0.0110 | 5038 (70.77) | 5019 (70.50) | 0.0059 |
| Dyslipidaemia | 528 (17.04) | 508 (16.40) | 0.0173 | 1809 (25.41) | 1796 (25.23) | 0.0042 |
| Diabetes | 1367 (44.13) | 1393 (44.96) | −0.0169 | 2462 (34.58) | 2444 (34.33) | 0.0053 |
| CKD | 1333 (43.03) | 1301 (41.99) | 0.0209 | 2826 (39.70) | 2808 (39.44) | 0.0052 |
| Liver disease | 44 (1.42) | 49 (1.58) | −0.0133 | 74 (1.04) | 77 (1.08) | −0.0041 |
| COPD | 287 (9.26) | 287 (9.26) | 0.0000 | 1280 (17.98) | 1266 (17.78) | 0.0051 |
| Rheumatic disease | 360 (11.62) | 348 (11.23) | 0.0122 | 1263 (17.74) | 1272 (17.87) | −0.0033 |
|
| ||||||
| Concomitant medications | ||||||
| CCBs | 1268 (40.93) | 1254 (40.48) | 0.0092 | 2432 (34.16) | 2453 (34.46) | −0.0062 |
| Diuretics | 1534 (49.52) | 1517 (48.97) | 0.0110 | 4221 (59.29) | 4166 (58.52) | 0.0157 |
| Beta-blockers | 790 (25.50) | 814 (26.28) | −0.0177 | 3533 (49.63) | 3588 (50.40) | −0.0155 |
| Statins | 1342 (43.32) | 1298 (41.90) | 0.0287 | 5145 (72.27) | 5199 (73.03) | −0.0170 |
| Antiplatelets/anticoagulants | 974 (31.44) | 971 (31.34) | 0.0021 | 5425 (76.20) | 5464 (76.75) | −0.0129 |
| Antidiabetics | 1002 (32.34) | 1036 (33.44) | −0.0234 | 1691 (23.75) | 1685 (23.67) | 0.0020 |
| Nitrates | 47 (1.52) | 39 (1.26) | 0.0221 | 2402 (33.74) | 2420 (33.99) | −0.0053 |
| NSAIDs | 427 (13.78) | 432 (13.94) | −0.0047 | 747 (10.49) | 730 (10.25) | 0.0078 |
|
| ||||||
| Electronic Frailty Index | 0.0509 | 0.1089 | ||||
| Fit | 2090 (67.46) | 2075 (66.98) | 2438 (34.25) | 2304 (32.36) | ||
| Mild | 843 (27.21) | 882 (28.47) | 2928 (41.13) | 3263 (45.84) | ||
| Moderate | 146 (4.71) | 133 (4.29) | 1403 (19.71) | 1245 (17.49) | ||
| Severe | 19 (0.61) | 8 (0.26) | 350 (4.92) | 307 (4.31) | ||
|
| ||||||
| Polypharmacy | 0.0241 | 0.0360 | ||||
| No polypharmacy | 814 (26.28) | 816 (26.34) | 715 (10.04) | 664 (9.33) | ||
| Polypharmacy (5–9 medications) | 1453 (46.90) | 1421 (45.87) | 2908 (40.85) | 2996 (42.08) | ||
| Excessive polypharmacy (≥10 medications) | 831 (26.82) | 861 (27.79) | 3496 (49.11) | 3459 (48.59) | ||
Unless otherwise stated.
SMD indicates difference in mean or proportion of covariates in the exposed versus control group, divided by the pooled standard deviation. SMD <0.2 indicates a negligible difference in covariates between both groups. ACEI = angiotensin-converting enzyme inhibitor. ADR = adverse drug reaction. ARB = angiotensin receptor blocker. CCB = calcium channel blocker. CKD = chronic kidney disease. COPD = chronic obstructive pulmonary disease. CVD = cardiovascular disease. NSAID = non-steroidal anti-inflammatory drug. SD = standard deviation. SMD = standardised mean difference.
ACEI/ARB-related ADRs, and the risk of a CVD event and all-cause mortality
CVD primary prevention cohort
During the mean follow-up time of 6.57 years (SD 3.96), 648 patients had CVD events: 366/3098 (11.81%) were in the ADR consultation group and 282/3098 (9.10%) were in the control group. Cox regression analysis showed that patients with an ACEI/ARB-related ADR had an increased risk of subsequent CVD event compared with users of ACEIs/ARBs without an ADR (aHR 1.22, 95% CI = 1.05 to 1.43) (Table 2). Similar results were observed for the secondary outcome, all-cause mortality. During the mean follow-up time of 6.93 years (SD 3.96), there were 1196 deaths; of these, 659 (21.27%) were in the ADR consultation group and 537 (17.33%) were in the control group. ACEI/ARB- related ADRs increased the risk of all- cause mortality (aHR 1.14, 95% CI = 1.01 to 1.27) (Table 2).
Table 2.
Adjusted hazard ratio and incidence rate per 1000 person–years (95% CI) for CVD events and all- cause mortality in CVD primary and secondary prevention cohorts
| Outcome | CVD primary prevention cohort | CVD secondary prevention cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADR group, N = 3098 | Control group, N = 3098 | ADR group, N = 7119 | Control group, N = 7119 | |||||||
| Event, n (%) | Incidence rate (95% CI) | Event, n (%) | Incidence rate (95% CI) | aHR (95% CI) | Event, n (%) | Incidence rate (95% CI) | Event, n (%) | Incidence rate (95% CI) | aHR (95% CI) | |
| Primary outcome | ||||||||||
| Composite CVD events | 366 (11.81) | 17.46 (15.76 to 19.34) | 282 (9.10) | 14.27 (12.70 to 16.04) | 1.22 (1.05 to 1.43) | 1574 (22.11) | 46.07 (43.85 to 48.41) | 1431 (20.10) | 41.12 (39.05 to 43.31) | 1.13 (1.05 to 1.21) |
| Myocardial infarction | 140 (4.52) | 6.43 (5.44 to 7.58) | 98 (3.16) | 4.81 (3.95 to 5.86) | 1.33 (1.03 to 1.72) | 667 (9.37) | 17.78 (16.42 to 19.26) | 510 (7.16) | 12.62 (11.49 to 13.86) | 1.32 (1.18 to 1.48) |
| Stroke/TIA | 250 (8.07) | 11.71 (10.34 to 13.26) | 198 (6.39) | 9.85 (8.57 to 11.32) | 1.19 (0.98 to 1.43) | 1041 (14.62) | 28.87 (27.17 to 30.68) | 1016 (14.27) | 28.13 (26.45 to 29.91) | 1.04 (0.95 to 1.13) |
| Secondary outcome | ||||||||||
| All-cause mortality | 659 (21.27) | 29.65 (27.47 to 32.00) | 537 (17.33) | 25.90 (23.80 to 28.19) | 1.14 (1.01 to 1.27) | 2792 (39.22) | 69.53 (67.00 to 72.16) | 2416 (33.94) | 60.38 (58.02 to 62.84) | 1.15 (1.09 to 1.21) |
ADR = adverse drug reaction. aHR = adjusted hazard ratio. CVD = cardiovascular disease. TIA = transient ischaemic attack.
CVD secondary prevention cohort
During the mean follow-up time of 4.84 years (SD 3.84), 3005 patients had recurrent CVD events in the secondary prevention cohort; 1574/7119 (22.11%) of these occurred in the ADR group and 1431/7119 (20.10%) in the control group. ACEI/ARB-related ADRs were associated with an increased risk of recurrent CVD events (aHR 1.13, 95% CI = 1.05 to 1.21) (Table 2).
Similarly, for the mortality outcome, during the mean follow-up time of 5.63 years (SD 3.95), there were 5208 deaths: 2792 (39.22%) and 2416 (33.94%) in the ADR and control groups, respectively. ACEI/ARB-related ADRs increased the risk of all-cause mortality (aHR 1.15, 95% CI = 1.09 to 1.21) (Table 2 and Supplementary Figure S3).
Subgroup analysis among patients with hypertension, CKD, and HF
Patients with hypertension who had ACEI/ARB-related ADRs had an increased risk of subsequent CVD events (aHR 1.13, 95% CI = 1.05 to 1.21) and all-cause mortality (aHR 1.16, 95% CI = 1.09 to 1.22). Consistent findings were observed among patients with CKD; ACEI/ARB-related ADRs were associated with CVD events (aHR 1.35, 95% CI = 1.22 to 1.50) and all-cause mortality (aHR 1.24, 95% CI = 1.16 to 1.33). Patients with HF had the highest incidence rates of CVD events and all-cause mortality when compared with the hypertension and CKD populations; however, patients with HF and ACEI/ARB-related ADRs had a similar risk of CVD events (aHR 1.12, 95% CI = 0.98 to 1.28), but increased risk of all-cause mortality (aHR 1.16, 95% CI = 1.07 to 1.25) compared with those without ADRs (Table 3 and Figure 1).
Table 3.
Subgroup analysis across different indications for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers
| Outcomes | ADR group | Control group | aHR (95% CI) | ||
|---|---|---|---|---|---|
| Event, n (%) | Incidence rate (95% CI) | Event, n (%) | Incidence rate (95% CI) | ||
| Patients with hypertension (n = 141 151)a | |||||
| Composite CVD events | 1494 (19.28) | 35.44 (33.69 to 37.29) | 1334 (17.21) | 31.67 (30.01 to 33.41) | 1.13 (1.05 to 1.21) |
| All-cause mortality | 2629 (33.92) | 55.18 (53.11 to 57.33) | 2217 (28.61) | 47.48 (45.54 to 49.50) | 1.16 (1.09 to 1.22) |
| Patients with CKD (n = 30 028)b | |||||
| Composite CVD events | 826 (19.82) | 47.49 (44.36 to 50.84) | 645 (15.48) | 35.03 (32.43 to 37.84) | 1.35 (1.22 to 1.50) |
| All-cause mortality | 1712 (41.07) | 85.65 (81.69 to 89.80) | 1404 (33.69) | 68.95 (65.44 to 72.65) | 1.24 (1.16 to 1.33) |
| Patients with HF (n = 12 646)c | |||||
| Composite CVD events | 438 (17.63) | 49.86 (45.41 to 54.76) | 420 (16.90) | 44.37 (40.32 to 48.82) | 1.12 (0.98 to 1.28) |
| All-cause mortality | 1327 (53.40) | 132.05 (125.13 to 139.35) | 1222 (49.18) | 113.91 (107.69 to 120.48) | 1.16 (1.07 to 1.25) |
Before propensity score matching: ADR group n = 7801, control group n = 132 683; after propensity score matching: ADR group n = 7750, control group n = 7750.
Before propensity score matching: ADR group n = 4223, control group n = 26 609; after propensity score matching: ADR group n = 4168, control group n = 4168.
Before propensity score matching: ADR group n = 2544, control group n = 10 102; after propensity score matching: ADR group n = 2485, control group n = 2485. ADR = adverse drug reaction. aHR = adjusted hazard ratio. CKD = chronic kidney disease. CVD = cardiovascular disease. HF = heart failure. RAAS = renin-angiotensin-aldosterone system.
Figure 1.
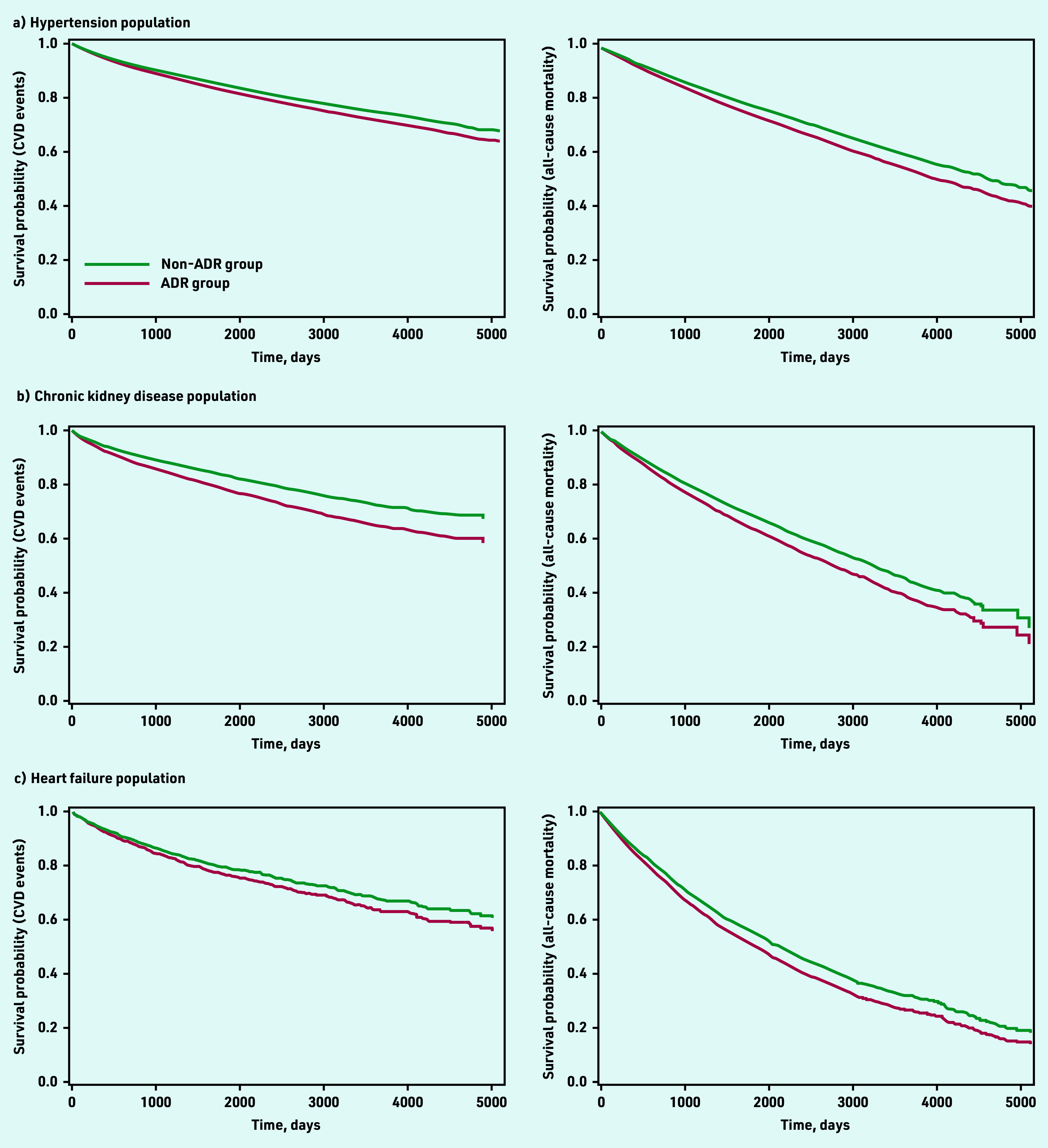
Kaplan-Meier survival curves comparing survival probability relating to CVD outcomes and all- cause mortality between ADR and non-ADR groups across different indications for ACEIs/ARBs: a) hypertension population; b) chronic kidney disease population; and c) heart failure population. ACEI = angiotensin-converting enzyme inhibitor. ADR = adverse drug reaction. ARB = angiotensin receptor blocker. CVD = cardiovascular disease.
Treatment-pattern changes following ACEI/ARB-related ADRs
Treatment-pattern changes in the year following the ADR are summarised in Table 4. Half (n = 4333, 50.19%) of the patients continued using ACEIs/ARBs, including switching from ACEIs to ARBs (n = 2228, 51.42%), continuing ACEIs (n = 921, 21.26%), continuing ARBs (n = 204, 4.71%), and switching from ACEI and ARB combinations to single ACEI/ARB drugs (n = 980, 22.62%). Overall, 13.17% of patients with ADRs used dual ACEI/ARB combinations, compared with only 1.22% of patients without ADRs on dual ACEI/ARB combinations. Most (n = 980, 86.19%) of them switched to single ACEI/ARB, with or without other antihypertensive drugs. The remaining half of the patients (n = 4301, 49.81%) discontinued ACEI/ARB use; the majority (n = 3695, 85.91%) were prescribed other antihypertensive drugs only, and a small percentage (n = 606, 14.09%) discontinued all antihypertensive drugs altogether. Cox regression analysis showed that the continued use of ACEIs/ARBs following ADR consultations did not lower the risk of CVD events (aHR 0.95, 95% CI = 0.85 to 1.05), but reduced the risk of all- cause mortality (aHR 0.88, 95% CI = 0.82 to 0.95) compared with the discontinuation of ACEIs/ARBs (Supplementary Table S2).
Table 4.
Treatment-pattern changes following ADRs relating to use of ACEIs/ARBs
| Treatment changes following ACEI/ARB-related ADR | Total, n (%) | CVD primary prevention, n (%) | CVD secondary prevention, n (%) |
|---|---|---|---|
| Total | 8634 | 2889 | 5745 |
|
| |||
| Continued RAASI therapy | 4333 (50.19) | 1679 (58.12) | 2654 (46.20) |
| Switching from ACEI to ARB | 2228 (51.42) | 864 (51.46) | 1364 (51.39) |
| Continuing on ACEI | 921 (21.26) | 232 (13.82) | 689 (25.96) |
| Continuing on ARB | 204 (4.71) | 81 (4.82) | 123 (4.63) |
| Switching from dual RAASI combination to single RAASI drug | 980 (22.62) | 502 (29.90) | 478 (18.01) |
|
| |||
| Cessation of RAASI therapy | 4301 (49.81) | 1210 (41.88) | 3091 (53.80) |
| Using other types of antihypertensive drugs only | 3695 (85.91) | 989 (81.74) | 2706 (87.54) |
| Cessation of all antihypertensive drugs | 606 (14.09) | 221 (18.26) | 385 (12.46) |
ACEI = angiotensin-converting enzyme inhibitor. ADR = adverse drug reaction. ARB = angiotensin receptor blocker. CVD = cardiovascular disease. RAASI = renin-angiotensin-aldosterone system inhibitor.
Sensitivity analysis
Similar findings were observed using the IPTW method, with ACEI/ARB-related ADRs being associated with an increased risk of subsequent CVD events and all-cause mortality in both primary and secondary prevention cohorts (Supplementary Table S3). In the secondary analysis, consistent results were observed when the window period was adjusted from 12 to 6 months: continued RAASI therapy was associated with reduced risk of all-cause mortality (aHR 0.88, 95% CI = 0.82 to 0.95) (Supplementary Table S4). Consistent findings were also observed among patients with complete ethnicity data (n = 68 591, 41.60%) (Supplementary Table S5). Patients with ADRs who continued using ACEIs/ARBs had an increased risk of CVD events compared with those who continued using ACEIs/ARBs and did not have ADRs (Supplementary Table S6).
DISCUSSION
Summary
Using longitudinal primary care medical records from 2004 to 2019, it was found that patients with ACEI/ARB-related ADR consultations had an increased risk of subsequent CVD events and all-cause mortality. The finding was relatively consistent across CVD history and different indications for ACEI/ARB use. In addition, it was found that the discontinuation of ACEIs/ARBs following an ADR was associated with an increased risk of all-cause mortality.
Strengths and limitations
To the authors’ knowledge, this is the first study to examinine the impact of ACEI/ARB-related ADR consultations on patients’ outcomes in UK primary care. A thorough analysis was conducted with stratification based on CVD history and across different indications for ACEI/ARB use. In addition, treatment-pattern changes following the ADRs were examined, which might help to improve understanding of how ADRs are managed in a real-world setting and ascertain whether practice complies with treatment guidelines.
However, the study has several limitations. ADR-related consultations in primary care, which were identified using standardised designated codes, were used as a proxy for the ADRs. ACEI/ARB-related ADRs were observed in ∼1% of users of ACEIs/ARBs, which is a lower rate than that of previous studies (up to 3.9%);13,14 this may be due to variability in ADR assessment and/or recording.26,27 In addition, the severity of ADRs addressed in the consultations could not be identified, which might have affected the decision to continue or discontinue the medication. However, a previous systematic review estimated that the majority of ADRs in the primary care setting were of mild- to-moderate severity, compared with those requiring urgent medical care or hospitalisation.38 A relatively long interval was also found between the ACEI/ARB initiation date and the ADR dates in the present study, and, as such, it is possible that the ADRs occurred after an increase in ACEI/ARB dose. Nevertheless, the authors were unable to capture the dose relationship data in the study.
Comparison with existing literature
Previous studies have reported that ADRs related to other cardiovascular drugs increased the risk of adverse cardiovascular outcomes.20,26 In addition, a study by Albani et al, which focused on patients with a history of acute coronary syndrome, showed that the intolerance to medications used for secondary CVD prevention, including ACEIs/ARBs, was independently associated with recurrent CVD events;39 this is consistent with the findings of the study presented here. Schmidt et al showed that elevated creatinine levels of ≥30% following ACEI/ARB use were associated with CVD events, mortality, and end- stage renal diseases.40 This echoes the findings of the current study, which indicate that closer monitoring for patients with potential ADRs is needed.
The findings of the present study show that half of the patients who experienced an ADR continued using ACEIs/ARBs and had a reduced risk of all-cause mortality compared with those who discontinued using ACEIs/ARBs. Several studies have examined the impact of ACEI/ARB discontinuation following a specific ADR.41–44 Leon et al showed that discontinuation of ACEI/ARB after hyperkalaemia was associated with an increased risk of mortality.43 Using target trial emulation, Xu et al also found that hyperkalaemia- related discontinuation was associated with an increased risk of adverse clinical outcomes, with the absolute risk difference for mortality being twice as high as that of CVD events.44 The decision to continue or discontinue ACEIs/ARBs following ADRs should be considered based on each patient’s circumstances. Additional treatment strategies are of importance to facilitate continued ACEI/ARB use following hyperkalaemia, and may include adequate monitoring, careful dosing, and the use of novel potassium binders, such as sodium zirconium cyclosilicate, which was found to be effective and well tolerated in patients with CKD, diabetes, and HF.45,46 Recently, UK guidance from the National Institute for Health and Care Excellence has recommended this agent for patients with advanced CKD and HF who cannot achieve an optimal dose of ACEIs/ARBs because of hyperkalaemia.47
The study presented here showed that more than half of patients who experienced an ACEI-related ADR switched to ARBs; this is in line with current clinical guidelines.5,23 When an ACEI-related ADR is confirmed or other causes have been ruled out, ACEI rechallenge — for example, using the same or other types of ACEI — is generally not recommended due to a high risk of recurrent reactions.48 Although a marginal risk of subsequent ADRs may still occur with the use of ARBs due to them having a generally similar pathway, several studies have reported that the tolerability of ARBs is excellent in patients with previous ACEI-related ADRs, with lower rate of discontinuation, cough, and angioedema.49–52 A Cochrane systematic review showed that the effectiveness of ARBs was found to be non-inferior compared with that of ACEIs.53
Since 2013, the use of dual ACEI and ARB combinations has not been endorsed because of an increased risk of ADRs, with no cardiovascular or mortality benefit.54,55 Existing evidence recommends ACEIs/ARBs with CCBs or a combination of two first- line drugs for high-risk patients, including patients with established CVD, renal disease, and those with markedly high baseline blood pressure.8,56,57 This combination showed superior efficacy with minimal ADRs for high- risk patients.58
Implications for practice
This study showed that ACEI/ARB-related ADRs increased the risk of subsequent CVD events and all-cause mortality so, in clinical practice, the monitoring of patients affected with ADRs should be performed more closely to mitigate the risk of adverse clinical outcomes.
Clinical guidelines recommend scheduled monitoring of renal function and serum potassium among users of ACEIs/ARBs,7,9,22 but a previous study showed that only 10% of users of ACEIs/ARBs in the UK received guideline-recommended clinical monitoring.59 A study by Raebel et al focusing on patients with diabetes and CKD further showed that those users of ACEI/ARBs who received potassium monitoring were less likely to experience severe ADRs.60 Early identification of ADRs through guideline-recommended laboratory monitoring may help to mitigate the subsequent burden of the ADRs.
The monitoring should not only include laboratory monitoring but also medication adherence, as previous studies have reported that ADRs negatively affected medication adherence.61,62 In the subgroup analysis among patients who continued using ACEIs/ARBs, patients with ADRs had an increased risk of CVD events, compared with those without ADRs, indicating the importance of additional monitoring for affected patients as their medication adherence might be compromised even after the treatment has been switched and/or modified, resulting in suboptimal treatment outcomes. In chronic diseases such as hypertension, CKD, and HF, medication adherence is of utmost importance for disease control.63–65 Both medication safety and adherence should be monitored vigilantly by healthcare professionals for patients with ADRs, particularly when the evidence is apparent that those affected by ADRs may have an increased risk of untoward clinical outcomes. This additional monitoring may be incorporated in a medication review/structured medication review for patients with chronic disease by primary care providers. Additional treatment strategies may also be required.
Funding
This study was supported by a research grant from Rosetrees Trust in the UK (reference number: CM705). Widya N Insani was funded by a scholarship from the Indonesia Endowment Fund for Education/Lembaga Pengelola Dana Pendidikan (reference number: 201908223215121). The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
The study protocol was approved by the IQVIA Medical Research Database Scientific Review Committee (reference number: 21SRC008).
Data
All relevant data are in the article and Supplementary Data files.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article:
REFERENCES
- 1.Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Che J, Malecki KC, Walsh MC, et al. Overall prescription medication use among adults: findings from the Survey of the Health of Wisconsin. WMJ. 2014;113(6):232–237. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang F, Mamtani R, Scott FI, et al. Increasing use of prescription drugs in the United Kingdom. Pharmacoepidemiol Drug Saf. 2016;25(6):628–636. doi: 10.1002/pds.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boffa RJ, Constanti M, Floyd CN, et al. Hypertension in adults: summary of updated NICE guidance. BMJ. 2019;367:l5310. doi: 10.1136/bmj.l5310. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 6.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence . Chronic kidney disease: assessment and management NG203. London: NICE; 2021. https://www.nice.org.uk/guidance/ng203 (accessed 24 Aug 2023). [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . Hypertension in adults: diagnosis and management NG136. London: NICE; 2022. https://www.nice.org.uk/guidance/ng136 (accessed 24 Aug 2023). [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence . Chronic heart failure in adults: diagnosis and management NG106. London: NICE; 2018. https://www.nice.org.uk/guidance/ng106 (accessed 24 Aug 2023). [PubMed] [Google Scholar]
- 10.Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174(5):773–785. doi: 10.1001/jamainternmed.2014.348. [DOI] [PubMed] [Google Scholar]
- 11.Probstfield JL, O’Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105(1 Suppl):10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database Syst Rev. 2018;4(4):CD001841. doi: 10.1002/14651858.CD001841.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brugts JJ, Arima H, Remme W, et al. The incidence and clinical predictors of ACE-inhibitor induced dry cough by perindopril in 27,492 patients with vascular disease. Int J Cardiol. 2014;176(3):718–723. doi: 10.1016/j.ijcard.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 14.Wetmore JB, Yan H, Horne L, et al. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant. 2021;36(5):826–839. doi: 10.1093/ndt/gfz263. [DOI] [PubMed] [Google Scholar]
- 15.Tsang C, Bottle A, Majeed A, Aylin P. Adverse events recorded in English primary care: observational study using the General Practice Research Database. Br J Gen Pract. 2013. DOI: [DOI] [PMC free article] [PubMed]
- 16.Byrd JB, Woodard-Grice A, Stone E, et al. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy. 2010;65(11):1381–1387. doi: 10.1111/j.1398-9995.2010.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fournier J-P, Sommet A, Bourrel R, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) and hypertension treatment intensification: a population-based cohort study. Eur J Clin Pharmacol. 2012;68(11):1533–1540. doi: 10.1007/s00228-012-1283-9. [DOI] [PubMed] [Google Scholar]
- 18.Maideen NMP, Balasubramanian R, Muthusamy S, Nallasamy V. An overview of clinically imperative and pharmacodynamically significant drug interactions of renin-angiotensin-aldosterone system (RAAS) blockers. Curr Cardiol Rev. 2022;18(6):e110522204611. doi: 10.2174/1573403X18666220511152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips CO, Kashani A, Ko DK, et al. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167(18):1930–1936. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- 20.Serban M-C, Colantonio LD, Manthripragada AD, et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69(11):1386–1395. doi: 10.1016/j.jacc.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Butt TF, Branch RL, Beesley L, Martin U. Managing hypertension in the very elderly: effect of adverse drug reactions (ADRs) on achieving targets. J Hum Hypertens. 2010;24(8):514–518. doi: 10.1038/jhh.2009.116. [DOI] [PubMed] [Google Scholar]
- 22.National Institute for Health and Care Excellence . Hypertension: angiotensin-converting enzyme inhibitors. London: NICE; 2023. https://cks.nice.org.uk/topics/hypertension/prescribing-information/angiotensin-converting-enzyme-inhibitors (accessed 24 Aug 2023). [Google Scholar]
- 23.National Institute for Health and Care Excellence . Hypertension: angiotensin-II receptor blockers. London: NICE; 2023. https://cks.nice.org.uk/topics/hypertension/prescribing-information/angiotensin-ii-receptor-blockers (accessed 24 Aug 2023). [Google Scholar]
- 24.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 25.Chapman S, Man K, Wei L. Adverse drug reactions in UK primary care consultations: a retrospective cohort study to evaluate impact on appointments and treatment discontinuation. Int J Pharm Pract. 2020;28(S1):37. [Google Scholar]
- 26.Insani WN, Whittlesea C, Ju C, et al. Statin-related adverse drug reactions in UK primary care consultations: a retrospective cohort study to evaluate the risk of cardiovascular events and all-cause mortality. Br J Clin Pharmacol. 2022;88(11):4902–4914. doi: 10.1111/bcp.15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang C, Majeed A, Banarsee R, et al. Recording of adverse events in English general practice: analysis of data from electronic patient records. Inform Prim Care. 2010;18(2):117–124. doi: 10.14236/jhi.v18i2.761. [DOI] [PubMed] [Google Scholar]
- 28.Blais JE, Akyea RK, Coetzee A, et al. Lipid levels and major adverse cardiovascular events in patients initiated on statins for primary prevention: an international population-based cohort study protocol. BJGP Open. 2021. DOI: [DOI] [PMC free article] [PubMed]
- 29.Inamdar AA, Inamdar AC. Heart failure: diagnosis, management and utilization. J Clin Med. 2016;5(7):62. doi: 10.3390/jcm5070062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei L, Ratnayake L, Phillips G, et al. Acid-suppression medications and bacterial gastroenteritis: a population-based cohort study. Br J Clin Pharmacol. 2017;83(6):1298–1308. doi: 10.1111/bcp.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171–184. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 32.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishtala PS, Salahudeen MS. Temporal trends in polypharmacy and hyperpolypharmacy in older New Zealanders over a 9-year period: 2005–2013. Gerontology. 2015;61(3):195–202. doi: 10.1159/000368191. [DOI] [PubMed] [Google Scholar]
- 34.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau WCY, Chan EW, Cheung C-L, et al. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317(11):1151–1158. doi: 10.1001/jama.2017.1363. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y, Moodie EEM, Abrahamowicz M. Comparison of approaches to weight truncation for marginal structural Cox models. Epidemiologic Methods. 2013;2(1):1–20. [Google Scholar]
- 37.Austin PC, Fine JP. Practical recommendations for reporting Fine–Gray model analyses for competing risk data. Stat Med. 2017;36(27):4391–4400. doi: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insani WN, Whittlesea C, Alwafi H, et al. Prevalence of adverse drug reactions in the primary care setting: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0252161. doi: 10.1371/journal.pone.0252161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albani S, Fabris E, Doimo S, et al. Early occurrence of drug intolerance as risk factor during follow-up in patients with acute coronary syndrome or coronary revascularization. Eur Heart J Cardiovasc Pharmacother. 2018;4(4):195–201. doi: 10.1093/ehjcvp/pvy017. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ. 2017;356:j791. doi: 10.1136/bmj.j791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beusekamp JC, Tromp J, Cleland JGF, et al. Hyperkalemia and treatment with RAAS inhibitors during acute heart failure hospitalizations and their association with mortality. JACC Heart Fail. 2019;7(11):970–979. doi: 10.1016/j.jchf.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 Suppl):S212–S220. [PubMed] [Google Scholar]
- 43.Leon SJ, Whitlock R, Rigatto C, et al. Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am J Kidney Dis. 2022;80(2):164–173. doi: 10.1053/j.ajkd.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Fu EL, Trevisan M, et al. Stopping renin-angiotensin system inhibitors after hyperkalemia and risk of adverse outcomes. Am Heart J. 2022;243:177–186. doi: 10.1016/j.ahj.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Amin AN, Menoyo J, Singh B, Kim CS. Efficacy and safety of sodium zirconium cyclosilicate in patients with baseline serum potassium level ≥5.5 mmol/L: pooled analysis from two phase 3 trials. BMC Nephrol. 2019;20(1):440. doi: 10.1186/s12882-019-1611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372(3):222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 47.National Institute for Health and Care Excellence . Sodium zirconium cyclosilicate for treating hyperkalaemia TA559. London: NICE; 2022. https://www.nice.org.uk/guidance/ta599/chapter/1-Recommendations (accessed 24 Aug 2023). [Google Scholar]
- 48.Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2012;12(4):263–277. doi: 10.1007/BF03261835. [DOI] [PubMed] [Google Scholar]
- 49.Granger CB, Ertl G, Kuch J, et al. Randomized trial of candesartan cilexetil in the treatment of patients with congestive heart failure and a history of intolerance to angiotensin-converting enzyme inhibitors. Am Heart J. 2000;139(4):609–617. doi: 10.1016/s0002-8703(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 50.Pfeffer MA, McMurray JJV, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 51.Tanser PH, Campbell LM, Carranza J, et al. Candesartan cilexetil is not associated with cough in hypertensive patients with enalapril-induced cough. Am J Hypertens. 2000;13(2):214–218. doi: 10.1016/s0895-7061(99)00165-x. [DOI] [PubMed] [Google Scholar]
- 52.Yusuf S, Teo K, Anderson C, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 53.Li ECK, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev. 2014;2014(8):CD009096. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 55.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 56.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. 2014;32(1):3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 57.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 58.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 59.Berhe DF, Taxis K, Haaijer-Ruskamp FM, et al. Impact of adverse drug events and treatment satisfaction on patient adherence with antihypertensive medication — a study in ambulatory patients. Br J Clin Pharmacol. 2017;83(9):2107–2117. doi: 10.1111/bcp.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin-angiotensin system blockade: a UK general practice-based cohort study. BMJ Open. 2017;7(1):e012818. doi: 10.1136/bmjopen-2016-012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med. 2010;25(4):326–333. doi: 10.1007/s11606-009-1228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tedla YG, Bautista LE. Drug side effect symptoms and adherence to antihypertensive medication. Am J Hypertens. 2016;29(6):772–779. doi: 10.1093/ajh/hpv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124(7):1124–1140. doi: 10.1161/CIRCRESAHA.118.313220. [DOI] [PubMed] [Google Scholar]
- 64.Ruppar TM, Cooper PS, Mehr DR, et al. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc. 2016;5(6):e002606. doi: 10.1161/JAHA.115.002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seng JJB, Tan JY, Yeam CT, et al. Factors affecting medication adherence among pre-dialysis chronic kidney disease patients: a systematic review and meta-analysis of literature. Int Urol Nephrol. 2020;52(5):903–916. doi: 10.1007/s11255-020-02452-8. [DOI] [PubMed] [Google Scholar]


