Abstract
Cancer remains the leading cause of death around the world. In cancer treatment, over 50% of cancer patients receive radiotherapy alone or in multimodal combinations with other therapies. One of the adverse consequences after radiation exposure is the occurrence of radiation-induced tissue fibrosis (RIF), which is characterized by the abnormal activation of myofibroblasts and the excessive accumulation of extracellular matrix. This phenotype can manifest in multiple organs, such as lung, skin, liver and kidney. In-depth studies on the mechanisms of radiation-induced fibrosis have shown that a variety of extracellular signals such as immune cells and abnormal release of cytokines, and intracellular signals such as cGAS/STING, oxidative stress response, metabolic reprogramming and proteasome pathway activation are involved in the activation of myofibroblasts. Tissue fibrosis is extremely harmful to patients' health and requires early diagnosis. In addition to traditional serum markers, histologic and imaging tests, the diagnostic potential of nuclear medicine techniques is emerging. Anti-inflammatory and antioxidant therapies are the traditional treatments for radiation-induced fibrosis. Recently, some promising therapeutic strategies have emerged, such as stem cell therapy and targeted therapies. However, incomplete knowledge of the mechanisms hinders the treatment of this disease. Here, we also highlight the potential mechanistic, diagnostic and therapeutic directions of radiation-induced fibrosis.
Keywords: Ionizing radiation, Organ fibrosis, Radiation-induced fibrosis, Myofibroblast, Diagnosis, Therapy
Introduction
Ionizing radiation has been widely utilized in various fields such as industry, medicine and the military. In cancer treatment, more 50% cancer patients undergo radiotherapy either alone or in conjunction with other therapies. However, patients who receive tumor radiotherapy may experience severe adverse effects due to medical exposure to ionizing radiation. Although fractional radiation and other accurate techniques have been extensively applied in radiotherapy, radiation-induced tissue fibrosis remains a serious concern [1–3]. In addition, accidental and occupational exposure to ionizing radiation can also induces tissue damage. Ionizing radiation is particularly harmful to major organs throughout the body, including the heart, lung and skin, resulting in both acute and chronic damage.
Ionizing radiation has been found to cause a range of biological effects, which include oxidative damage to biological molecules like DNA, lipid, proteins and metabolites [4]. The accumulation of DNA damage and reactive oxygen species (ROS) can trigger cell death via various mechanisms, such as apoptosis, necrosis, necroptosis and ferroptosis. Additionally, ionizing radiation can lead to senescence and autophagy [5]. Ionizing radiation can have both acute and chronic biological effects, with organ fibrosis being a late symptom that occurs after exposure.
Fibrosis is the process of normal tissue components being destroyed and replaced by disordered collagen fibers and matrix. The process is typically marked by an increase in the production and deposition of extracellular matrix components (ECM), as well as a significant accumulation of myofibroblasts. Fibrotic tissue remodeling often results in organ failure and is linked to increased morbidity and mortality rates [6]. Radiation-induced tissue fibrosis causes irreversible damage to tissues and organs, exacerbating normal tissue damage around the tumor, and significantly limiting the available options for radiotherapy dose and frequency. However, the mechanism behand radiation-induced fibrosis remains elusive. Therefore, it is crucial to elucidate the regulatory mechanism underlying the development of radiation-induced tissue fibrosis to mitigate the adverse effects of radiotherapy and improve its therapeutic efficacy.
Radiation-induced fibrosis is a condition where fibroblasts remain activated and there is an excessive build-up of extracellular matrix [7]. Unfortunately, there are currently no specific or effective treatments available. We reviewed the mechanisms associated with radiation-induced fibrosis and non-radiation-induced fibrotic diseases and found that there are some commonalities between the two, such as pro-fibrotic mechanisms and cells involved in the regulation of fibrosis. Compared to radiation-induced fibrosis, non-radiation-induced fibrosis has been richly studied. The aim of this review is to summarize the mechanisms involved in radiation-induced fibrosis and non-radiation-induced fibrotic diseases, and to contribute to the study of the mechanisms of radiation-induced fibrosis. In addition, potential treatments for radiation-induced fibrosis will be discussed.
The utilization of ionizing radiation in medicine
Ionizing radiation is extensively employed in medical practice for tumor radiotherapy, non-tumor radiotherapy (such as keloid) and diagnostic imaging. This type of radiation encompasses high-energy electromagnetic waves (such as X-rays and γ-rays) and high-energy particles, including ɑ-particles, β-particles, protons, neutrons and electrons. Different types of radiation have varying levels of penetration and harm to the human body. X-rays and γ-rays are highly penetrating and can cause significant damage, while ɑ-particles and β-particles are less penetrating and less harmful when exposed externally [8]. It is vital to note that ionizing radiation is not harmful to the human body below a certain threshold dose. However, exceeding this limit can cause severe tissue and organ damage or even death [9]. Exposure of the human body to ionizing radiation not only directly damages DNA but also triggers an imbalance in oxidative stress, leading to oxidative DNA damage [10] (Fig. 1). This damage initiates the DNA damage repair process in cells, which may result in apoptosis, mitotic catastrophe, and senescence if the DNA damage repair is insufficient. In addition, ionizing radiation can induce an inflammatory response, which may cause tissue damage due to cell death and local inflammation. In tumor radiotherapy, ionizing radiation is used to kill tumor cells, but it can also damage the normal tissue surrounding the tumor.
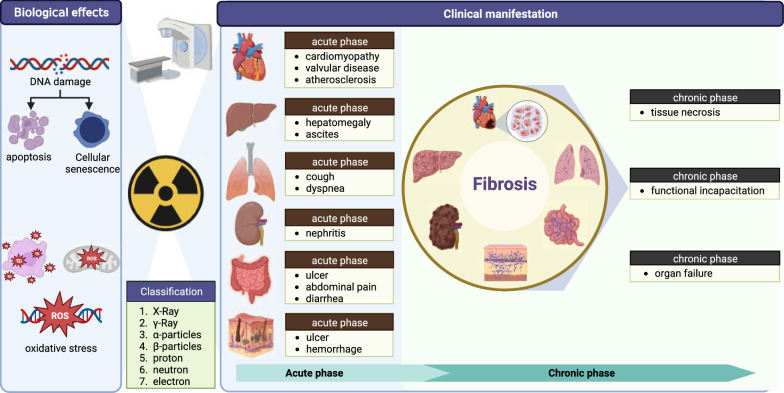
Fig. 1.
Biological effects of ionizing radiation on human normal tissues. In radiotherapy, ionizing radiation causes a variety of biological effects that result in cellular damage. Ionizing radiation causes cellular DNA breakage, leading to apoptosis and senescence. In addition, ionizing radiation causes oxidative damage due to an imbalance of oxidative stress. During cancer treatment, ionizing radiation can cause damage to the heart, liver, lung, kidney, intestines, skin and other organs. The stages of damage are mainly divided into acute and chronic fibrosis. (Figure was created with https://www.Biorender.com)
Physicians utilize ionizing radiation’s cytotoxic effects to eliminate tumor cells and enhance cancer patient’s survival rate. Nevertheless, although radiotherapy patients reap the benefits of ionizing radiation, they also experience late side effects that can significantly affect their quality of life [11] (Fig. 1). Fibrosis frequently caused by chronic inflammation and oxidative aging, is one of the most prevalent late side effects of radiotherapy [12]. In tumor radiotherapy, there are many factors that affect radiation-induced fibrosis, including the dose of radiation and the volume of tissue irradiated. Genetic factors also play an important role. For example, the increased risk of fibrosis after radiotherapy in breast cancer patients is associated with the ATM gene [3]. The ATM gene is involved in the repair of double-stranded DNA breaks. The progression of fibrosis can lead to organ failure and greatly impact the quality of life for radiotherapy patients. While ionizing radiation can cause fibrosis in almost any organ, the susceptibility varies. Patients undergoing radiotherapy for chest tumors are particularly at risk for lung and heart damage. The heart is generally known to be resilient to radiation, however, exposure to high doses of ionizing radiation can result in radiation-induced heart disease (RIHD). This condition is characterized by cardiomyopathy, valvular disease, and atherosclerosis. Furthermore, prolonged radiation exposure can lead to myocardial fibrosis, which in turn can cause arrhythmias, myocardial ischemia, and ultimately cardiomyopathy [13]. Radiation pneumonitis is a potential side effect of radiotherapy for thoracic tumors. On the other hand, radiation-induced pulmonary fibrosis is a late side effect that occurs when patients with chest tumors receive radiotherapy. Research shows that about 50% of lung cancer patients may develop radiation pneumonitis after receiving radiotherapy [14]. However, the prevalence of radiation-induced pulmonary fibrosis is only 16–28% [15]. Most patients with radiation pneumonitis recover with treatment, and only a few progresses to the fibrosis stage. Such patients will have symptoms such as decreased lung function and respiratory failure [16]. In addition to the damage to the lungs and heart, patients with abdominal tumors who undergo radiotherapy also experience harm to their liver, kidneys, and intestines which negatively impact their care. High doses of ionizing radiation can cause acute liver enlargement and ascites [17]. Additionally, it can lead to cause atrophy of the intestinal mucosa, ulceration, and symptoms of enteritis, including abdominal pain and diarrhea [18]. According to research, approximately 90% of patients experience some form of skin reaction as a result of radiotherapy, making the skin the most vulnerable area of the body to damage [19] (Fig. 1).
While the acute side effects of radiotherapy can be managed by modifying the treatment regimen, patients may still experience long-term side effects of ionizing radiation. These late effects can occur in any organ of the body and can be particularly challenging for patients (Fig. 1). Among these late effects, organ fibrosis is the most prevalent and can cause significant damage. The process of fibrosis is complex, involving factors such as inflammatory response and oxidative stress [20]. Currently, there is no standard landmark for the development of radiation-induced fibrosis. Therefore, exploring the main regulatory mechanisms involved in the development of radiation-induced fibrosis can lead to the discovery of effective treatment options for this condition.
Mechanisms of radiation-induced fibrosis
Fibrosis is an irreversible process that can affect any organ. Since the mechanisms of fibrosis may be similar across different organs, studies focused on fibrotic disease in one organ can provide insight into the mechanisms and potential treatments for fibrosis in other organs. Fibrosis is regulated by various factors, including chronic inflammation, oxidative damage, metabolic reprogramming, and cellular senescence, working together in a synergistic manner. The activation of fibroblasts into myofibroblasts and the subsequent secretion of collagen are the primary events in tissue fibrosis. This process can be triggered by various factors such as transforming growth factors (TGFs), interleukins (ILs), and connective tissue growth factors. The pro-fibrotic microenvironment is also characterized by the presence of immune cells, including macrophages and T cells. The process involves multiple cells and cytokines making it a complex phenomenon. While research has made significant progress in understanding the mechanisms of inflammation, immune cells and cytokines that lead to fibrosis, translating these findings into clinical therapeutic approaches remains a challenge [21]. Although there are etiologic and clinical differences among fibrotic diseases, inflammatory stimulation, myofibroblast activation, and immune cell interactions are present in the development of fibrosis [22]. To better understand the mechanisms of radiation-induced fibrosis, it is necessary to first gain insight into the common factors and pathways that induce fibrosis.
Cellular mechanisms of fibrosis
Myofibroblast-the manipulator of fibrosis
Organ fibrosis is a complex process that involves the interaction of multiple cells and cytokines. Myofibroblasts play a crucial role in this process and are the main cells responsible for extracellular matrix secretion [23] (Fig. 2). When activated myofibroblasts secrete α-smooth muscle actin (α-SMA) and type I, III and IV collagen, which are key components of the extracellular matrix [24]. Integrins link α-SMA stress fibers to collagen, resulting in high contractility that stiffens the tissue microenvironment. This can eventually lead to tissue organ sclerosis and dysfunction [25]. While high levels of α-SMA are a characteristic of myofibroblast activation, not all activated myofibroblasts exhibit high levels of α-SMA. Some α-SMA-negative myofibroblasts can also contribute to tissue fibrosis by producing stress fibers made up of β-actin [26]. Activated fibroblasts produce matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which play a crucial role in remodeling the extracellular matrix [27]. Multiple signaling stimuli can activate fibroblasts into myofibroblasts, with the most significant chemical signal being transforming growth factors-β (TGF-β). Additionally, changes in extracellular matrix composition can activate fibroblast transformation through cell surface receptors [28]. Recent studies have shown that myofibroblasts can be derived not only from tissue-resident fibroblasts, but also from hepatic stellate cells (HSC), endothelial cells, pericytes, and smooth muscle cells, through process such as epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EndoMT) [29–32] (Fig. 2). This phenomenon explains the high abundance of myofibroblasts observed in organs affected by fibrosis, such as the heart, liver and kidney. During the process of wound healing, the persistence of myofibroblasts can lead to fibrous repair. However, it is possible for myofibroblasts to dedifferentiate into fibroblasts, which can prevent the formation of scars [33]. To our knowledge, there have been no reports on the treatment of fibrosis through the dedifferentiation of myofibroblasts.
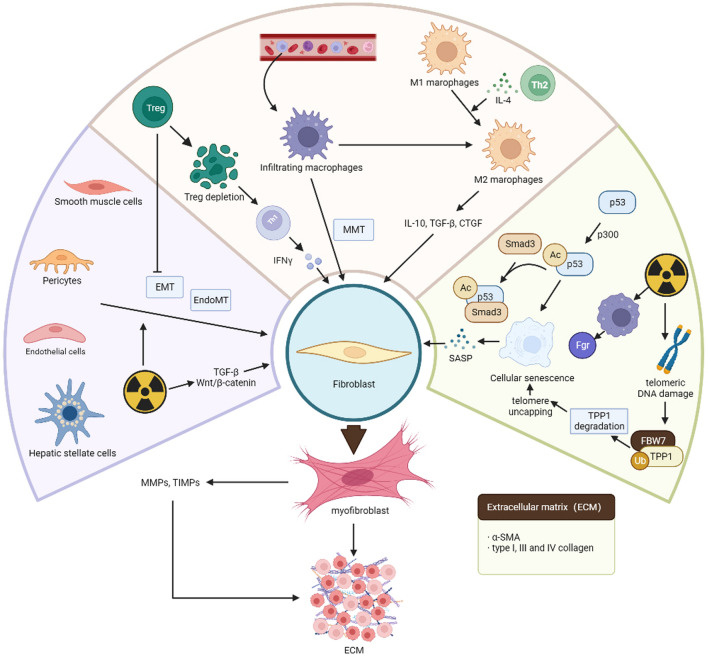
Fig. 2.
Various cells regulate the development of fibrosis. The process of fibrosis is accompanied by a variety of cellular changes. The formation of myofibroblasts is a central part of fibrosis. Cells such as fibroblasts, hepatic stellate cells and endothelial cells can be transformed into myofibroblasts and participate in the development of fibrosis. In addition, immune cells such as migrating infiltrating macrophages and T cells play a role in regulating fibrosis. Cellular senescence induced by ionizing radiation also plays an important role in fibrosis. (Figure was created with https://www.Biorender.com)
Apart from bleomycin- and carbon tetrachloride-induced fibrosis models, radiation-induced organ fibrosis is also primary driven by myofibroblasts [34, 35]. Myofibroblast activation is promoted by ionizing radiation through the activation of various signals such as TGF-β and Wnt/β-catenin [36]. Ionizing radiation can activate fibroblasts, endothelial cells and vascular smooth muscle cells, transforming them into myofibroblasts. These cells are involved in the secretion of ECM [37] and can cause irreversible fibrosis in organs. This chronic phenotype is a result of tumor radiotherapy and severely limits the efficacy of radiotherapy. To effectively treat radiation-induced fibrosis, it is crucial to understand the source and activation mechanism of myofibroblasts, as well as the regression of these cells. This knowledge will aid in developing targeted therapies for the condition.
Participants in myofibroblast activation-immunocytes
Role of macrophage infiltration and polarization in fibrosis
In contrast to certain pro-fibrotic cells that reside in organs, infiltrating cells from nearby tissues or those that travel through the bloodstream are crucial inactivating myofibroblasts. Immune cell like macrophages and T cells are the most notable example of these cells. Prolonged inflammation triggered by ionizing radiation is a significant contributor to fibrosis. In inflamed tissues and organs, a significant number of inflammatory cells migrate and infiltrate, secreting inflammatory factors and activating myofibroblasts. Among them cells, macrophages are the most representative. Monocyte-derived macrophages are a crucial factor in radiation-induced fibrosis, as their migratory infiltration plays a significant role [38] (Fig. 2). Leve et al. found that in a CD73−/− mouse model increased CD73/adenosine signaling, and hyaluronic acid promote macrophage recruitment and activate the macrophage fibrotic phenotype, which is involved in the development of radiation-induced fibrosis [39]. It is noteworthy that there are differences between tissue-infiltrating macrophages and resident macrophages. According to a study on radiation-induced lung fibrosis, it is discovered that tissue-infiltrating macrophages are capable of activating fibroblasts, while resident macrophages are not [39]. Furthermore, the removal of tissue-infiltrating macrophages has been shown to have an antifibrotic effect. The use of clinically approved colony-stimulating factor receptor-1 (CSF1R) neutralizing antibodies can also eliminate macrophage infiltration and decrease the extent of fibrosis. The role of colony-stimulating factor 1 (CSF1), which is a ligand of CSF1R, extends beyond simply recruiting macrophages. It also plays a crucial role in promoting another pro-fibrotic mechanism, namely macrophage polarization [40].
Macrophages can be categorized into two phenotypes: a pro-inflammatory M1 type and an anti-inflammatory M2 type [41]. M2 macrophages are vital in the progression of fibrosis [42]. They can secrete transforming growth factor-β (TGF-β) and IL-10 to promote the conversion of fibroblasts into myofibroblasts [43]. Additionally, they participate in fibroblast proliferation and migration by secreting connective tissue growth factor (CTGF) [44]. The conversion of macrophages into M2 is stimulated by various factors and promotes tissue fibrosis development. Additionally, M1 can also be converted to M2 phenotype when stimulated by Th2-type cytokines like IL-4. This conversion result in the secretion of pro-fibrotic factors such as TGF-β and platelet-derived growth factor (PDGF) [45].
In a mouse model of unilateral ureteral obstruction, a study found that macrophages can directly transform into myofibroblasts and contribute to fibrosis progression [46]. This process is referred to as macrophage-myofibroblast transition (MMT). However, there have been no reports of MMT in radiation-induced fibrosis models. In conclusion, tissue-infiltrating macrophages and macrophage polarization are important mechanisms of ionizing radiation-induced fibrosis. Inhibition of fibrosis-associated macrophage infiltration and modulation of macrophage polarization are novel strategies for the treatment of fibrosis.
Regulatory T cells modulate fibrosis
While macrophages are known to be crucial in the fibrotic process, the role of regulatory T cells (Treg) in regulating fibrosis should not be overlooked. Treg, which is a subpopulation of CD4+ T cells, have been found to play a critical role in radiation fibrosis [47]. Depletion of Treg leads to an increase in Th17 cells and a large amount of Th1 cytokines, such as interferon-γ secretion. This process inhibits fibroblast proliferation and reduces collagen production [48, 49] (Fig. 2). In a mouse model of Th1 cytokine deficiency, fibrosis was found to be significantly increased [50]. Treg not only regulate radiogenic fibrosis through cytokines, but they also inhibit EMT and reducing fibrosis [47]. Although the mechanism by which Treg regulate fibrosis is not fully understood, it has been found that they are involved in myofibroblast activation through extracellular signaling molecules such as TGF-β and Wnt. The mechanism of Treg regulation of fibrosis remain elusive.
The relationship between cellular senescence and fibrosis
Recent studies have shown that aging contributes to fibrosis [51, 52]. The key regulator of senescence namely p53 play a crucial in this process [53]. Originally identified as a tumor suppressor, p53 can activate p21 to promote cellular senescence [54]. Additionally, p53 can form a complex with Smad3 to contribute to fibrosis development [55]. In a study, it was found that p300, an acetyltransferase, plays a role in promoting senescence-associated fibrosis through the p53-Smad3 complex with the p53/p21 pathway. The acetylation of p53 induced by p300 is critical process that initiates fibrosis [56].
Numerous experiments have demonstrated that ionizing radiation induces cellular senescence, with radiation-induced macrophage senescence playing a significant role in radiation-induced fibrosis. Senescent macrophages secrete senescence-associated secretory phenotypes (SASP), such as pro-inflammatory cytokines, matrix metalloproteinases, and chemokines, that encourage fibroblasts to develop fibrogenic phenotypes [57]. Cellular senescence is characterized by its interaction with the environment. Senescent cells produce an extracellular matrix that is abundant in pro-fibrotic factors, which further promote fibroblast differentiation [58]. Additionally, senescent fibroblasts can induce senescence in neighboring fibroblasts, leading to an exacerbation of senescence-associated fibrosis [59]. In a study on irradiated mice, single-cell RNA-Seq revealed that macrophages and neutrophils undergo senescence and express elevated levels of the tyrosine kinase Fgr. This observation indicates that Fgr may play a role in development of radiation-induced fibrosis [60]. Moreover, ionizing radiation elicits a response that damages telomeric DNA. The E3 ubiquitin protein ligase FBW7 (F-box and WD40 repeat domain-containing 7) binds to and degrades telomeric protection protein 1 (TPP1), which leads to telomere uncapping and induces senescence and fibrosis [61].
However, cellular senescence is mechanism that inhibits cell proliferation. In the case of myofibroblasts, inducing fibroblast senescence compromises their function [62]. This leads to loss of myofibroblast phenotype. However, the relationship between cellular senescence and fibrosis is context-dependent and requires further investigation. Therefore, further research is necessary to fully understand the role of cellular senescence in the development of fibrosis.
Activation of extracellular signaling molecules in myofibroblasts
Transforming growth factor-β (TGF-β) during tissue fibrosis
Radiotherapy uses ionizing radiation to kills tumor cells, but it also alters cytokine levels and damages surrounding normal tissue. Among these changes, TGF-β is the most important. TGF-β induces the expression of inflammatory and fibrotic genes in normal cells, causing damage to normal tissue and interfering with the efficacy of radiotherapy [63, 64] (Fig. 3). As mentioned above, Treg cells secrete TGF-β, which plays a crucial role in regulating fibrosis [65]. TGF-β has three major isoforms, namely TGF-β1, TGF-β2, and TGF-β3. Among these, TGF-β1 is the most predominant pro-fibrotic factor and facilitates the transition of fibroblasts to myofibroblasts, thereby contributing to promotes the development of fibrosis [66].
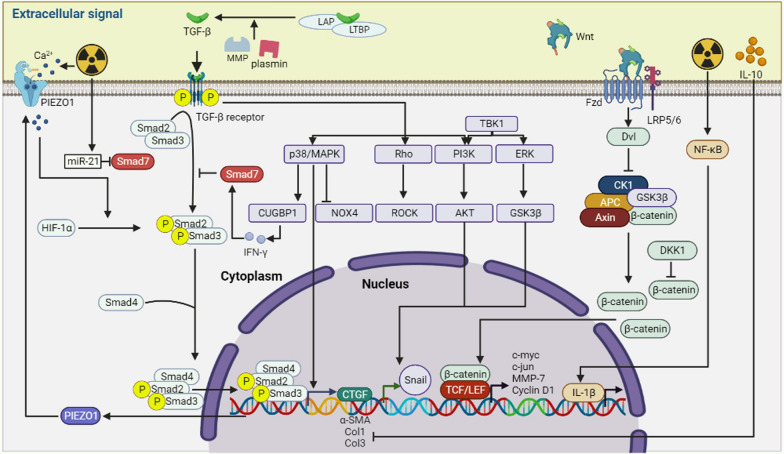
Fig. 3.
Extracellular signaling regulates fibrosis. The process of fibrosis induced by ionizing radiation is mediated by several extracellular molecules. One of the most important groups is the cytokine family, TGF-β, an important factor in the regulation of fibrosis, which can be involved in regulating the expression of fibrosis-related genes through Smad-dependent and Smad-independent pathways. In addition, extracellular Wnt signaling pathway may also be involved in regulating fibrosis gene expression by activating intracellular β-catenin signaling pathway. Cytokines such as IL-1β and IL-10 are also involved in regulating fibrosis gene expression. (Figure was created with https://www.Biorender.com)
It is currently believed that TGF-β exists mainly as a latent TGF-β/LAP/LTBP complex, which remain inactive. However, under certain pathological conditions, this complex can be cleaved by fibrinolytic enzymes, matrix metalloproteinases, and other agents, leading to the release TGF-β [67, 68]. Once released, TGF-β1 binds to type II TGF-β receptors and phosphorylates type I TGF-β receptors. TGF-β receptors of type I are activated and recruit Smad2/3, which then binds to Smad4 to form a ternary complex. This complex translocates into the nucleus, where it regulates the expression of target genes including collagen coding genes [69–71]. The activation of Smad signaling by TGF-β1 leads to the conversion of fibroblasts to myofibroblasts and promotes the expression of α-SMA and collagen [72]. The pro-fibrotic effect of TGF-β1 was validated through the use of CRISPR/Cas9 technology to knock down TGF-β1 in mice [73]. Furthermore, reduced radiation-induced fibrosis was also observed in Smad3 KO mice [74]. During radiation-induced fibrosis, the highly activated TGF-β/Smad pathway, and reduce levels of Smad7 led to increased expression of fibrotic genes. This is especially relevant since Smad7 has been shown to be a potent inhibitor of the TGF-β/Smad pathway [75, 76]. NF-κB is a crucial inflammatory regulator that can promote the development of radiation-induced fibrosis by regulating TGF-β signaling [77].
Lipoxin A4 (LXA4), is a potent antioxidant that is produced from arachidonic acid. Studies have shown that LXA4 can attenuate liver fibrosis by targeting the TGF-β/Smad pathway [78]. Additionally, radiation has been shown to increase the expression of the LXA4 receptor (FPR2), while LXA4 itself decreases NF-κB and Smad binding element promoter activity [79]. Hypoxia-inducible factor-1α (HIF-1α) plays a crucial role in reducing radiation-induced ROS production and regulating TGF-β signaling. Its regulation is influenced by calcium signaling [80]. PIEZO1, a calcium channel, is known to increase its expression in response to ionizing radiation, resulting in an increase in inward Ca2+ flux. Increased levels of cytoplasmic Ca2+ can boost TGF-β1 signaling mediated by HIF-1α. The activated signaling pathway can release C/EBPβ from the PIEZO1 promoter by inhibiting the expression of the transcription factor C/EBPβ [81]. On the other hand, unlike the effects of LXA4 and HIF-1α, a non-coding RNA (miR-21) can interfere with antioxidants. Moreover, radiation induced the expression of miR-21, which in turn activates the TGF-β/Smad signaling pathway and suppresses the expression of Smad7. This ultimately results in the development and progression of fibrosis [82].
In addition to the canonical TGF-β/Smad pathway, other pathways such as PI3K/Akt, mitogen-activated protein kinase (MAPK), and AMP-activated protein kinase (AMPK) have been found to be activated in cardiac fibrosis models. This was discovered by constructing circRNA expression profiles and circRNA-associated ceRNA networks [83]. These pathways are part of the Smad-independent signaling pathway. Researchers found that Inhibition of p38/MAPK/Akt reduced the level of NADPH oxidase 4 (NOX4), which in turn inhibited radiation-induced myofibroblast activation [84]. The pro-fibrotic effects of MAPK have a wider impact than just bile duct ligation-induced liver fibrosis model, TGF-β signaling can cause significant activation of HSCs and stimulate high levels of CUG-binding protein 1 (CUGBP1) in HSCs through the p38/MAPK pathway. This leads to reduced expression of interferon-γ (IFN-γ). IFN-γ has been observed to induce the expression- of Smad7, which subsequently inhibits the TGF-β/Smad cascade response [85–87]. Along with various Smad-independent pathways, the PI3K/Akt is a signaling pathway associated with cell proliferation and metabolism. Inhibition of this pathway has also been demonstrated to decrease the transformation of fibroblasts into myofibroblasts [88]. EMT is a crucial factor in the activation of myofibroblasts. The activation of Smad-independent signaling pathways such as Akt and ERK/GSK3β/Snail signaling plays a significant role in this process. Studies have shown that TANK-binding kinase 1 (TBK1) can activate both signaling pathways to promote EMT, which eventually leads to organ fibrosis [89]. The Rho/Rock (Rho-associated convoluted helix kinase) signaling pathway is associated with cell differentiation and proliferation, and it is a Smad-independent signaling pathway that is closely related to radiation-induced fibrosis [90]. TGF-β, as the major signaling molecule for myofibroblast activation, has been shown to promote the development of radiation-induced fibrosis through multiple Smad-dependent and Smad-independent pathways. However, some inhibitors of the TGF-β pathway, such as Smad7, have also been shown to have anti-fibrotic effects. Current studies have focused on the crosstalk between TGF-β and other fibrosis-related pathways that regulate fibrosis, such as HIF-1α. Therefore, mastering the interactions between these pathways is of critical importance for the treatment of fibrosis.
The regulatory role of Wnt signaling in fibrosis
In recent years, numerous studies have demonstrated that Wnt signaling molecules play an important role in tissue fibrosis including heart, liver, kidney and other organs [91–93]. β-catenin is a component of the Wnt signaling pathway, which is important for embryonic development. Normally, β-catenin in the cytoplasm is phosphorylated by a disruption complex consist of APC, Axin, CK1α, and GSK-3β. This leads to degradation by proteasome-dependent ubiquitination, resulting in low levels of cytoplasmic β-catenin that cannot activate downstream genes [94, 95]. Dishevelled (Dsh) is phosphorylated upon binding of Wnt ligands to Frizzled (Fzd) and low-density lipoprotein receptor-associated protein 5/6 (LRP5/6) on the target cell membrane [96]. This phosphorylated Dsh then promotes the phosphorylation of GSK-3β, which in turn inhibits its activity and reduces the degradation of β-catenin [97]. The accumulation of cytoplasmic β-catenin leads to its translocation to the nucleus where it binds to nuclear T-cell factor (TCF)/lymphoid enhancer-binding factor (Lef). This binding initiates the transcription of downstream target genes such as Wnt-1-induced secreted protein (WISP1), cyclin D1, c-myc, c-jun, and MMP-7, which promote fibrosis progression [98–100] (Fig. 3). The Wnt/β-catenin signaling pathway, is a known pro-fibrotic pathway, has been shown to contribute to the progression of radiation-induced skin fibrosis [101]. MiR-17-5p, a miRNA highly expressed in hepatocellular carcinoma, activates the Wnt/β-catenin pathway and exacerbates radiation-induced liver fibrosis [102]. This highlights the significance of the Wnt/β-catenin pathway in the development of radiation-induced fibrosis.
The Dickkopf (DKK) family is a group of glycoproteins that are secreted. This family consists of five major types: DKK1-4 and Dkkl1 (soggy) [103]. DKK1 is known to be an antagonist of the Wnt/β-catenin pathway and can significantly reduce β-catenin activity, which in turn blocks the process of fibrosis [104, 105]. Additionally, human adipose-derived mesenchymal stromal cells (Ad-MSCs) can produce DKK1, which helps in reducing radiation fibrosis through the Wnt/β-catenin pathway [106]. Although the Wnt/β-catenin pathway shows great potential in the treatment of radiation-induced fibrosis, there is a lack of research in this area. Further exploration of the role of this pathway in radiation-induced fibrosis could provide valuable insights for developing effective treatment strategies.
Connective tissue growth factor (CCN2/CTGF), a key regulator of fibrosis
The TGF-β/Smad signaling pathway is a potent pathway that leads to fibrosis. When exposed to ionizing radiation, this pathway induces the expression of α-SMA, collagen, and other fibrosis-related factors such as CTGF [107]. CTGF (CCN2) is a member of the stromal cell protein family, is a well-studied fibrosis-related factor. It is a secreted protein that is rich in cysteine and has been shown to play a crucial role in wound healing and organ fibrosis [108, 109]. The TGF-β1/Smad/CTGF signaling pathway has been found to activate of hepatic stellate cells in a radiation-induced liver fibrosis [110]. Furthermore, TGF-β can induce the expression of CTGF through Smad-independent TGF-β signaling pathway, such as MAPK signaling and the Rho/ROCK pathway. CTGF, in turn, can activate of myofibroblasts [111–113]. In the angiotensin II-induced myocardial fibrosis model, Dorn et al. discovered that only CCN2 derived from fibroblasts can effectively stimulate the activation of cardiac fibroblasts and promote fibrosis. CCN2 from cardiomyocytes, on the other hand, does not have this function. As a result, CTGF is believed to be an autocrine regulator [114]. Although CTGF's role in fibrosis is well established, its regulation in radiation-induced fibrosis has not been confirmed. Currently, there are several strategies being explored to target CTGF for fibrosis treatment [115, 116]. However, the exact mechanism by which CTGF promotes fibrosis in the context remains unclear.
Role of the interleukin family members in fibrotic diseases
The inflammatory response caused by radiation is a significant mechanism for tissue damage, and cytokines from the interleukin family play a crucial role in mediating this response. Prolonged exposure to ionizing radiation can result in severe tissue fibrosis due to chronic inflammation [3]. IL-1β, secreted mainly by macrophages and dendritic cells, is the most crucial pro-inflammatory factor [117]. It binds to the IL-1β receptor on the membrane surface, stimulating the production of more IL-1β, ultimately leading to the development of fibrosis [118]. Additionally, under inflammatory conditions, IL-1β acts as a potent pro-EndMT factor, promoting the transformation of endothelial cells into activated fibroblasts and contributing to the occurrence of fibrosis [119]. Radiation can activate the NF-κB signaling pathway, leading to the production of large amounts of IL-1β. This cytokine can then recruit immune cells to the site of injury [120, 121] (Fig. 3). The CC chemokine ligand CCL2 plays a crucial role in this process. In radiation-induced lung fibrosis, IL-1β produced by lung epithelial cells, macrophages and fibroblasts stimulates the production of CCL2. The resulting increase in CCL2 levels attracts more CCR+ macrophages to the site of injury, exacerbating the development of radiation-induced fibrosis [122].
Interleukin 10 (IL-10) is a cytokine that has both anti-inflammatory and anti-fibrotic effects. It can inhibit the production of type I and III collagen (Col 1 and Col3), while also increasing the expression of MMP and inhibiting the transformation of fibroblasts into myofibroblasts. Additionally, IL-10 promotes the differentiation of macrophages into a regenerative phenotype, which further contributes to its antifibrotic role. These findings are supported by studies referenced as [123, 124] (Fig. 3). According to a study, the use of bone marrow mesenchymal stem cells was found to increase the number of anti-inflammatory CD163+ macrophages and promote the expression of IL-10 in activated macrophages in irradiated mice. This, in turn, helped in attenuating radiation-induced skin fibrosis [125]. The interleukin family consists of many members that are abundant in the inflammatory environment induced by ionizing radiation and are mainly secreted by immune cells. Different interleukins can exert pro- and anti-fibrotic effects. Their specific regulatory fibrotic effects need to be further investigated.
Intracellular changes in fibrosis-associated cells
Progression of fibrosis due to oxidative stress imbalance
Ionizing radiation is known to cause cellular damage through oxidative stress (Fig. 4). This occurs when free radical production becomes abnormal and damages the antioxidant system. Free radicals are classified into two types: reactive oxygen species (ROS) and reactive nitrogen species (RNS). The production of ROS from water in the cytoplasm, specifically hydroxyl radicals (·OH) and superoxide anions (O2−·), is induced by ionizing radiation [7]. In addition, ionizing radiation can disrupt the mitochondrial electron transport chain as well as superoxide dismutase and glutathione antioxidant enzymes, leading to a significant accumulation of ROS [126].
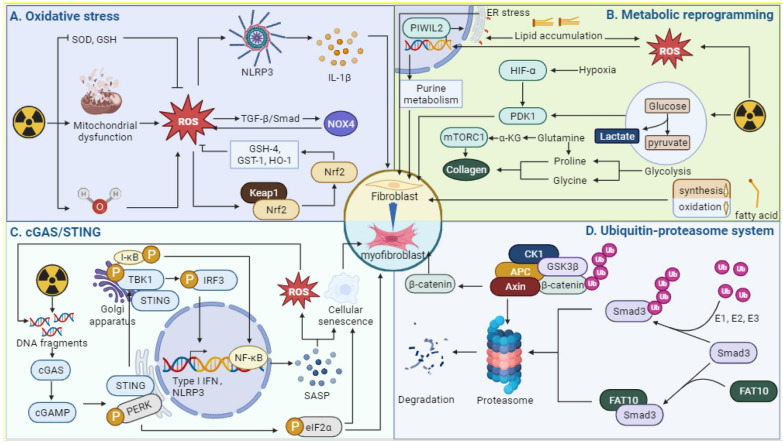
Fig. 4.
Changes in intracellular components in fibrosis. A Ionizing radiation promotes fibrosis by generating ROS and disrupting the activity of antioxidant enzymes such as SOD and GSH. B Ionizing radiation can alter intracellular glucose metabolism, amino acid metabolism, and lipid metabolism pathways through metabolic reprogramming, thereby promoting fibrosis. C DNA damage caused by ionizing radiation can result in large amounts of DNA fragmentation. These free DNA fragments activate the cGAS/STING pathway and promote fibrosis. D The ubiquitin–proteasome pathway and the FAT-10 proteasome pathway mediate the degradation of key fibrosis signaling molecules such as Smad3 and β-catenin, thereby promoting fibrosis. (Figure was created with https://www.Biorender.com)
Mitochondria are organelles responsible for generating energy within cells and are closely involved in regulating oxidative stress. Several studies have shown that dysfunction of mitochondria and imbalanced oxidative stress can contribute to the development of organ fibrosis. Specifically, mitochondrial dysfunction can lead to the production of reactive oxygen species (mtROS), which promote fibrosis, while oxidative stress imbalance can result in mitochondrial dysfunction, damage to mitochondrial DNA, and other factors that promote fibrosis [127, 128]. ROS plays a significant role in promoting myofibroblast activation by increasing the expression of NOX4 through the TGF-β/Smad pathway [129]. Additionally, ROS can also activate NOD-like receptor protein 3 (NLRP3) and its downstream factor IL-1β [130]. This highlights the crucial role of ROS in the activation of fibrosis. The upregulation of DPYSL4, a mitochondrial super-complex-related gene, in post-illumination lung epithelial cells stimulates the production of ATP and NADPH, leading to a reduction in pro-fibrotic ROS production [130]. Mitochondrial enzymes also play a crucial role in regulating ROS levels. In a mouse model of CCI4-induced liver fibrosis, the enzyme p66Shc was discovered to promote the production of ROS, which activates inflammatory vesicles NLRP3 and is involved in the progression of fibrosis. This was reported in a study with reference [131].
Nuclear factor erythroid-2-related factor 2 (Nrf2) regulates intracellular antioxidant and plays a crucial role in reducing ionizing radiation-induced oxidative damage. In normal conditions, Nrf2 remains inactive as it binds to cytoplasmic Kelch-like ECH-related protein 1 (Keap1). However, when there is an imbalance in intracellular oxidative stress, the Nrf2 pathway gets activated, promoting the expression of downstream antioxidant proteins such as glutathione peroxidase-4, GST-1, and HO-1. These proteins work together to reduce ROS levels [132, 133]. Lowering ROS levels can reduce TGF-β signaling and consequently decrease radiographic fibrosis [134]. However, in Nrf2−/− mice, collagen secretion is increased due to activated pro-fibrosis signaling [135]. Additionally, the aberrant expression of receptor-interacting protein kinase-3 (RIPK3), a factor associated with necroptosis, can lead to mitochondrial dysfunction [136]. Studies have demonstrated that Nrf2 can impede RIPK3-induced mitochondrial dysfunction, which in turn suppresses ROS the production of ROS and accumulation of collagen [135]. In addition, zinc is a trace element with antioxidant properties and a crucial component of both superoxide dismutase (SOD) and MMPs. A deficiency in zinc results in reduced levels of ROS, which can contribute to the progression of fibrosis [137, 138]. Mitochondrial damage is a significant contributor to the buildup of ROS within the cell. This damage can be mitigated by the cellular antioxidant defense mechanism known as mitochondrial autophagy [139].
Although zinc and mitochondrial autophagy have been extensively studied in models of fibrosis induced by non-radiological factors, their role in in protecting against radiation-induced fibrosis remains unclear. Therefore, further research is required to investigate the mechanisms of cellular antioxidant damage protection in such cases.
cGAS/STING pathway-identifier of DNA damage
Ionizing radiation and the resulting oxidative damage can cause breaks in both nuclear and mitochondrial DNA, ultimately leading to cell death. This process results in the scattering of numerous DNA fragments throughout the cytoplasm. However, the cell has a mechanism to address this DNA fragmentation called the Stimulator of Interferon Genes (STING). STING is a crucial signaling molecule for intrinsic immunity and is primary located in the endoplasmic reticulum. When cells are exposed to Ionizing radiation, cellular and mitochondrial DNA damage occurs, leading to the release of free DNA into the cytoplasm. This free DNA is then detected by the cytoplasmic dsDNA recognizer cyclic guanosine adenosine phosphate synthase (cGAS), which catalyzes cyclic dinucleotide 2′3' cyclic GMP-AMP (cGAMP). The cGAS/STING pathway play a crucial role in radiation-induced injury by activating downstream STING, which then translocate to the Golgi apparatus and activates TBK1. The activation of TBK1 lead to the activation of interferon regulatory factor 3 (IRF3), which induces the expression of type I interferon and pro-inflammatory cytokines [140, 141]. Thus, the cGAS/STING pathway plays an important role in radiation-induced injury (Fig. 4). The involvement of STING pathway in DNA damage caused by ionizing radiation-induced fibrosis remains unclear.
A recent study showed that the cGAS/STING pathway is enriched in radiation-induced fibrosis [142]. Furthermore, the role of the STING pathway in liver, kidney and lung fibrosis has also been demonstrated [143–145]. Conversely, drug inhibition of STING/TBK1 signaling has been shown to reduce the conversion of bone marrow fibroblasts and macrophages into myofibroblasts [146], thus presenting is a promising pathway for targeted fibrosis therapy. Ferroptosis is a type of cell death that is dependent on iron and causes oxidative DNA damage. The damage can activate the STING pathway and ultimately lead to fibrosis [147]. Like the effects of ionizing radiation. In a study by Wang et al. found that mtDNA released from mitochondrial damage can promote fibrosis development by activating NLRP3 through cGAS/STING signaling. The process of endoplasmic reticulum stress can be regulated by the ER stress signal X-box binding protein 1 (XBP1) and its downstream factor BCL2/adenovirus E1B interaction protein 3 (BNIP3) [148]. It is necessary to note that this type of stress often occurs during the production of large amounts of extracellular matrix. Previous studies have shown that STING is an important regulator of ER stress and that it can lead to fibrosis [149]. Notably, the researchers observed elevated expression levels of three proteins, namely phosphorylated inositol-requiring kinase (p-IRE-1α), phosphorylated eukaryotic initiation factor 2α (p-eIF2α), and phosphorylated protein kinase RNA-like endoplasmic reticulum kinase (p-PERK), in a mouse model of cardiac fibrosis. This finding suggests that these three proteins may play a crucial role in the development of this pro-fibrotic process. IRE-1α is the upstream molecule of the endoplasmic reticulum stress signal XBP1 and plays a key role in its activation [148]. In contrast, two proteins, eIF2α and PERK, are associated with a non-classical STING pathway. In this pathway, the STING signal can directly activate PERK at the endoplasmic reticulum, which in turn phosphorylate eIF2α, regulating the selective expression of certain proteins. This differs from the conventional STING-TBK1-IRF3 pathway. The cGAS/STING pathway is crucial in regulating cellular senescence and fibrosis [150]. As highlight in previous research on the importance of cellular senescence in fibrosis. Notably, our findings indicate that this pathway serves as a key regulator of senescence [151]. Previous research used Oroxylin A to inhibit the production of methionine metabolites, specifically the methyl donor S-adenosylmethionine, leading to a reduction in DNA methyltransferase (DNMT)-mediated methylation of the cGAS gene [152]. Additionally, activated STING play a role in promoting NF-κB/I-κB activation, which further contributes to STING pathway activation and exacerbates ROS-induced oxidative DNA damage [152]. This promotes cellular senescence, but ultimately presents an anti-fibrotic function. This suggests that the relationship between senescence and fibrosis remains to be discussed.
Surprisingly, the relationship between the cGAS/STING pathway and fibrosis is controversial. While pharmacological activation of STING signaling has been found to play an anti-fibrotic role in mouse liver and kidney [153]. However, overexpression of STING attenuates the development of fibrosis by inhibiting autophagy [154]. In summary, the production of cytoplasmic free DNA is an important feature of ionizing radiation-induced damage. And the cGAS/STING pathway plays an important role in radiation-induced fibrosis because of its role in recognizing DNA fragments. Recent studies have identified some of the downstream effects of STING, such as cellular senescence and NLRP3 activation. However, to develop anti-fibrotic drugs targeting the STING pathway, further dissection of the fibrosis-related effects downstream of the STING pathway is needed.
Metabolic reprogramming in fibrosis
The process of fibrosis results in the production of a significant amount of matrix, including ɑ-SMA and collagen. This is generally considered an anabolic process that consumes energy, leading to an increase in energy metabolism and anabolism [155]. Mitochondria plays a crucial role in this process. The organism undergoes metabolic reprogramming as an adaptive process in response to external stimuli (Fig. 4). This process involves various metabolic pathways, including gluconeogenesis, amino acid metabolism and lipid metabolism. While the process of energy changes is complex, a study on cardiac fibrosis, using a proteomic approach found that ionizing radiation has an impact on cardiac energy metabolism in mice, ultimately affecting the onset of cardiac fibrosis [156]. Due to energy for cell proliferation, repair, synthesis and secretion are primary produced through aerobic mitochondrial respiration. Pro-fibrotic factors, including TGF-β and ionizing radiation, have been shown to reprogram cellular metabolism and promote myofibroblast formation through the enhancement of aerobic glycolysis [157–159]. This process leads to a significant increase in pyruvate dehydrogenase 1 (PDK1) during glycolysis. HIF-1α is a key factor in regulating anaerobic glycolysis to adapt to hypoxic environments and induces myofibroblast differentiation by targeting PDK1 [160]. The end product of glycolysis (lactic acid) has been identified as a promoter of collagen hydroxylation through proline hydroxylase activity [161]. Glucose transporter protein 1 (GLUT1), which is the most abundant glucose transporter protein in mammals, plays a crucial role in glucose transport. In a study conducted by Cho et al., it was discovered that GLUT1-dependent glycolysis could promote pulmonary fibrosis induced by bleomycin [162]. Moreover, the pro-fibrotic effect of GLUT1-dependent glycolysis was found to be closely linked with the inflammatory sensor AIM2 [163]. Additionally, a separate study discovered that purine metabolism, in addition to gluconeogenesis, plays a role in the advancement of fibrosis [164]. As previously mentioned, Nrf2, is a crucial regulator of radiogenic fibrosis. It can enhance the expression of Piwi-like RNA-mediated gene silencing 2, which in turn activates purine metabolism to repair the damage caused by ionizing radiation [165].
In addition to promoting fibrosis, glycolysis can also provide essential amino acids such as proline and glycine for collagen synthesis [166]. The serine-glycine pathway can convert glycerol-3-phosphate, a glycolytic intermediate, to produce a significant amount of glycine, which is the primary component of collagen synthesis [166]. And proline can be converted from glutamine [167]. Glutamine catabolism is a crucial topic as it serves as a biosynthetic precursor and energy donor. Its catabolism is closely linked to the progression of fibrosis [168]. The conversion of glutamine to glutamate is catalyzed by glutaminase in this process. Furthermore, α-ketoglutarate can be produced from glutamine and this compound can activate the downstream rapamycin complex 1 (mTORC1), which in turn promotes collagen synthesis as indicated by reference [169].
Apart from sugar and amino acid metabolism, lipid metabolism also plays a significant role in the fibrotic response. Generally, fibrosis leads to a reduction in fatty acid oxidation (FAO) and arise in fatty acid synthesis [170]. In a mouse model of unilateral ureteral obstruction, the activation of signal transducer and activator of transcription 6 (STAT6) inhibits FAO, leading to lipid accumulation and the development of fibrosis [170]. After knocking down STAT6, the effect was no longer present, indicating its dependence on STAT6. Additionally, radiation-induced skin fibrosis was found to be exacerbated by the impairment of FAO [159]. Furthermore, increased fatty acid synthesis has also been shown to have a pro-fibrotic effect [171]. However, the pro-fibrotic mechanisms of lipid metabolism disorders are not limited to these findings and require further investigation [172]. The uptake of triglyceride-rich very low-density lipoproteins into the cell is promoted, which leads to excessive intracellular lipid accumulation. This accumulation of lipids causes the production of ROS and endoplasmic reticulum stress, ultimately resulting in fibrosis. This process involves the activation of the type 2 immune response mediated by group 2 innate lymphoid cells of the pancreas, which is triggered by IL-33 secreted by pancreatic stellate cells [173]. Fibrosis is essentially an anabolic process in which large amounts of extracellular matrix are synthesized. Alterations in glucose metabolism, amino acid metabolism, and lipid metabolism have been shown to play an important role in radiographic fibrosis. Alterations in cellular metabolism not only provide energy for the initiation of fibrosis, but also provide some essential raw materials for fibrosis. Thus, alterations in metabolic pathways are an important mechanism of ionizing radiation-induced fibrosis.
Ubiquitin–proteasome system-potential regulators of fibrotic pathways
The progression of fibrosis involves various signaling molecules, and their abnormal expression or degradation is crucial in the body's resistance to fibrosis. Several studies have demonstrated the involvement of the ubiquitin–proteasome system (UPS), a protein degradation pathway, in the progression of fibrosis [174–176] (Fig. 4). Application of the proteasome inhibitor Delanzomib in a mouse model of renal fibrosis showed potential anti-fibrotic therapeutic effects [177]. Another study conducted on radiation-induced skin injury in rats revealed that the UPS is involved in the induction of radiation-induced fibrosis [178]. The UPS is mainly composed of ubiquitin, ubiquitin-activating enzyme E1, ubiquitin-binding enzyme E2, ubiquitin-protein ligase E3, 26S proteasome and ubiquitin-dissociating enzyme (DUB) [179]. The UPS pathway consists of two main steps: first, E1, E2 and E3 catalyze the ubiquitination of target protein. Second, the ubiquitinated protein is degraded by the 26S proteasome [180]. This pathway plays an important role in maintaining the normal cellular state by removing abnormal and damaged proteins. The 26S proteasome serves as the site of protein degradation in the UPS. The pro-fibrotic signal TGF-β can induce myofibroblast transformation by activating 26S proteasome. This activation is associated with the regulatory subunit Rpn6 [181].
The ubiquitin–proteasome system has been found to affect the onset of fibrosis by interfering with various pro-fibrotic pathways such as TGF-β/Smad, Wnt/β-catenin, and the transcriptional co-activator Yes-associated protein (YAP). A recent study discovered that the degradation of Smad3 through the proteasome by human leukocyte antigen F-associated transcript 10 (FAT10) could impede the development of fibrosis [182]. This process described does not involve protein ubiquitination and therefore cannot be referred to as UPS. It has been given a new name: the FAT10-proteasome system (FPS) (Fig. 4). While UPS has been shown to be effective in inhibiting fibrosis through Smad3 degradation, other means such as the regulation of tissue fibrosis [183]. Alkylation repair homolog 5 (ALKBH5) is an important protein that regulates tissue fibrosis. In a mouse model of pulmonary fibrosis exposed to PM2.5 [184], reduction of ALKBH5 activated the pro-fibrotic signaling pathway YAP, which subsequently increased the expression of P4HA2. P4HA2 is a crucial enzyme for collagen formation [185]. Alongside E3 ubiquitin ligases, DUB, another member of the UPS, also contributes significantly to the progression of fibrosis. DUB, a regulatory protein, plays a vital role in the regulation of fibrotic signaling pathways by preventing the degradation of target proteins via deubiquitinating [186]. One such DUB, ubiquitin-specific protein hydrolase 2 (USP2, a DUB) can deubiquitinate β-catenin, which leads to increased β-catenin entry into the nucleus and promoting the expression of fibrotic genes [187]. Therefore, utilizing the ability of the UPS to degrade target proteins may offer a promising research direction for the treatment of fibrosis.
Mechanism of radiation-induced organ fibrosis
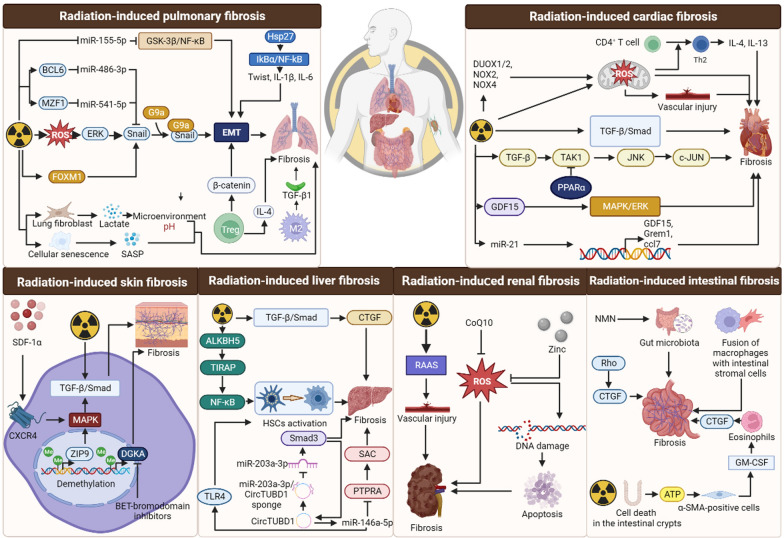
Long-term exposure to ionizing radiation can result in fibrosis of various organs such as the heart, liver, lungs and skin. The degree of radiation-induced organ fibrosis is influenced by the radiation dose, the mode of exposure, and individual genetic susceptibility [188]. The radiosensitivity of different organs varies due to a variety of factors. And sensitive organs are more prone to developing fibrotic changes with prolonged exposure to ionizing radiation. Radiation-induced fibrosis leads to both structural changes and functional failure of organs. While the specific organs affected by radiation damage may vary, there are similarities in the cellular and molecular mechanisms underlying radiogenic fibrosis across different organs [189] (Fig. 5).
Fig. 5.
Mechanisms of radiation-induced fibrosis in various tissues. Ionizing radiation causes fibrosis in the lungs, heart, skin, liver, kidney and intestine by modualting a variety of molecules involved in cytokine signaling, collagen biosynthesis, oxidative stress response and epigenetic regulation. (Figure was created with https://www.Biorender.com)
Radiation-induced pulmonary fibrosis
Radiation-induced pulmonary fibrosis is a frequently observed clinical complication in patients receiving radiotherapy for thoracic cancer. This condition usually manifests several months after radiation treatment. The process of epithelial-mesenchymal transition triggered by ionizing radiation is a key mechanism responsible for the development of radiation-induced pulmonary fibrosis [190]. Ionizing radiation triggers the generation of significant levels of ROS, which in turn modulates the formation of complexes between Snail and G9a via the ROS/ERK pathway. This process leads to an increase in H3K9 methylation at the E-cadherin promoter, ultimately resulting in the inhibition of E-cadherin expression [191]. The inhibition of EMT and reduction of fibrosis are the ultimate outcomes. The promotion of fibrosis in ionizing radiation-induced EMT is facilitated by Heat shock protein 27 which promotes NF-kB release through the IkBα-NFkB pathway and inducing pro-fibrotic factors such as Twist, IL-1β and IL-6 [192]. Furthermore, the upregulation of transcription factor forkhead box M1 (FOXM1) is observed in alveolar epithelial cells after irradiation. FOXM1 activates Snail expression by binding to its promoter, promote, which in turn promotes EMT and increases the degree of lung fibrosis after irradiation [193]. Recent studies on non-coding RNAs have revealed that several miRNAs are involved in regulating EMT and contribute to the development of radiation-induced pulmonary fibrosis. For example, ionizing radiation can activate myeloid zinc finger protein 1 (MZF1) and activated MZF1 can inhibit the production of miR-541-5p precursors and thus reduce miR-541-5p levels. This also leads to the expression of Slug (Snail2), a key gene in EMT, which allows the exacerbation of pulmonary fibrosis after radiation exposure [194]. Moreover, ionizing radiation stimulation activates B-cell lymphoma 6 protein (BCL6) from the BTB/POZ zinc finger protein family, which inhibits the expression of downstream miR-486-3p and increases the expression of Snail, thus promoting EMT [195]. According to research, radiation exposure leads to a reduction in miR-155-5p levels in alveolar epithelial cells. This, in turn, activates GSK-3β, which is the target of miR-155-5p, and facilitates the phosphorylation of the NF-κB subunit p65. This activation of the GSK-3β/NF-κB pathway ultimately results in pulmonary EMT and the development of pulmonary fibrosis [196]. There are numerous other mechanisms implicated in radiation-induced pulmonary fibrosis. Sphingosine-1-phosphate receptor 3 (SIPR3) is a vital pro-fibrotic factor that can lead to radiation-induced pulmonary fibrosis. It does so by activating TGF-β1 signaling and increasing the expression of Snail. However, this process can be reversed by inhibiting miR-495-3p (target gene for SIPR3). This funding presents a new approach to treating radiation-induced pulmonary fibrosis [197].
In addition to miRNAs, Treg and macrophages are crucial factors in the regulation of radiation-induced pulmonary fibrosis. Treg can worsen radiation-induced pulmonary fibrosis by promoting β-catenin-mediated EMT, which severely restricts the use of radiotherapy [47]. Treg cells have been shown to produce Th2 cytokines, including IL-4, which have potent pro-fibrotic effects [198]. It has been suggested that the regulation of cytokines by Treg cells may be dependent on the activation of M2-type macrophages [199]. After radiation exposure, the lung is infiltrated by a significant number of M2 macrophages These macrophages produce high levels of TGF-β, which in turn promotes the mesenchymal transformation of lung epithelial cells [197, 200]. Phosphorylation of STAT3 mediates inhibition of Rho-associated convoluted helix kinase (ROCK), which in turn inhibits the conversion of macrophages to M2 and attenuates the extent of post-irradiation lung fibrosis [201].
Ionizing radiation has been shown to induce cellular senescence and secrete tyrosine kinase Fgr to promote the development of pulmonary fibrosis [60]. Senescent macrophages secrete SASP, such as tumor necrosis factor α, IL-1α, which are crucial in the development of pulmonary fibrosis [57]. Similarly, when type II alveolar cells are exposed to ionizing radiation, they undergo senescence and secrete a significant amount of SASP in response to IGF-1 signaling. This SASP induces IL-13, which is a powerful M2-inducing factor that promotes the development of pulmonary fibrosis [202].
ROS production is the canonical mechanism of tissue damage caused by ionizing radiation. Several studies have confirmed the role of ROS in the progression of radiation-induced fibrosis. The production of ROS triggered by radiation activates downstream NLRP3 inflammatory vesicles, resulting in increased production of IL-β by lung epithelial cells, which directly affects the transformation of fibroblasts into myofibroblasts. At the same time, ionizing radiation also activates the p53-mediated mitochondrial super-complex-related gene DPYSL4, which increases intracellular ATP levels and reduces ROS levels, thereby inhibiting NLRP3 activation [130, 203]. However, the classical pro-fibrotic signal, TGF-β, is also essential to investigate the mechanism of radiation-induced lung fibrosis. It is well known that the microenvironment in which cells are located is also a risk factor for fibrosis. Studies have shown that radiation can induce lung fibroblasts to secrete high amounts of lactate, leading to acidification of the extracellular environment and subsequent activation of pro-fibrotic TGF-β [204]. Current research on radiation-induced pulmonary fibrosis has focused on EMT, Treg, cellular senescence and ROS production, with insufficient research on metabolic alterations, proteasomal and other pathways. Therefore, further investigation of the mechanisms of lung fibrosis in patients with thoracic tumors is needed.
Radiation-induced skin fibrosis
Radiation-induced skin fibrosis is a common consequence of cancer radiotherapy and is caused by the prolonged presence of radiation dermatitis. Therefore, it is crucial to understand the mechanism of radiation-induced skin damage to enhance the effectiveness of cancer radiotherapy. In the study, some researchers examined the effect of ionizing radiation on gene expression profiles of human skin fibroblasts. This research indicate that genes associated with differentiation and inflammatory responses were active in irradiated human skin fibroblasts, where cell proliferation was inhibited [205]. Prolonged inflammation is an important mechanism for the development of radiation-induced fibrosis [3]. Inflammatory factors such as IL-1 and IL-6 have long been shown to promote the development of fibrosis [206].
According to research, TGF-β is a necessary pro-fibrotic signal for radiation-induced skin fibrosis to occur [207]. The interaction between stromal cell-derived factor-1α and its receptor CXCR4 activate the TGF-β/Smad signaling pathway through downstream MAPK signaling, ultimately leading to the promotion of fibrosis [208]. Inhibition of upstream platelet-derived growth factor receptor β has been found to reduce TGF-β expression, offering a promising anti-fibrotic pathway [209]. Another study has shown that low molecular weight fucoidan derived from brown seaweed can also inhibit TGF-β/Smad signaling [210]. Radiation-induced skin fibrosis is regulated by four core components, including EZR, MSN, CDC42 and ACTB, which play a significant role in the actin cytoskeleton [210]. A genome-wide analysis of radiation-induced skin fibrosis tissues revealed that ionizing radiation induces CpG dinucleotide demethylation in the exon 1 of the zinc transporter protein ZIP9, thereby recruiting the transcription factor Sp1 to promote ZIP9 expression [211]. Overexpression of ZIP9 activates the TGF-β/Smad signaling pathway and promotes the development of fibrosis, and Wnt/β-catenin is another classical pro-fibrotic pathway. Inhibition of this pathway has been found to attenuate radiation-induced skin fibrosis. Furthermore, Wnt/β-catenin also inhibits the conversion of epithelial and endothelial cells into myofibroblasts, which ultimately resists fibrosis [101].
Another group found that decreased methylation levels of diacylglycerol kinase α (DGKA) enhancer allow recruitment of the transcription factor early growth response 1 (EGR1), promoting increased radiation-induced DGKA expression [212]. The development of radiation-induced skin fibrosis is promoted by the epigenetic regulation of DGKA. To counteract this, de-epigenetic regulation of DGKA enhancers such as BET-bromodomain inhibitors are utilized to decrease the likelihood of radiation-induced skin fibrosis [213, 214].
Radiation-induced liver fibrosis
During radiotherapy for hepatocellular carcinoma, the liver tissue being irradiated often undergoes fibrotic changes. HSCs are the primary instigators of liver fibrosis and they release a range of pro-fibrotic factors in response to radiation stimulation that encourage liver fibrotic changes [215]. TGF-β1 is one of them, which can promote HSC activation and liver fibrosis by activating downstream Smad signaling [216]. Like fibrosis in other organs, activated TGF-β1 signaling can further exacerbate radiation-induced liver fibrosis by stimulating ROS and CTGF production [110, 217]. The activation of the TGF-β signaling pathway requires the activation of Smad3 by TGF-β receptors. However, the process can be inhibited by high levels of intracellular Smad7 which in turn blocks the TGF-β/Smad pathway [218]. Therefore, targeted inhibition of TGF-β signaling can be a potential treatment for radiation-induced liver fibrosis [219]. In addition, a study showed that the Hedgehog signaling pathway may promote radiation-induced activation of hepatic stellate cells and play an important role in radiation-induced liver fibrosis [220].
The effects of radiation on cells can be manifested as DNA damage and oxidative damage. When exposed to ionizing radiation, mitochondrial DNA can be damaged, leading to ROS production, increased secretion of SASP and p53 activation, which eventually manifests as severe liver fibrosis [221]. Proteomics studies revealed that radiation can lead to the expression of α-SMA, CTGF, and other proteins. Additionally, bioinformatics analysis has revealed that radiation can increase the expression of the CDC20 gene [29]. When the CDC20 gene was knocked down, liver tissue showed an increase in p21 expression, which in turn inhibited HSC proliferation, HSC senescence and fibrosis [222, 223]. In epigenetic regulation, radiation promotes the expression of demethylase α-ketoglutarate-dependent dioxygenase ALKBH5 in hepatic stellate cells, resulting in toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) mRNA demethylation, which in turn activates NF-κB signaling to promote hepatic stellate cell activation [224]. Increased expression of ALKBH5 resulted in the aggregation of monocytes and the promotion of macrophage M2 phenotype through the interaction of CCL5 and CCR5. This effect was further promoted by CCL5 secreted by M2 macrophages [224].
Radiation-induced liver fibrosis involves several non-coding RNAs that regulate its progression. Among them, circTUBD1 plays a crucial role as a regulator. In the presence of radiation, circTUBD1 forms a molecular sponge with miR-203a-3p, which in turn regulates the expression of Smad3. The increased Smad3 positively regulates circTUBD1, leading to the continuous accumulation of intracellular Smad3 and exacerbation of liver fibrosis [225, 226]. In addition, circTUBD1 also affects TLR4 signaling through miR-146a-5p, which promotes the secretion of pro-fibrotic factors such as IL-1β and TNF-α by post-illumination hepatic stellate cells [227]. For miR-146a-5p, it can inhibit PTPRA (protein tyrosine phosphatase receptor type A) gene expression, which leads to downstream SRC inactivation and inhibits radiation-induced fibrosis [228]. In all, radiation-induced liver fibrosis is a common toxic side effect in patients undergoing abdominal radiotherapy. The use of ionizing radiation for the treatment of liver cancer may also induce liver fibrosis is known to be a risk factor for liver cancer and should be minimized.
Radiation-induced cardiac fibrosis
Radiation-induced cardiac fibrosis is a process in which large amounts of collagen are deposited in the heart after exposure to ionizing radiation. In addition to radiation-induced pulmonary fibrosis, radiation-induced cardiac fibrosis is another common toxic effect in patients treated with radiotherapy for thoracic tumors. Attempts to mitigate ionizing radiation-induced cardiac fibrotic changes will reduce the incidence of arrhythmias and heart failure. The conversion of cardiac fibroblasts into myofibroblasts is the main hallmark of radiation-induced cardiac fibrosis [229]. Like the previously described radiation-induced lung and skin fibrosis, TGF-β1 and CTGF are involved in the regulation of cardiac fibrosis [230]. In addition to activating TGF-β1/Smad3 signaling and promoting fibrosis progression, ionizing radiation may also regulate fibrosis through Smad-independent pathways such as PI3K/AkT and Rho/ROCK [231]. Peroxisome proliferator-activated receptor α (PPARα) is a member of the intranuclear receptor transcription factor superfamily that regulates the expression of target genes and has the function of regulating cardiac energy metabolism [232]. Transcriptomics and proteomics have shown that PPARα is involved in the regulation of radiation-induced cardiac fibrosis and is associated with TGF-β signaling [233]. PPARα is shown to exert an antifibrotic effect by inhibiting TAK1 phosphorylation in the Smad-independent TGF-β pathway. However, radiation has shown to decrease in PPARα expression, which in turn promotes TAK1 activation and downstream JNK and c-Jun activation, ultimately leading to the upregulation of fibrotic genes [234, 235]. The Smad-independent TGF-β pathway is the only one associated with PPAR’s ability to counteract radiation-induced fibrosis. However, the TGF-β/Smad pathway is not affected [234]. Growth differentiation factor 15, a member of the TGF-β superfamily is overexpression radiation-induced cardiac fibroblasts. This overexpression promotes radiation-induced cardiac fibrosis through the Smad-independent MAPK/ERK pathway [236].
Additionally, radiation-induced myocardial fibrosis is associated with cardiac energy metabolism. In a study comparing differentially expressed proteins before and after radiation-induced injury, it was discovered that the proteins primary associated with energy metabolism and fibrosis [156]. Subsequent research confirmed that radiation causes mitochondrial damage in cardiomyocytes, promoting the development of cardiac fibrosis. AMPKα is an intracellular energy receptor involved in the regulation of redox homeostasis [237]. Activation of AMPK has been shown to counteract the imbalance of oxidative stress in the heart caused by radiation, which can prevent myocardial fibrosis remodeling [238]. In a study using the agonist adropin, encoded by the energy homeostasis-related gene Enho, adropin promotes downstream PI3K/Akt signaling through activation of vascular endothelial growth factor receptor 2 [239]. Activated PI3K/Akt signaling decreases the expression of NOX4, eNOS and cleaved-Caspase 3, which attenuates radiation-induced myocardial fibrosis [239].
Oxidative stress is also an important factor in the regulation of fibrosis. Ionizing radiation causes an imbalance between oxidants such as NADPH oxidase and reductants such as superoxide dismutase, resulting in a large amount of ROS [240]. NADPH oxidases mainly include NADPH1-5, DUOX1 and DUOX2, are mainly responsible for this imbalance. Research has demonstrated that radiation can cause an upregulation of DUOX1/2 in cardiac tissue [241]. The induction of IL-4 or MAPK-mediated IL-13 can initiate this process [242, 243]. Furthermore, the expression of NOX2 and NOX4 is stimulated by radiation [241]. However, the excessive production of oxidase can lead to the overproduction of ROS, which can ultimately contribute to the development of myocardial fibrosis. In addition, the overproduction of ROS can also oxidize lipids, resulting in the production of lipid peroxides that can cause further damage to myocardial tissue and blood vessels [244, 245]. Vascular injury plays a crucial role in myocardial fibrosis, and abnormal platelet activation increases the risk of both vascular injury and radiation-induced myocardial fibrosis. However, the presence of platelet tissue factor pathway inhibitor-α can effectively inhibit the coagulation process, thereby reducing the severity of radiation-induced myocardial fibrosis [246]. The imbalance of oxidative stress affects immune cells and can indirectly regulate fibrosis. Ionizing radiation can induce a large amount of ROS, which activates the conversion of CD4+ T cells into Th2 lymphocytes. These lymphocytes secrete potent pro-fibrotic factors like IL-4 and IL-13 [247].
Non-coding RNAs are another important regulator of cardiac fibrosis. It was found that miR-21 is a pro-fibrotic factor that is highly expressed in response to radiation stimulation, and that high levels of miR-21 promote the expression of extracellular matrix regulatory genes GDF15, Grem1 and CCL7 [248]. A previous study identified a substance with antifibrotic effects, molecular hydrogen, which acts by decreasing miR-21 levels as well as increasing miR-15b levels [249]. Recent studies have highlighted the significance of sensory nerves in regulating radiation-induced cardiac fibrosis. These nerves can have a dual role in the process, acting as both anti-fibrotic agents by modulating mast cells and a pro-fibrotic role by increasing the expression of the injury-associated nuclear receptor NR4A1/2 [250]. Therefore, the effect of sensory nerves on radiation-induced fibrosis needs to be further investigated.
Radiation-induced renal fibrosis
Radiation-induced renal fibrosis is a massive proliferation of fibrous tissue in the kidney caused by radiation, resulting in loss of renal function. It was previously reported that radiation can activate the renin–angiotensin–aldosterone system (RAAS), which in turn leads to vascular injury and promotes structural fibrosis in the kidney [251]. Radiation-induced oxidative stress imbalance is a significant mechanism of fibrosis. In addition, the excessive ROS oxidize lipids, which subsequently promote the expression of fibrotic factors [189]. These excess ROS cannot be scavenged, leading to DNA damage. The damaged DNA, in turn promotes apoptosis of kidney cells, further exacerbating radiation-induced renal fibrosis [252]. The fat-soluble vitamin coenzyme Q10 (CoQ10) may reduce excessive oxidative stress and lipid peroxidation, thus playing a role in reducing radiation-induced renal fibrosis [253]. Zinc is a micronutrient with antioxidant properties, and a recent study has found that zinc nanoparticles can be used to treat radiation-induced renal fibrosis by reducing oxidative stress [254]. Endoglin, a co-receptor for transforming growth factor is a potential pro-fibrotic factor. Mice with low expression of endoglin exhibit reduced levels of IL-1β and IL-6, both of which are pro-fibrotic factors [255].
Radiation-induced intestinal fibrosis
The intestine is one of the most sensitive organs to ionizing radiation. Radiation-induced bowel injury often occurs during radiotherapy of patients with abdominal tumors. Like other organs, radiation-induced injury to the intestine is classified as acute and chronic. Acute radiation-induced injury manifests as an acute gastrointestinal syndrome such as intestinal mucosal rupture, bleeding, abdominal pain, and diarrhea, whereas chronic radiation-induced injury manifests as fibrosis [256]. Ionizing radiation damage to the intestinal mucosa is mainly caused by inducing inflammation of the small intestinal epithelium and apoptosis and cellular DNA damage [257].
Radiation-induced intestinal fibrosis is triggered by the interaction of multiple cytokines, immune cells, and enterocytes [258]. The pro-fibrotic factor CTGF adversely affects radiation-induced intestinal fibrosis, while attenuating Rho kinase signaling attenuates CTGF expression and inhibits fibrosis-like changes in intestinal smooth muscle cells [113, 259]. It is important to note that previous studies have not taken into consideration the role of intestinal flora in this relationship between intestinal fibrosis and ionizing radiation. While ionizing radiation causes intestinal fibrosis, it also leads to changes in the composition and β-diversity of the intestinal flora. Using nicotinamide mononucleotide (NMN) to treat mice with damaged intestines after irradiation, NMN was found to remodel the structure of the intestinal flora and alter its metabolic pathways, thereby reducing the extent of intestinal fibrosis [260].
Immune cells and their interactions with intestinal cells are also important pro-fibrotic factors. Fusion of bone marrow-derived macrophages with intestinal stromal cells promotes radiation-induced intestinal fibrosis [261]. Eosinophils also have been implicated in the regulation of radiation-induced intestinal fibrosis. Ionizing radiation causes intestinal crypt cell death, which leads to an increase in extracellular ATP levels, which in turn activates the secretion of C–C motif chemokine 11 (CCL11) by surrounding α-SMA-positive cells. CCL11 then interacts with CCR3 and recruits eosinophils [262]. In addition, activated α-SMA + cells promote eosinophil expression of TGF-β1 through granulocyte–macrophage colony-stimulating factor signaling and promote the development of radioactive intestinal fibrosis [262]. Lgr5+ intestinal stem cells have been identified as playing a crucial role in regenerative repair of radiation-induced intestinal injury [263], but their involvement in fibrosis remains unclear.
Diagnosis of radiation-induced fibrosis
The progression and severity of fibrotic disease is contingent upon the amount of fibrosis present within the organ [264]. As such, early detection and diagnosis of fibrosis is crucial. The gold standard for diagnosing fibrosis is through histology, which is typically obtained through fine-needle biopsies or surgical specimens [265]. Histologic scoring methods vary from organ to organ and usually depend on the etiology of the fibrosis. However, histologic assessment of fibrosis still has serious limitations, such as percutaneous liver or kidney biopsy, which carries risks including bleeding [266]. Biopsy results may represent only a small fraction of the organs in which fibrosis is distributed [267]. As a result, many non-invasive methods have been developed. Some of them are used in clinical practice to assess the progressive stages of fibrosis. These techniques include imaging methods, mechanical and functional tests, serum biomarkers, and genetic risk factors for fibrosis.
MRI and CT
Imaging techniques are widely used in patients with chronic diseases where fibrosis is suspected. In the liver, MRI-derived proton density fat fraction has been widely used to quantify liver fat content and has been the focus of early clinical trials of antifibrotic drugs in non-alcoholic steatohepatitis [268]. Cardiac MRI is an essential tool in clinical algorithms due to its ability to assess various aspect of the heart such as cardiac wall thickness, myocardial mass, late gadolinium enhancement and myocardial extracellular volume fraction. The latter is particularly important as it is closely linked to myocardial fibrosis, making cardiac MRI a valuable diagnostic tool for detecting and monitoring heart conditions [269]. Regarding pulmonary fibrosis, high-resolution chest computed tomography (CT) provides diagnostic and prognostic information in patients with pulmonary fibrosis, and various radiologic parameters have been established to stage disease severity, making lung biopsy rarely necessary [270]. For example, homogeneous ground-glass attenuation, representing early radiation pneumonitis, can be detected on CT imaging a few weeks after completion of therapy, even in the absence of findings on chest radiography [271–273].
Elastography techniques
New elastography techniques are available for the assessment of liver fibrosis. These methods include ultrasound-based transient elastography (TE, also called fibroscan), acoustic radiation force impulse imaging (ARFI) and shear wave elastography (SWE), and MRI-based elastography (MRE) [265]. These methods have been widely used in patients with chronic liver disease, and this technique has been recommended for use in the diagnosis of liver fibrosis [274]. For renal fibrosis, similar ultrasound and MR elastography approaches are currently being adapted [264]. In contrast, cardiac fibrosis can be typically diagnosed by functional testing and traditional methods such as volumetric tracings, echocardiography or cardiac MRI.
Biomarkers
Although circulating biomarkers of fibrosis are simple, reproducible and inexpensive compared to histological, imaging or mechanical assessments, their major drawbacks include a general lack of specificity for fibrosis, a large overlap between stages of fibrosis progression, and the presence of many confounding factors of individual markers. In pulmonary fibrosis, fibrosis-related factors such as chemokines (IL-8, CCL18), proteases (MMP-1, MMP-7) or growth factors have been suggested to have diagnostic potential for fibrosis, but their utility in clinical routine has not been established [275]. In renal fibrosis, potential renal fibrosis biomarkers include TGF-β, monocyte chemoattractant protein-1 (MCP-1) and MMP-2, or urinary excretion of collagen III and related proteins such as the N-terminal pro-peptide of type III collagen [276]. In the specific field of liver fibrosis, numerous biomarkers have been suggested and are currently being utilized to a certain degree. These include hyaluronic acid and the N-terminal pro-peptide of type III collagen, also known as PIIINP [265]. The best diagnostic and prognostic accuracy can be achieved when biomarkers are used in predictive models and when different risk factors are integrated with information from multiple biomarkers [277].
In the realm of fibrosis biomarker research, there are several innovative to explore, including mitochondrial DNA, circulating miRNA, lncRNA and microbiome signatures found in stool samples [275]. A promising avenue of investigation involves identifying biomarkers that accurately reflect the pathogenic process of fibrogenesis or fibrolysis. The different collagens and collagen fragments that are being released into the circulating might therefore have exceptional potential as biomarkers [278]. New imaging approaches that combine visualization with molecular targeting have already shown promising results in molecular MRI for animal models of liver, kidney, heart and lung fibrosis [279].
Diagnosis by PET-CT
The use of radionuclides to detect fibrotic lesions is an emerging field. Positron emission tomography (PET)-CT is a relatively advanced imaging technique in the field of nuclear medicine. It can reflect the signal of lesions by detecting the concentration of radiotracer in the lesion area [280]. 18F-fluciclatide is a radiotracer that can bind to αvβ3 integrin transmembrane receptors and activate extracellular matrix modifications. Its uptake by tissues can reflect the activity of fibrosis, but also angiogenesis and inflammatory responses, which greatly reduces the specificity of 18F-fluciclatide [281]. In recent years, studies have focused on fibroblast activating protein. This is a protein that is low expressed in normal tissues and significantly increased in fibrotic tissues and is an ideal target to mark and detect myofibroblast activation [282, 283]. Based on this, radiolabeled fibroblast activation protein inhibitor was used to assess and quantify the expression of fibroblast activating protein [284]. The [68 Ga] Ga-FAPI-04 PET-CT was originally used for tumour imaging, but an increasing number of studies are now demonstrating its potential value in the diagnosis of cardiac, pulmonary, renal, intestinal and hepatic fibrosis, with the advantage of being non-invasive and sensitive [14, 285–288]. In addition, fibrosis occurs later than fibroblast activation and FAP expression; therefore, the use of [68 Ga] Ga-FAPI-04 PET-CT facilitates the early diagnosis of fibrosis [286, 289]. Interestingly, a recent study used PET-MR in combination with [68 Ga] Ga-FAPI to detect intestinal fibrosis in patients with Crohn's disease [290]. Currently, radiotracers have not been used to detect radiation-induced fibrosis. And the technique is only in preclinical studies and more evidence is needed to prove its clinical application.
Advances in the treatment of radiation-induced fibrosis
Increasingly advanced modern radiotherapy has virtually minimized exposure to radiation of normal tissues, but radiation-induced fibrosis is still a common and severe adverse chronic event. Although amifostine is so far the only thiol derivative approved by Food and Drug Administration (FDA) for treatment of radiation-induced damage, its use is limited for wide clinical application due to toxicity at the therapeutic dose. Unfortunately, at present, no more effective measures can completely alleviate or reverse tissue fibrosis, so symptomatic treatments for fibrosis-related symptoms are commonly adopted, like non-steroidal anti-inflammatory drugs (NSAIDs) for muscular pain, injection of botulinum toxin for dystonia, and oxygen therapy or mechanical ventilation for respiratory failure. Therefore, prevention is the crucial first step in mitigating radiation-induced fibrosis. In addition to careful consideration of the dose and timing of radiotherapy, it is important to eliminate any identified risk factors. Smoking, for example, has been linked to a significantly increased incidence and severity of radiation-induced fibrosis, likely due to impaired oxygenation and elevated carboxyhemoglobin levels [291, 292]. For decades, a variety of medications have been studied for the treatment or prevention of radiation-induced tissue fibrosis and most of them yielded promising results (Table 1).
Table 1.
Current medications studied for radiation-induced fibrosis
| Name | Molecular type | Target/action | Site | Stage of project trial | References |
|---|---|---|---|---|---|
| SOD-TAT | Ligand-modified SOD | Scavenging ROS and free radicals | Lung | Preclinical study | [296] |
| AEOL10113 | Nonenzymatic SOD mimic | Suppressing redox-regulated pathways | Lung, rectum, prostate | Preclinical study | [300, 302, 305] |
| Melatonin | Indole-derived hormone | Scavenging ROS and RNS and activating antioxidative enzymes | Lung | Preclinical study | [307] |
| Combined PTX and Vit E | A methylxanthine derivative and a vitamin | antioxidant, anti-inflammatory and suppressing the TGF-β pathway | Neck and chest | In clinical use | [314–317] |
| Triptolide | Diterpenoid epoxide | Inhibiting the activity of alveolar macrophages and the IKKβ/NF-κB pathway | Lung | Preclinical study | [324] |
| SKI2162, EW-7197, LY2157299, LY2109761 | TGFβR I inhibitor | Blocking TGF-β receptor I | Lung, skin | Preclinical study | [325–328] |
| P144 | Hydrophobic peptide | Binding to soluble TGF-β1 | Muscle | Preclinical study | [329] |
| Imatinib | Receptor tyrosine kinase inhibitor | Blocking PDGF receptor | Lung, skin | Preclinical study | [331] |
| Pamrevlumab | Monoclonal antibody | Blocking CTGF receptor | Lung | Preclinical study | [105] |
| sLRP6E1E2 | Soluble Wnt receptor | Binding to extracellular Wnt ligands | Skin | Preclinical study | [101] |
| Curcumin | Natural polyphenol | Activating antioxidative enzymes, inhibiting NF-κB pathway and downregulating inflammatory factors | Lung, skin | Preclinical study | [364, 366] |
| Rosmarinic acid | Natural polyphenol | Inhibiting RhoA/Rock pathway | Lung, parotid glands | Preclinical study | [370, 371] |
| GSPs | Plant extract | Ameliorating mitochondrial dysfunction and inhibiting MAPK pathway | Lung, breast | In clinical trials (NCT00041223) | [377] |
| Pirfenidone | Pyridone analog | Inhibiting TGF-β1/Smad3 pathway | Lung, skin | In clinical trials (NCT03902509) | [385] |
| Nintedanib | Receptor tyrosine kinase inhibitor | VEGF receptor, FGF receptor and PDGF receptor | Lung | Preclinical study | [386] |
| Esomeprazole | Proton pump inhibitor | inducing of MAPK/Nrf2/HO1 pathway and inhibiting of DDAH/iNOS pathway | Skin | Preclinical study | [345] |
| Metformin | activating AMPK and Inhibiting respiratory chain complex I | Lung, heart, skin | Preclinical study | [390–394] | |
| Losartan | ACEI | inhibiting TGF-β/Smad signaling pathway | Heart, breast | In clinical trials (NCT05637216) (NCT05607017) | [354] |
| Deferoxamine | Iron chelator | Inhibiting HIF-1α | Skin | Preclinical study | [356–358] |
| Methoxyestradiol | Metabolite of estradiol | inhibiting HIF-1α | Lung, skin | Preclinical study | [359, 361] |
| MSCs | secreting anti-inflammatory and antifibrotic factors | Lung, parotid glands | In clinical trials (NCT02513238) (NCT02277145) | [400] |
SOD superoxide dismutase; PTX pentoxifylline; Vit E vitamin E; GSPs Grape seed proanthocyanidins; MSCs Mesenchymal stem cells
Antioxidant treatment
Oxidative stress is one of the central pathophysiological mechanisms in radiation damage, so lots of radio-prophylactics are developed based on free radical scavenging or antioxidation. SOD is the most principal endogenous defense against oxygen radicals [293]. The clinical application of SOD is limited due to its large molecular weight, which prevents it from passing through the cell membrane freely [294]. Additionally, a recent study found that the topical SOD was not effective in treating post-radiation fibrosis [295].
Over recent years, varied ligand-modified SODs and nonenzymatic SOD mimics have been synthesized to overcome the handicaps of native SODs. HIV-1 TAT protein transduction domain (YGRKKRRQRRR) is usually utilized to carry larger molecules across the cell membrane. A recombinant protein SOD-TAT that fuses hCuZn-SOD with TAT enhanced pulmonary antioxidant ability and thus reduced radiation-induced pulmonary fibrosis [296]. Furthermore, a bifunctional antioxidant enzyme named GST-TAT-SOD fused with glutathione-S-transferase (GST) and TAT-SOD was constructed, which manifested a strong radioprotective activity against whole-body irradiation in mice [297].
A series of Mn (III) porphyrin-based SOD mimics such as MnTE-2-PyP5+ (AEOL10113) and MnTDE-2-ImP5+ (AEOL10150) have been assessed in various preclinical models as radioprotectants. Several studies suggested that these Mn porphyrins ameliorated radiation-induced lung and rectum fibrosis by suppressing redox-regulated pathways including HIF-1α, NF-κB, and TGF-β [298–302]. Previous studies have suggested that Mn porphyrins protect normal tissues from radiation-induced damage, and act as chemo-/radio-sensitizers in tumor cells [303, 304]. However, a study showed that AEOL-10150 increases the survival in lethal lung radiation injury in rhesus macaques but does not improve the incidence of pneumonitis or the severity of fibrosis [305].
Melatonin has been identified as a potent antioxidant that can directly scavenge ROS and RNS. Additionally, it can indirectly stimulate antioxidative enzymes and suppress pro-oxidative enzymes [306]. Numerous studies have demonstrated that melatonin can reduce radiation-induced pneumonitis and lung fibrosis [307, 308]. In addition, the ease with which melatonin penetrates all cell types and low toxicity attributed to the instinctive metabolic pathway might be the natural advantage of melatonin. Meanwhile, in vitro studies have revealed the antitumor activity of melatonin when used alongside irradiation [306].
Other kinds of antioxidant nutrients such as α-lipoic acid [309, 310] and coenzyme Q10 [311] are also demonstrated to be beneficial to radiation-induced fibrosis in preclinical models. Although the protective effect of antioxidants such as α-tocopherol (vitamin E; Vit E) during radiation therapy is well-established in radiation-induced mucositis, their effects on the human body are not yet fully understood. One essential concern is to safeguard normal tissues from radiation damage while not interfering with tumor treatment. However, previous studies have highlighted that the use of high doses of antioxidants like vitamin E; Vit E during radiation therapy may give rise to a higher local recurrence rate in patients with head and neck tumors [312, 313].
Anti-inflammatory treatment
Plentiful studies demonstrated that combined treatment with anti-inflammatory pentoxifylline (PTX) and vitamin (Vit) E can reverse radiation-induced cervicothoracic fibrosis by suppressing the TGF-β pathway. Marked efficacy can be observed clinically after three months of treatment with combined PTX (800 mg/d) and Vit E (1,000 IU/d). Fibrosis continued to be alleviated along with the time of treatment, and the mean time to maximal treatment effect was 24 months [314, 315]. There was a risk of a rebound effect if treatment was too short. Thus, treatment of fewer than 3 months is not recommended in patients with severe radiation-induced fibrosis [316]. A study, in which the median time from radiotherapy to the trial showed that 6-month treatment of Vit E and pentoxifylline did not provide any benefits [317]. Poor efficacy is presumably ascribed to relatively shorter treatment and more severe or older established fibrosis. Additionally, combined treatment with PTX and Vit E started 1–3 months after radiotherapy has been proven to prevent fibrosis [318]. Recently, Erbium: YAG fractional laser ablation has been exploited for cutaneous co-delivery of PTX and d-α-tocopherol succinate. This method has shown to temporarily impair the stratum corneum and increase the bioavailability of PTX in the skin potentially providing an effective alternative to systemic exposure [319, 320]. Triptolide (TPL) is an herb-purified diterpenoid epoxide with potent anti-inflammatory and immunosuppressive effects. TPL mitigated radiation-induced pulmonary fibrosis in a mouse model, and possible mechanisms have been explored [321, 322]. TPL could inhibit the infiltration, Inflammatory factors secretion, and phagocytosis of alveolar macrophages and eventually decrease paracrine activation of myofibroblasts [323]. TPL also inhibited the IKKβ/NF-κB pathway and thus reduces LOX production in fibroblasts [324].
Targeted treatment for fibrosis
Targeted treatment for the TGF-β1 signaling pathway
As we discussed earlier, the TGF-β1 signaling pathway plays a crucial role in the development of fibrosis. Targeted inhibition of these correlated signaling pathways is a promising therapeutic method. Several novel TGF-β1 receptor kinase inhibitors, including SKI2162 [325], vactosertib (EW-7197) [326], galunisertib (LY2157299) [327] and LY2109761 [328], are demonstrated to specifically block the TGF-β1 signaling pathway and hence suppress radiation-induced murine pulmonary and skin fibrosis. P144 is a hydrophobic peptide that can directly binds to soluble TGF-β1, thus block the binding of TGF-β1 to the TGF-β1 type I receptor. Intravenous administration of P144 clearly reduced radiation-induced muscle fibrosis in rabbits [329]. Some prospective studies of topical P144 in patients with systemic sclerosis have been completed, but these inhibitors of TGF-β signaling pathway have not been tested for efficacy on radiation-induced fibrosis on humans.
Targeted treatment against the PDGF signaling pathway
In a study, several receptor tyrosine kinase inhibitors (RTKIs) that target platelet-derived growth factor receptor-α (PDGFR-α) and PDGFR-β were also found to reduce lung fibrosis after radiation injury and prolong animal survival [330]. Among these inhibitors, Imatinib, commonly used for the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors, was shown to attenuate radiation-induced pulmonary fibrosis in mice whether it was administered before radiation or after acute inflammation [331]. In irradiated skin, treatment with imatinib was found to not only reduce the activation of PDGFR but also decreased the levels of TGF-β [209]. Combing the inhibition of TGF-β1 and PDGF signaling may be of more positive effect [327].
Targeted treatment against CTGF
CTGF plays a critical role in radiation-induced fibrogenesis. Pamrevlumab (FG-3019) is a fully human recombinant monoclonal antibody against CTGF and is in an ongoing phase 3 trial (NCT03955146) in patients with idiopathic pulmonary fibrosis (IPF) [332]. Blocking CTGF with FG-3019 could prevent and reverse pulmonary fibrosis after thoracic irradiation with prolonged survival and improved lung function in mice. A significant finding is that lung remodeling, which had already occurred by 16 weeks after irradiation, was still reversed and lung function was restored with FG-3019 treatment [115].
Targeted treatment against the Rho/ROCK signaling pathway
Targeting of Rho/ROCK signaling pathway is another way to inhibit the effect of CTGF. Many preclinical studies have shown that ROCK inhibitors alleviate pulmonary fibrosis through a variety of potential mechanisms [201]. Statins are discovered to downregulate CTGF expression by inhibition of Rho protein is prenylation, and thereby repress fibrosis [333]. In preclinical models, both pravastatin and lovastatin have been shown to reduce radiation-induced fibrosis [334–337]. A prospective trial was conducted to evaluate the effectiveness of pravastatin treating with delayed cutaneous and subcutaneous grade-2 radiation-induced fibrosis in patients. In a study involving 60 patients, pravastatin 40 mg/d for 12 months for 12 months. The results demonstrated that this treatment successfully reversed established radiation-induced cutaneous and subcutaneous fibrosis, as evidenced by a decrease in thickness and clinical signs of fibrosis [338].
Targeted treatment for the Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is a significant target in fibrotic reactions and there have been numerous strategies to specifically block this pathway. XAV939 can preserve the β-catenin degradation complex’s integrity, which ultimately result in the degradation of β-catenin and the inhibition of Wnt/β-catenin signaling. It was shown that XAV939 significantly improved the pulmonary fibrosis induced by bleomycin and increased mouse survival [339]. In another study, researchers found that radiation-induced dermal fibrosis can be improved by inhibiting the Wnt/β-catenin signaling pathway. This was achieved by adenovirus gene therapy, which expressed a Wnt antagonist that binds to Wnt ligands [101].
Proton pump inhibitors
Clinical data revealed that proton pump inhibitors (PPIs) are associated with favorable outcomes in patients with IPF and, in fact, antioxidant and anti-inflammatory activity of PPIs has been reported in preclinical models [340, 341]. Previous research demonstrated the pleiotropic effect of esomeprazole, which significantly suppressed lung inflammation and fibrosis in vivo and in vitro through induction of the MAPK/Nrf2/HO1 pathway and inhibition of the DDAH/iNOS pathway [342–344]. In addition to assessing the effectiveness of an esomeprazole topical product, also known as dermaprazole on radiation-induced dermatitis, the study found that dermaprazole was able to speed up the healing process of wounds and reduce scarring on the irradiated skin [345]. Another recent study revealed that esomeprazole can enhance the anti-fibrotic efficacy of pirfenidone through complementary molecular mechanisms [346].
Angiotensin-converting enzyme inhibitors
Angiotensin-converting enzyme inhibitors (ACEIs) have been shown to impact fibrosis in various tissues, although the exact mechanism remains unclear. It may be linked to the inhibition of the TGF-β1/Smad signaling pathway [347]. Clinical studies have confirmed the effectiveness of losartan in treating of IPF and keloid [348, 349]. Some kinds of ACEIs mitigated fibrosis after thorax irradiation in preclinical models [350–353]. A retrospective study showed that ACEIs decrease the risk of radiation pneumonitis in lung cancer patients receiving thoracic irradiation [354]. There have been two undergoing clinical trials that aim to investigate whether losartan can prevent breast (NCT05637216), or cardiac muscle (NCT05607017) fibrosis induced by radiotherapy in patients with breast cancer.
HIF-1α inhibitors
Deferoxamine (DFO), an iron chelator approved for treating iron-overload-associated diseases, can limit the iron-dependent generation of ROS and the degradation of HIF-1α. HIF-1α is a transcription factor that upregulates pro-angiogenic genes, including vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS). It has been shown that DFO mitigates radiation-induced hypovascularity and improves bone regeneration and soft tissue elasticity [355, 356]. Topical DFO has been developed for skin application and has been shown to improve cutaneous vascularity and tissue pliability in an irradiated nude mouse model [357]. In comparison to treatment after recovery from irradiation, prophylactic treatment with transdermal DFO has been found to be more effective in decreasing levels of markers of ROS and apoptosis, improving tissue perfusion, and mitigating radiation-induced skin fibrosis [358]. Another HIF-1α inhibitor, 2-Methoxyestradiol (2-ME), which is a metabolite of estradiol, has been approved as an anti-cancer drug that inhibits tubulin polymerization [359]. In a study conducted on irradiated mice, it was found that 2-ME had positive effects on radiation-induced pulmonary injury and fibrosis. The mice model improved survival rates and better pulmonary functions. These effects were attributed to the inhibition of radiation-induced inflammation and vascular EndMT which depends on HIF-1α [30, 360, 361]. The protective effects of 2-ME were also observed in the skin of irradiated mice on the vascular endothelium.
Natural products
In recent years, there has been a growing interest in natural products that have healthcare benefits. These products are often considered to be more cost-effective, less toxic, and have a wider therapeutic window than l artificial drugs. Several plant extracts that contain antioxidant polyphenols have been found to have both radio-sensitization and radioprotection effects, making them a safe and effective adjuvant in radiotherapy. Curcumin (diferuloylmethane) ameliorated radiation-induced pulmonary, heart, liver, and skin damage and fibrosis, but does not protect tumor cells from radiation killing [362–366]. At present, randomized controlled trials have been conducted to evaluate the efficacy of curcumin to treat radiation dermatitis and oral mucositis. Curcumin mouthwash was equally effective and safe in prevention of the radiation-induced oral mucositis as the standard medication benzydamine (a kind of NSAID) [367]. Oral curcumin, compared with placebo, did not demonstrate a detectable benefit for radiation dermatitis in breast cancer patients [368, 369]. Rosmarinic acid (RA) substantially reduced radiation-induced fibrosis of the parotid glands and lungs. The possible mechanism is that RA upregulates miR-19b-3p, which targets myosin phosphatase target subunit 1 (MYPT1), and thereby attenuates RhoA/Rock signaling [370, 371]. Grape seed proanthocyanidins (GSPs) have been found to protect against radiation damage to various tissues by ameliorating mitochondrial dysfunction and suppressing MAPK Signaling Pathway [372–376]. However, a phase 2 trial administering GSPs orally failed to show efficacy in treating breast induration in patients who underwent radiotherapy for breast cancer [377]. In addition, there are also quercetin [378] and its monoglycoside [379], naringenin [380], and resveratrol [381], all of which are shown with radioprotective and antifibrosis effects. The exact pharmacological mechanisms are still unclear though, the extensive biological functions of these natural products may just match the intricate mechanisms of fibrosis.
Pirfenidone and nintedanib
The pathophysiologic events induced by radiation are like those that occur in idiopathic pulmonary fibrosis (IPF) [328]. Both pirfenidone and nintedanib are small-molecule oral drugs and their ability of retarding the rate of progression of IPF have been approved by FDA. Unfortunately, these two drugs do not completely stop the progression of IPF [382]. Pirfenidone selectively downregulates the expression of TGF-β1, PDGF, and other fibrosis-associated cytokines, and nintedanib is a tyrosine kinase inhibitor targeting the VEGF receptor, FGF receptor, and PDGF receptor. Animal experiments have shown that pirfenidone alleviates radiation-induced pulmonary and intestinal fibrosis and reduces the expression of the TGF-β1/Smad3 signaling pathway [107, 383, 384]. According to a small-scale pilot study treatment with Pirfenidone resulted in a slight improvement in limited activity caused by after-radiotherapy fibrosis [385]. A phase 2 clinical trial (NCT03902509) is now underway and aims to confirm the efficacy of pirfenidone in the treatment of post-radiation lung injury. Nintedanib histologically reduced radiation-induced lung fibrosis in mice [386]. There have been two phase 2 clinical studies that aim to evaluate the effect of nintedanib on radiation pneumonia. One of the two (NCT02452463) was terminated prematurely owing to low accrual, while another (NCT02496585) is still ongoing. However, nintedanib inhibits wound healing. A concerning case study reported, a patient with head and neck cancer who was undergoing radiotherapy and was also taking nintedanib for their IPF. This patient experienced a significant delay in their recovery from radiation mucositis, highlighting the potential negative effects of nintedanib on wound healing [387]. This might suggest that nintedanib should not be used during the acute injury and repair phase after radiotherapy.
Metformin
Metformin is the most widely prescribed medicine to treat type 2 diabetes mellitus, also manifests radio-sensitization for tumors and radioprotection for normal tissue [388]. Targeting mitochondria and subsequent activation of AMPK are considered the hinge of pleiotropic actions of metformin [389]. Recent studies indicate that metformin can attenuate radiation-induced fibrosis in the lung [390–392], heart [393] and skin [394]. The proposed explanation suggests that metformin candecrease mitochondrial ROS production by inhibiting of respiratory chain complex I, activate the DNA repair pathway through AMPK activation, and reverse the upregulation of NADPH oxidases such as NOX4 and DUOX1/2 induced by radiation. While there have been several randomized clinical trials aimed at testing the radio-sensitization effect of metformin, there has yet to be a clinical study specifically designed to test its radioprotective effects.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are multipotent stem cells that can be isolated from numerous stromal tissues within the body and can differentiate into adipocytes, chondroblasts or osteoblasts in vitro. MSCs exhibit immunomodulatory and paracrine functions along with low immunogenicity and easy accessibility. Adipose-derived MSCs (Ad-MSCs), bone marrow-derived MSCs (BM-MSCs), and umbilical cord-derived MSC (UC-MSCs) are the commonly used sources for the extraction of MSCs. In recent years, there have been lots of preclinical studies demonstrating that the production of MSCs plays a crucial role in radiation-induced fibrosis. In a study conducted on mice, it was found that systemic infusion of BD-MSCs altered the phenotype of macrophages and suppressed local inflammation in a TNF-receptor 2-dependent manner. This resulted in the inhibition of cutaneous radiation-induced fibrosis [125]. Similarly, Ad-MSCs were found to attenuate radiation-induced murine colon fibrosis by releasing hepatocyte growth factor (HGF) and tumor necrosis factor-stimulated gene 6 (TSG-6) [395]. The anti-fibrotic effects of Ad-MSCs on irradiated lungs by stimulating the endogenous secretion of HGF and PGE2 [396]. It was recently found that Ad-MSCs medium inhibited the induction of EMT via Wnt/β-catenin signaling in vitro and in vivo, which was mediated by Ad-MSCs-secreted DKK-1, a potent antagonist of the canonical Wnt pathway [106]. A study illustrated that injection of Ad-MSCs within two hours and one week of thoracic irradiation resulted in beneficial outcomes in the treatment of radiation-induced pulmonary fibrosis compared to one-time injection [397]. In addition, UC-MSCs modified with SOD3 or decorin (DCN) are found to show more beneficial effects on radiation-induced fibrosis compared to conventional MSCs. These cell-based gene therapies can realize the combined delivery of UC-MSCs and SOD3 or DCN, which are respectively an antioxidant enzyme in extracellular spaces and a natural inhibitor of TGF-β signaling. Meanwhile, administration of these UC-MSCs within hours after irradiation resulted in much better improvement than that after dozens of days [398, 399]. MSCs are tested clinically for the treatment of radiation-induced xerostomia (NCT02513238) and pulmonary fibrosis (NCT02277145). However, in addition to these promising results, we must confront the current limitations of MSC-based therapy. Safety is the most concerned issue as the pro-tumorigenic effect is found in MSCs [400]. Fortunately, from the existing clinical data, MSC-based therapy may be safe. The heterogeneity of MSCs that impacts quality control is another of the major obstacles to the realization of clinical application at present. Besides, there is no sufficient data that fully answer problems such as the optimal source and culture conditions of MSCs, and the best route and dose of administration. In short, the feasibility and efficacy of MSCs in the treatment of radiation-induced fibrosis in humans are difficult to be confirmed in the short term.
Conclusions and future directions
Although radiotherapy technology has advanced to a point where accuracy can be precisely controlled, the surrounding normal tissues are still affected by radiation. The current understanding of fibrosis mechanisms has primarily centered around oxidative stress, cytokines, and cellular interactions related to fibrosis. In addition, the emerging roles of metabolic reprogramming, the cGAS/STING pathway, and the proteasome pathway in radiation-induced fibrosis have been extensively studied. Radiation-induced fibrosis in each organ has many common mechanisms of occurrence as well as its own unique mechanisms. Myofibroblasts are the main body of fibrosis. Factors that activate myofibroblasts can essentially cause extracellular matrix remodeling and fibrosis to occur. For example, TGF-β, IL-1β, CTGF, and other fibrosis-promoting factors are present in various organs, including the heart, liver, lung, kidney, skin, and intestine. It is noteworthy that some mechanisms regulating fibrosis are bidirectional. For example, SASP secreted by senescent macrophages can promote the development of fibrosis, but senescence of myofibroblasts leads to the loss of the myofibroblast phenotype. In this paper, in addition to the mechanism of ionizing radiation-induced fibrosis, we also explain the mechanism of fibrosis induction by other factors such as bleomycin and unilateral ureteral ligation. We found that some cells and some signaling pathways involved in radiation-induced fibrosis and non-radiation-induced fibrosis may be the same. For example, they both need to promote fibrosis formation by activating fibroblasts. In addition, they can both promote the development of fibrosis through signaling pathways such as TGF-β and Wnt/β-catenin. However, the commonality of the two still needs to be proved by much research. Research on non-radiation-induced fibrosis is relatively more abundant. This may provide some research ideas for radiation-induced fibrosis.
Radiation-induced fibrosis is a late toxic side effect of radiotherapy received by cancer patients. Given the adverse health effects of fibrosis, early diagnosis is crucial. In recent years, in addition to traditional imaging modalities such as serum marker testing, histological examination, CT and MRI, nuclear medicine techniques have opened a new era to achieve fibrosis visualization. For example, the effective combination of PET and radiotracer has enabled non-invasive, efficient and sensitive fibrosis diagnosis, making early fibrosis easier to detect. Unfortunately, traditional histologic and serologic tests lack specificity and sensitivity. Also, the emerging nuclear medicine technology is currently only in the preclinical research stage. Finally, the lack of understanding of the mechanisms involved has hindered the development of effective therapeutic tools. Presently, available treatments primarily rely on anti-inflammatory and antioxidant treatments, but their efficiency is very limited, and it is difficult to reverse the organ fibrosis and organ dysfunction that has occurred. Some targeted therapies and stem cell therapies, such as mesenchymal stem cells, show great promise for clinical application. However, much work remains to be done to understand the mechanisms underlying the development of radiation-induced fibrosis to develop precise and effective therapies.
Acknowledgements
Not applicable.
Abbreviations
- RIF
Radiation-induced fibrosis
- ROS
Reactive oxygen species
- ECM
Extracellular matrix components
- RIHD
Radiation-induced heart disease
- TGFs
Transforming growth factors
- ILs
Interleukins
- α-SMA
α-Smooth muscle actin
- MMPs
Matrix metalloproteinases
- TIMPs
Tissue inhibitors of metalloproteinases
- HSC
Hepatic stellate cell
- EMT
Epithelial-mesenchymal transition
- EndoMT
Endothelial-mesenchymal transition
- CSF1R
Colony-stimulating factor receptor-1
- CSF1
Colony-stimulating factor 1
- CTGF/CCN2
Connective tissue growth factor
- PDGF
Platelet-derived growth factor
- MMT
Macrophage-myofibroblast transition
- Treg
Regulatory T cell
- SASP
Senescence-associated secretory phenotypes
- FBW7
F-box and WD40 repeat domain-containing 7
- TPP1
Telomeric protection protein 1
- LXA4
Lipoxin A4
- HIF-1α
Hypoxia-inducible factor-1α
- MAPK
Mitogen-activated protein kinase
- AMPK
AMP-activated protein kinase
- NOX4
NADPH oxidase 4
- CUGBP1
CUG-binding protein 1
- TBK1
TANK-binding kinase 1
- LRP5/6
Low-density lipoprotein receptor-associated protein 5/6
- WISP1
Wnt-1-induced secreted protein
- RNS
Reactive nitrogen species
- NLRP3
NOD-like receptor protein 3
- Nrf2
Nuclear factor erythroid-2-related factor 2
- Keap1
Kelch-like ECH-related protein 1
- RIPK3
Receptor-interacting protein kinase-3
- SOD
Superoxide dismutase
- STING
Stimulator of Interferon Genes
- cGAS
Cyclic guanosine adenosine phosphate synthase
- cGAMP
Cyclic dinucleotide 2′3′ cyclic GMP-AMP
- IRF3
Interferon regulatory factor 3
- XBP1
X-box binding protein 1
- BINP3
BCL2/adenovirus E1B interaction protein 3
- DNMT
DNA methyltransferase
- PDK1
Pyruvate dehydrogenase 1
- GLUT1
Glucose transporter protein 1
- mTORC1
Rapamycin complex 1
- FAO
Fatty acid oxidation
- STAT6
Signal transducer and activator of transcription 6
- UPS
Ubiquitin–proteasome system
- DUB
Ubiquitin-dissociating enzyme
- YAP
Yes-associated protein
- FAT10
F-associated transcript 10
- FPS
FAT10-proteasome system
- ALKBH5
Alkylation repair homolog 5
- FOXM1
Forkhead box M1
- MZF1
Myeloid zinc finger protein 1
- BCL6
B-cell lymphoma 6 protein
- SIRP3
Sphingosine-1-phosphate receptor 3
- ROCK
Rho-associated convoluted helix kinase
- DGKA
Diacylglycerol kinase α
- PPARα
Peroxisome proliferator-activated receptor α
- RAAS
Renin–angiotensin–aldosterone system
- CoQ10
Coenzyme Q10
- NMN
Nicotinamide mononucleotide
- CCL11
C–C motif chemokine 11
- CAF
Cancer-associated fibroblast
- FSTL1
Follistatin-like 1
- PET
Positron emission tomography
- Vit E
Vitamin E
- PTX
Pentoxifylline
- TPL
Triptolide
- RTKIs
Receptor tyrosine kinase inhibitors
- IPF
Idiopathic pulmonary fibrosis
- RA
Rosmarinic acid
- GSPs
Grape seed proanthocyanidins
- PPIs
Proton pump inhibitors
- ACEIs
Angiotensin-converting enzyme inhibitors
- DFO
Deferoxamine
- VEGF
Vascular endothelial growth factor
- eNOS
Endothelial nitric oxide synthase
- 2-ME
Methoxyestradiol
- MSCs
Mesenchymal stem cells
- Ad-MSCs
Adipose-derived mesenchymal stem cells
- BM-MSCs
Bone marrow-derived mesenchymal stem cells
- UC-MSCs
Umbilical cord-derived mesenchymal stem cells
- HGF
Hepatocyte growth factor
Author contributions
Framework of the article was designed by SYZ and CLL. The manuscript was done by ZXY, CYX and SHZ and revised by BS. The figures and table are done by ZXY, CYX and CC. All authors read and approved the final manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (82073477), the Young Talent Project of China National Nuclear Corporation and Scientific Fund for Distinguished Young Scholars in Sichuan Province (2022JDJQ0051).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors state no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Changlong Li, Email: changlongli@scu.edu.cn.
Shuyu Zhang, Email: zhang.shuyu@hotmail.com, Email: zhangshuyu@scu.edu.cn.
References
- 1.Berry CE, Abbas DB, Lintel HA, Churukian AA, Griffin M, Guo JL, et al. Adipose-derived stromal cell-based therapies for radiation-induced fibrosis. Adv Wound Care (New Rochelle). 2022 doi: 10.1089/wound.2022.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binatti E, Zoccatelli G, Zanoni F, Donà G, Mainente F, Chignola R. Effects of combination treatments with astaxanthin-loaded microparticles and pentoxifylline on intracellular ROS and radiosensitivity of J774A. 1 macrophages. Molecules. 2021;26(17):5152. doi: 10.3390/molecules26175152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obrador E, Salvador-Palmer R, Villaescusa JI, Gallego E, Pellicer B, Estrela JM, et al. Nuclear and radiological emergencies: biological effects, countermeasures and biodosimetry. Antioxidants. 2022;11(6):1098. doi: 10.3390/antiox11061098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodamoradi E, Hoseini-Ghahfarokhi M, Amini P, Motevaseli E, Shabeeb D, Musa AE, et al. Targets for protection and mitigation of radiation injury. Cell Mol Life Sci. 2020;77(16):3129–3159. doi: 10.1007/s00018-020-03479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distler JHW, Györfi AH, Ramanujam M, Whitfield ML, Königshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15(12):705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 7.Wang B, Wei J, Meng L, Wang H, Qu C, Chen X, et al. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed Pharmacother. 2020;121:109560. doi: 10.1016/j.biopha.2019.109560. [DOI] [PubMed] [Google Scholar]
- 8.Christensen DM, Livingston GK, Sugarman SL, Parillo SJ, Glassman ES. Management of ionizing radiation injuries and illnesses, part 3: radiobiology and health effects of ionizing radiation. J Am Osteopath Assoc. 2014;114(7):556–565. doi: 10.7556/jaoa.2014.109. [DOI] [PubMed] [Google Scholar]
- 9.Mu H, Sun J, Li L, Yin J, Hu N, Zhao W, et al. Ionizing radiation exposure: hazards, prevention, and biomarker screening. Environ Sci Pollut Res Int. 2018;25(16):15294–15306. doi: 10.1007/s11356-018-2097-9. [DOI] [PubMed] [Google Scholar]
- 10.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1–2):48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant AK, Banegas MP, Martinez ME, Mell LK, Murphy JD. Trends in radiation therapy among cancer survivors in the United States, 2000–2030. Cancer Epidemiol Biomarkers Prev. 2017;26(6):963–970. doi: 10.1158/1055-9965.EPI-16-1023. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys BD. Mechanisms of renal fibrosis. Annu Rev Physiol. 2018;80:309–326. doi: 10.1146/annurev-physiol-022516-034227. [DOI] [PubMed] [Google Scholar]
- 13.Wang KX, Ye C, Yang X, Ma P, Yan C, Luo L. New insights into the understanding of mechanisms of radiation-induced heart disease. Curr Treat Options Oncol. 2023;24(1):12–29. doi: 10.1007/s11864-022-01041-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Yang X, Liu H, Luo W, Liu H, Lv T, et al. Value of [(68)Ga]Ga-FAPI-04 imaging in the diagnosis of renal fibrosis. Eur J Nucl Med Mol Imaging. 2021;48(11):3493–3501. doi: 10.1007/s00259-021-05343-x. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Wu Z, Ning W. Advances in molecular mechanisms and treatment of radiation-induced pulmonary fibrosis. Transl Oncol. 2019;12(1):162–169. doi: 10.1016/j.tranon.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of clinical oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 17.Zhou YJ, Tang Y, Liu SJ, Zeng PH, Qu L, Jing QC, et al. Radiation-induced liver disease: beyond DNA damage. Cell Cycle. 2023;22(5):506–526. doi: 10.1080/15384101.2022.2131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy—pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11(8):470–479. doi: 10.1038/nrgastro.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray FN, Simmons BJ, Wolfson AH, Nouri K. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb) 2016;6(2):185–206. doi: 10.1007/s13555-016-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 21.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. 2020;587(7835):555–566. doi: 10.1038/s41586-020-2938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenti MV, Di Sabatino A. Intestinal fibrosis. Mol Aspects Med. 2019;65:100–109. doi: 10.1016/j.mam.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180(4):1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hortells L, Johansen AKZ, Yutzey KE. Cardiac fibroblasts and the extracellular matrix in regenerative and nonregenerative hearts. J Cardiovasc Dev Dis. 2019;6(3):29. doi: 10.3390/jcdd6030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinz B, McCulloch CA, Coelho NM. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp Cell Res. 2019;379(1):119–128. doi: 10.1016/j.yexcr.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Moulin V, Castilloux G, Auger FA, Garrel D, O'Connor-McCourt MD, Germain L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res. 1998;238(1):283–293. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- 28.Czubryt MP. Cardiac fibroblast to myofibroblast phenotype conversion—an unexploited therapeutic target. J Cardiovasc Dev Dis. 2019;6(3):28. doi: 10.3390/jcdd6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan B, Chen Y, Wu Z, Zhang L, Zhuang Y, Zhao X, et al. Proteomic profiling of human hepatic stellate cell line LX2 responses to irradiation and TGF-β1. J Proteome Res. 2019;18(1):508–521. doi: 10.1021/acs.jproteome.8b00814. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Hong ZY, Nam JK, Lee HJ, Jang J, Yoo RJ, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin Cancer Res. 2015;21(16):3716–3726. doi: 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- 31.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Coen M, Gabbiani G, Bochaton-Piallat ML. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 2011;31(11):2391–2396. doi: 10.1161/ATVBAHA.111.231548. [DOI] [PubMed] [Google Scholar]
- 33.Ko UH, Choi J, Choung J, Moon S, Shin JH. Physicochemically tuned myofibroblasts for wound healing strategy. Sci Rep. 2019;9(1):16070. doi: 10.1038/s41598-019-52523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishida Y, Kuninaka Y, Mukaida N, Kondo T. Immune mechanisms of pulmonary fibrosis with bleomycin. Int J Mol Sci. 2023;24(4):3149. doi: 10.3390/ijms24043149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, et al. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol. 2014;5:167. doi: 10.3389/fphar.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35(2):83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 37.Pakshir P, Hinz B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018;68:81–93. doi: 10.1016/j.matbio.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11(11):1386–1394. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Leve S, Wirsdörfer F, Cappuccini F, Schütze A, Meyer AV, Röck K, et al. Loss of CD73 prevents accumulation of alternatively activated macrophages and the formation of prefibrotic macrophage clusters in irradiated lungs. Faseb j. 2017;31(7):2869–2880. doi: 10.1096/fj.201601228R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 42.Yao Y, Wang Y, Zhang Z, He L, Zhu J, Zhang M, et al. Chop deficiency protects mice against bleomycin-induced pulmonary fibrosis by attenuating M2 macrophage production. Mol Ther. 2016;24(5):915–925. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Lin SC, Chen G, He L, Hu Z, Chan L, et al. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol. 2013;24(10):1644–1659. doi: 10.1681/ASN.2013030217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang SM, Wei CY, Wang Q, Wang L, Lu L, Qi FZ. M2-polarized macrophages mediate wound healing by regulating connective tissue growth factor via AKT, ERK1/2, and STAT3 signaling pathways. Mol Biol Rep. 2021;48(9):6443–6456. doi: 10.1007/s11033-021-06646-w. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Yang F, Chang Y, Zhang C, Xiong Y, Wang X, Ma X, et al. UUO induces lung fibrosis with macrophage-myofibroblast transition in rats. Int Immunopharmacol. 2021;93:107396. doi: 10.1016/j.intimp.2021.107396. [DOI] [PubMed] [Google Scholar]
- 47.Xiong S, Pan X, Xu L, Yang Z, Guo R, Gu Y, et al. Regulatory T cells promote β-catenin–mediated epithelium-to-mesenchyme transition during radiation-induced pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 2015;93(2):425–435. doi: 10.1016/j.ijrobp.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 48.Xiong S, Guo R, Yang Z, Xu L, Du L, Li R, et al. Treg depletion attenuates irradiation-induced pulmonary fibrosis by reducing fibrocyte accumulation, inducing Th17 response, and shifting IFN-γ, IL-12/IL-4, IL-5 balance. Immunobiology. 2015;220(11):1284–1291. doi: 10.1016/j.imbio.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Paun A, Bergeron ME, Haston CK. The Th1/Th17 balance dictates the fibrosis response in murine radiation-induced lung disease. Sci Rep. 2017;7(1):11586. doi: 10.1038/s41598-017-11656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linard C, Billiard F, Benderitter M. Intestinal irradiation and fibrosis in a Th1-deficient environment. Int J Radiat Oncol Biol Phys. 2012;84(1):266–273. doi: 10.1016/j.ijrobp.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 51.Mylonas KJ, O’Sullivan ED, Humphries D, Baird DP, Docherty M-H, Neely SA, et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci Transl Med. 2021;13(594):eabb0203. doi: 10.1126/scitranslmed.abb0203. [DOI] [PubMed] [Google Scholar]
- 52.Kellogg DL, Kellogg DL, Jr, Musi N, Nambiar AM. Cellular senescence in idiopathic pulmonary fibrosis. Curr Mol Biol Rep. 2021;7(3):31–40. doi: 10.1007/s40610-021-00145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao C, Guan X, Carraro G, Parimon T, Liu X, Huang G, et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med. 2021;203(6):707–717. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang E, Guo Q, Gao H, Xu R, Teng S, Wu Y. Metformin and resveratrol inhibited high glucose-induced metabolic memory of endothelial senescence through SIRT1/p300/p53/p21 pathway. PLoS ONE. 2015;10(12):e0143814. doi: 10.1371/journal.pone.0143814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overstreet JM, Samarakoon R, Meldrum KK, Higgins PJ. Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity. Cell Signal. 2014;26(7):1427–1436. doi: 10.1016/j.cellsig.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao XY, Lai YY, Luo XS, Peng DW, Li QQ, Zhou HS, et al. Acetyltransferase p300 regulates atrial fibroblast senescence and age-related atrial fibrosis through p53/Smad3 axis. Aging Cell. 2023;22(1):e13743. doi: 10.1111/acel.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su L, Dong Y, Wang Y, Wang Y, Guan B, Lu Y, et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021;12(6):527. doi: 10.1038/s41419-021-03811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fafián-Labora JA, O'Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30(8):628–639. doi: 10.1016/j.tcb.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee A, Epperly MW, Shields D, Hou W, Fisher R, Hamade D, et al. Ionizing irradiation-induced Fgr in senescent cells mediates fibrosis. Cell Death Discov. 2021;7(1):349. doi: 10.1038/s41420-021-00741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Chen R, Li G, Wang Z, Liu J, Liang Y, et al. FBW7 mediates senescence and pulmonary fibrosis through telomere uncapping. Cell Metab. 2020;32(5):860–77.e9. doi: 10.1016/j.cmet.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 62.López-Antona I, Contreras-Jurado C, Luque-Martín L, Carpintero-Leyva A, González-Méndez P, Palmero I. Dynamic regulation of myofibroblast phenotype in cellular senescence. Aging Cell. 2022;21(4):e13580. doi: 10.1111/acel.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farhood B, Khodamoradi E, Hoseini-Ghahfarokhi M, Motevaseli E, Mirtavoos-Mahyari H, Eleojo Musa A, et al. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol Res. 2020;155:104745. doi: 10.1016/j.phrs.2020.104745. [DOI] [PubMed] [Google Scholar]
- 64.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 2015;75(11):2232–2242. doi: 10.1158/0008-5472.CAN-14-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreau JM, Velegraki M, Bolyard C, Rosenblum MD, Li Z. Transforming growth factor-β1 in regulatory T cell biology. Sci Immunol. 2022;7(69):eabi4613. doi: 10.1126/sciimmunol.abi4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Batlle E, Massagué J. Transforming growth factor-β signaling in immunity and cancer. Immunity. 2019;50(4):924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/j.matbio.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, Yang T, Lu DW, Zhao H, Feng YL, Chen H, et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–681. doi: 10.1016/j.biopha.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 70.Boerma M, Wang J, Sridharan V, Herbert JM, Hauer-Jensen M. Pharmacological induction of transforming growth factor-beta1 in rat models enhances radiation injury in the intestine and the heart. PLoS ONE. 2013;8(7):e70479. doi: 10.1371/journal.pone.0070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin R, Yi S, Gong L, Liu W, Wang P, Liu N, et al. Inhibition of TGF-β signaling with halofuginone can enhance the antitumor effect of irradiation in Lewis lung cancer. Onco Targets Ther. 2015;8:3549–3559. doi: 10.2147/OTT.S92518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229(2):298–309. doi: 10.1002/path.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhen S, Qiang R, Lu J, Tuo X, Yang X, Li X. TGF-β1-based CRISPR/Cas9 gene therapy attenuates radiation-induced lung injury. Curr Gene Ther. 2022;22(1):59–65. doi: 10.2174/1566523220666201230100523. [DOI] [PubMed] [Google Scholar]
- 74.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160(3):1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang C, Zhao H, Li BL, Fu G, Liu H, Cai JM, et al. CpG-oligodeoxynucleotides may be effective for preventing ionizing radiation induced pulmonary fibrosis. Toxicol Lett. 2018;292:181–189. doi: 10.1016/j.toxlet.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Shu G, Dai C, Yusuf A, Sun H, Deng X. Limonin relieves TGF-β-induced hepatocyte EMT and hepatic stellate cell activation in vitro and CCl(4)-induced liver fibrosis in mice via upregulating Smad7 and subsequent suppression of TGF-β/Smad cascade. J Nutr Biochem. 2022;107:109039. doi: 10.1016/j.jnutbio.2022.109039. [DOI] [PubMed] [Google Scholar]
- 77.Verma S, Dutta A, Dahiya A, Kalra N. Quercetin-3-rutinoside alleviates radiation-induced lung inflammation and fibrosis via regulation of NF-κB/TGF-β1 signaling. Phytomedicine. 2022;99:154004. doi: 10.1016/j.phymed.2022.154004. [DOI] [PubMed] [Google Scholar]
- 78.Bai Y, Wang J, He Z, Yang M, Li L, Jiang H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor β (TGF-β)/smad pathway and pro-inflammatory cytokines. Med Sci Monit. 2019;25:3069–3076. doi: 10.12659/MSM.914860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H, Park SH, Han SY, Lee YS, Cho J, Kim JM. LXA(4)-FPR2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with TGF-β/Smad signaling. Cell Death Dis. 2020;11(8):653. doi: 10.1038/s41419-020-02846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azimi I. The interplay between HIF-1 and calcium signalling in cancer. Int J Biochem Cell Biol. 2018;97:73–77. doi: 10.1016/j.biocel.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 81.Huang JQ, Zhang H, Guo XW, Lu Y, Wang SN, Cheng B, et al. Mechanically activated calcium channel PIEZO1 modulates radiation-induced epithelial-mesenchymal transition by forming a positive feedback with TGF-β1. Front Mol Biosci. 2021;8:725275. doi: 10.3389/fmolb.2021.725275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwon OS, Kim KT, Lee E, Kim M, Choi SH, Li H, et al. Induction of MiR-21 by stereotactic body radiotherapy contributes to the pulmonary fibrotic response. PLoS ONE. 2016;11(5):e0154942. doi: 10.1371/journal.pone.0154942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu X, Jiang YN, Wang WJ, Zhang J, Shang DS, Sun CB, et al. Comprehensive circRNA expression profile and construction of circRNA-related ceRNA network in cardiac fibrosis. Biomed Pharmacother. 2020;125:109944. doi: 10.1016/j.biopha.2020.109944. [DOI] [PubMed] [Google Scholar]
- 84.Yang Q, Zhang P, Liu T, Zhang X, Pan X, Cen Y, et al. Magnesium isoglycyrrhizinate ameliorates radiation-induced pulmonary fibrosis by inhibiting fibroblast differentiation via the p38MAPK/Akt/Nox4 pathway. Biomed Pharmacother. 2019;115:108955. doi: 10.1016/j.biopha.2019.108955. [DOI] [PubMed] [Google Scholar]
- 85.Wu X, Wu X, Ma Y, Shao F, Tan Y, Tan T, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7:13498. doi: 10.1038/ncomms13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weng H, Mertens PR, Gressner AM, Dooley S. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46(2):295–303. doi: 10.1016/j.jhep.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 87.Mann DA, Marra F. Fibrogenic signalling in hepatic stellate cells. J Hepatol. 2010;52(6):949–950. doi: 10.1016/j.jhep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 88.Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, et al. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol. 2018;58(2):208–215. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qu H, Liu L, Liu Z, Qin H, Liao Z, Xia P, et al. Blocking TBK1 alleviated radiation-induced pulmonary fibrosis and epithelial-mesenchymal transition through Akt-Erk inactivation. Exp Mol Med. 2019;51(4):1–17. doi: 10.1038/s12276-019-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Monceau V, Pasinetti N, Schupp C, Pouzoulet F, Opolon P, Vozenin MC. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets. 2010;11(11):1395–1404. doi: 10.2174/1389450111009011395. [DOI] [PubMed] [Google Scholar]
- 91.Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q, et al. Wnt/β-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29(1):182–193. doi: 10.1681/ASN.2017040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rong X, Liu J, Yao X, Jiang T, Wang Y, Xie F. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Res Ther. 2019;10(1):98. doi: 10.1186/s13287-019-1204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, Bouros D. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest. 2019;156(2):383–391. doi: 10.1016/j.chest.2019.04.114. [DOI] [PubMed] [Google Scholar]
- 94.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo j. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 96.Barrott JJ, Cash GM, Smith AP, Barrow JR, Murtaugh LC. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc Natl Acad Sci USA. 2011;108(31):12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 98.Myung SJ, Yoon JH, Gwak GY, Kim W, Lee JH, Kim KM, et al. Wnt signaling enhances the activation and survival of human hepatic stellate cells. FEBS Lett. 2007;581(16):2954–2958. doi: 10.1016/j.febslet.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 99.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 100.Wang C, Dai J, Sun Z, Shi C, Cao H, Chen X, et al. Targeted inhibition of disheveled PDZ domain via NSC668036 depresses fibrotic process. Exp Cell Res. 2015;331(1):115–122. doi: 10.1016/j.yexcr.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 101.Lee DW, Lee WJ, Cho J, Yun CO, Roh H, Chang HP, et al. Inhibition of Wnt signaling pathway suppresses radiation-induced dermal fibrosis. Sci Rep. 2020;10(1):13594. doi: 10.1038/s41598-020-70243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasan HF, Abdel-Rafei MK, Galal SM. Diosmin attenuates radiation-induced hepatic fibrosis by boosting PPAR-γ expression and hampering miR-17-5p-activated canonical Wnt-β-catenin signaling. Biochem Cell Biol. 2017;95(3):400–414. doi: 10.1139/bcb-2016-0142. [DOI] [PubMed] [Google Scholar]
- 103.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, Qiu SS, Shao Y, Song HH, Li GL, Lu W, et al. Dickkopf-1 has an inhibitory effect on mesenchymal stem cells to fibroblast differentiation. Chin Med J (Engl) 2016;129(10):1200–1207. doi: 10.4103/0366-6999.181974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo Y, Sun L, Xiao L, Gou R, Fang Y, Liang Y, et al. Aberrant Wnt/beta-catenin pathway activation in dialysate-induced peritoneal fibrosis. Front Pharmacol. 2017;8:774. doi: 10.3389/fphar.2017.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shao L, Zhang Y, Shi W, Ma L, Xu T, Chang P, et al. Mesenchymal stromal cells can repair radiation-induced pulmonary fibrosis via a DKK-1-mediated Wnt/beta-catenin pathway. Cell Tissue Res. 2021;384(1):87–97. doi: 10.1007/s00441-020-03325-3. [DOI] [PubMed] [Google Scholar]
- 107.Sun YW, Zhang YY, Ke XJ, Wu XJ, Chen ZF, Chi P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-beta1/Smad/CTGF signaling pathway. Eur J Pharmacol. 2018;822:199–206. doi: 10.1016/j.ejphar.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 108.Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62(5):1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Z, Wang M, Cao L, Liu R, Ren Y, Li L, et al. Interference with connective tissue growth factor attenuated fibroblast-to-myofibroblast transition and pulmonary fibrosis. Ann Transl Med. 2022;10(10):566. doi: 10.21037/atm-22-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nie X, Yu Q, Li L, Yi M, Wu B, Huang Y, et al. Kinsenoside protects against radiation-induced liver fibrosis via downregulating connective tissue growth factor through TGF-β1 signaling. Front Pharmacol. 2022;13:808576. doi: 10.3389/fphar.2022.808576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ding ZY, Jin GN, Liang HF, Wang W, Chen WX, Datta PK, et al. Transforming growth factor β induces expression of connective tissue growth factor in hepatic progenitor cells through Smad independent signaling. Cell Signal. 2013;25(10):1981–1992. doi: 10.1016/j.cellsig.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 112.Ejaz A, Epperly MW, Hou W, Greenberger JS, Rubin JP. Adipose-derived stem cell therapy ameliorates ionizing irradiation fibrosis via hepatocyte growth factor-mediated transforming growth factor-β downregulation and recruitment of bone marrow cells. Stem Cells. 2019;37(6):791–802. doi: 10.1002/stem.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bourgier C, Haydont V, Milliat F, François A, Holler V, Lasser P, et al. Inhibition of Rho kinase modulates radiation induced fibrogenic phenotype in intestinal smooth muscle cells through alteration of the cytoskeleton and connective tissue growth factor expression. Gut. 2005;54(3):336–343. doi: 10.1136/gut.2004.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dorn LE, Petrosino JM, Wright P, Accornero F. CTGF/CCN2 is an autocrine regulator of cardiac fibrosis. J Mol Cell Cardiol. 2018;121:205–211. doi: 10.1016/j.yjmcc.2018.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, et al. Effects of CTGF blockade on attenuation and reversal of radiation-induced pulmonary fibrosis. J Natl Cancer Inst. 2017;109(8):djw339. doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 116.Sternlicht MD, Wirkner U, Bickelhaupt S, Lopez Perez R, Tietz A, Lipson KE, et al. Radiation-induced pulmonary gene expression changes are attenuated by the CTGF antibody Pamrevlumab. Respir Res. 2018;19(1):14. doi: 10.1186/s12931-018-0720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El Kasmi KC, Vue PM, Anderson AL, Devereaux MW, Ghosh S, Balasubramaniyan N, et al. Macrophage-derived IL-1β/NF-κB signaling mediates parenteral nutrition-associated cholestasis. Nat Commun. 2018;9(1):1393. doi: 10.1038/s41467-018-03764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 119.Hong OK, Lee SS, Yoo SJ, Lee MK, Kim MK, Baek KH, et al. Gemigliptin inhibits interleukin-1β-induced endothelial-mesenchymal transition via canonical-bone morphogenetic protein pathway. Endocrinol Metab (Seoul) 2020;35(2):384–395. doi: 10.3803/EnM.2020.35.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Tar. 2017;2(1):1–9. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 122.Groves AM, Johnston CJ, Williams JP, Finkelstein JN. Role of infiltrating monocytes in the development of radiation-induced pulmonary fibrosis. Radiat Res. 2018;189(3):300–311. doi: 10.1667/RR14874.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shi JH, Guan H, Shi S, Cai WX, Bai XZ, Hu XL, et al. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res. 2013;305(4):341–352. doi: 10.1007/s00403-013-1314-0. [DOI] [PubMed] [Google Scholar]
- 124.Mohammadi S, Ravanbakhsh H, Taheri S, Bao G, Mongeau L. Immunomodulatory microgels support proregenerative macrophage activation and attenuate fibroblast collagen synthesis. Adv Healthc Mater. 2022;11(11):e2102366. doi: 10.1002/adhm.202102366. [DOI] [PubMed] [Google Scholar]
- 125.Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, et al. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 2013;31(10):2231–2241. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lafargue A, Degorre C, Corre I, Alves-Guerra MC, Gaugler MH, Vallette F, et al. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic Biol Med. 2017;108:750–759. doi: 10.1016/j.freeradbiomed.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 127.Hur W, Kang BY, Kim SM, Lee GW, Kim J-H, Nam M-K, et al. Serine protease HtrA2/Omi deficiency impairs mitochondrial homeostasis and promotes hepatic fibrogenesis via activation of hepatic stellate cells. Cells. 2019;8(10):1119. doi: 10.3390/cells8101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lozhkin A, Vendrov AE, Ramos-Mondragón R, Canugovi C, Stevenson MD, Herron TJ, et al. Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biol. 2022;57:102474. doi: 10.1016/j.redox.2022.102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng Q, Li C, Yang CF, Zhong YJ, Wu D, Shi L, et al. Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-β1/Smad and NOX4/ROS pathways. Chem Biol Interact. 2019;299:131–139. doi: 10.1016/j.cbi.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 130.Rao X, Zhou D, Deng H, Chen Y, Wang J, Zhou X, et al. Activation of NLRP3 inflammasome in lung epithelial cells triggers radiation-induced lung injury. Respir Res. 2023;24(1):25. doi: 10.1186/s12931-023-02331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Y, Wang Z, Feng D, Zhao H, Lin M, Hu Y, et al. p66Shc contributes to liver fibrosis through the regulation of mitochondrial reactive oxygen species. Theranostics. 2019;9(5):1510–1522. doi: 10.7150/thno.29620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bai Y, Wang W, Wang L, Ma L, Zhai D, Wang F, et al. Obacunone attenuates liver fibrosis with enhancing anti-oxidant effects of GPx-4 and inhibition of EMT. Molecules. 2021;26(2):318. doi: 10.3390/molecules26020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Wang H, Wang B, Wei J, Zheng Z, Su J, Bian C, et al. Sulforaphane regulates Nrf2-mediated antioxidant activity and downregulates TGF-β1/Smad pathways to prevent radiation-induced muscle fibrosis. Life Sci. 2022;311(Pt B):121197. doi: 10.1016/j.lfs.2022.121197. [DOI] [PubMed] [Google Scholar]
- 135.Ge C, Hu L, Lou D, Li Q, Feng J, Wu Y, et al. Nrf2 deficiency aggravates PM(2.5)-induced cardiomyopathy by enhancing oxidative stress, fibrosis and inflammation via RIPK3-regulated mitochondrial disorder. Aging (Albany NY). 2020;12(6):4836–4865. doi: 10.18632/aging.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sureshbabu A, Patino E, Ma KC, Laursen K, Finkelsztein EJ, Akchurin O, et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI insight. 2018;3(11):e98411. doi: 10.1172/jci.insight.98411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Q, Xue Y, Fu Y, Bao B, Guo MY. Zinc deficiency aggravates oxidative stress leading to inflammation and fibrosis in lung of mice. Biol Trace Elem Res. 2022;200(9):4045–4057. doi: 10.1007/s12011-021-03011-7. [DOI] [PubMed] [Google Scholar]
- 138.Cao JW, Duan SY, Zhang HX, Chen Y, Guo M. Zinc deficiency promoted fibrosis via ROS and TIMP/MMPs in the myocardium of mice. Biol Trace Elem Res. 2020;196(1):145–152. doi: 10.1007/s12011-019-01902-4. [DOI] [PubMed] [Google Scholar]
- 139.Kong H, Jiang CY, Hu L, Teng P, Zhang Y, Pan XX, et al. Morphine induces dysfunction of PINK1/Parkin-mediated mitophagy in spinal cord neurons implying involvement in antinociceptive tolerance. J Mol Cell Biol. 2019;11(12):1056–1068. doi: 10.1093/jmcb/mjz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3(5):100324. doi: 10.1016/j.jhepr.2021.100324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loo TM, Miyata K, Tanaka Y, Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111(2):304–311. doi: 10.1111/cas.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang M, Fan Q, Hei TK, Chen G, Cao W, Meng G, et al. Single-cell transcriptome analysis of radiation pneumonitis mice. Antioxidants. 2022;11(8):1457. doi: 10.3390/antiox11081457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shen R, Yang K, Cheng X, Guo C, Xing X, Sun H, et al. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ Pollut. 2022;300:118986. doi: 10.1016/j.envpol.2022.118986. [DOI] [PubMed] [Google Scholar]
- 144.Chung KW, Dhillon P, Huang S, Sheng X, Shrestha R, Qiu C, et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 2019;30(4):784–99.e5. doi: 10.1016/j.cmet.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Han B, Wang X, Wu P, Jiang H, Yang Q, Li S, et al. Pulmonary inflammatory and fibrogenic response induced by graphitized multi-walled carbon nanotube involved in cGAS-STING signaling pathway. J Hazard Mater. 2021;417:125984. doi: 10.1016/j.jhazmat.2021.125984. [DOI] [PubMed] [Google Scholar]
- 146.Zeng H, Gao Y, Yu W, Liu J, Zhong C, Su X, et al. Pharmacological inhibition of STING/TBK1 signaling attenuates myeloid fibroblast activation and macrophage to myofibroblast transition in renal fibrosis. Front Pharmacol. 2022;13:940716. doi: 10.3389/fphar.2022.940716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Su W, Gao W, Zhang R, Wang Q, Li L, Bu Q, et al. TAK1 deficiency promotes liver injury and tumorigenesis via ferroptosis and macrophage cGAS-STING signalling. JHEP Rep. 2023;5(5):100695. doi: 10.1016/j.jhepr.2023.100695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Q, Bu Q, Liu M, Zhang R, Gu J, Li L, et al. XBP1-mediated activation of the STING signalling pathway in macrophages contributes to liver fibrosis progression. JHEP Rep. 2022;4(11):100555. doi: 10.1016/j.jhepr.2022.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Y, Chen W, Wang Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed Pharmacother. 2020;125:110022. doi: 10.1016/j.biopha.2020.110022. [DOI] [PubMed] [Google Scholar]
- 150.Zhang D, Liu Y, Zhu Y, Zhang Q, Guan H, Liu S, et al. A non-canonical cGAS-STING-PERK pathway facilitates the translational program critical for senescence and organ fibrosis. Nat Cell Biol. 2022;24(5):766–782. doi: 10.1038/s41556-022-00894-z. [DOI] [PubMed] [Google Scholar]
- 151.Yang H, Wang H, Ren J, Chen Q, Chen ZJ. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA. 2017;114(23):E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhao D, Gao Y, Su Y, Zhou Y, Yang T, Li Y, et al. Oroxylin A regulates cGAS DNA hypermethylation induced by methionine metabolism to promote HSC senescence. Pharmacol Res. 2023;187:106590. doi: 10.1016/j.phrs.2022.106590. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Zhang Y, Ren Y, Yang X, Ben H, Zhao F, et al. Pharmacological targeting of cGAS/STING-YAP axis suppresses pathological angiogenesis and ameliorates organ fibrosis. Eur J Pharmacol. 2022;932:175241. doi: 10.1016/j.ejphar.2022.175241. [DOI] [PubMed] [Google Scholar]
- 154.Xiong R, Li N, Chen L, Wang W, Wang B, Jiang W, et al. STING protects against cardiac dysfunction and remodelling by blocking autophagy. Cell Commun Signal. 2021;19(1):109. doi: 10.1186/s12964-021-00793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saito S, Deskin B, Rehan M, Yadav S, Matsunaga Y, Lasky JA, et al. Novel mediators of idiopathic pulmonary fibrosis. Clin Sci (Lond) 2022;136(16):1229–1240. doi: 10.1042/CS20210878. [DOI] [PubMed] [Google Scholar]
- 156.Xu P, Yi Y, Luo Y, Liu Z, Xu Y, Cai J, et al. Radiation-induced dysfunction of energy metabolism in the heart results in the fibrosis of cardiac tissues. Mol Med Rep. 2021;24(6):1–16. doi: 10.3892/mmr.2021.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Smith ER, Wigg B, Holt SG, Hewitson TD. TGF-β1 modifies histone acetylation and acetyl-coenzyme A metabolism in renal myofibroblasts. Am J Physiol Renal Physiol. 2019;316(3):F517–F529. doi: 10.1152/ajprenal.00513.2018. [DOI] [PubMed] [Google Scholar]
- 158.Yu H, Zhu J, Chang L, Liang C, Li X, Wang W. 3-Bromopyruvate decreased kidney fibrosis and fibroblast activation by suppressing aerobic glycolysis in unilateral ureteral obstruction mice model. Life Sci. 2021;272:119206. doi: 10.1016/j.lfs.2021.119206. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X, Psarianos P, Ghoraie LS, Yip K, Goldstein D, Gilbert R, et al. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat Metab. 2019;1(1):147–157. doi: 10.1038/s42255-018-0008-5. [DOI] [PubMed] [Google Scholar]
- 160.Goodwin J, Choi H, Hsieh MH, Neugent ML, Ahn JM, Hayenga HN, et al. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58(2):216–231. doi: 10.1165/rcmb.2016-0186OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Comstock JP, Udenfriend S. Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proc Natl Acad Sci USA. 1970;66(2):552–557. doi: 10.1073/pnas.66.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cho SJ, Moon JS, Lee CM, Choi AM, Stout-Delgado HW. Glucose transporter 1-dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am J Respir Cell Mol Biol. 2017;56(4):521–531. doi: 10.1165/rcmb.2016-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cho SJ, Moon JS, Nikahira K, Yun HS, Harris R, Hong KS, et al. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax. 2020;75(3):227–236. doi: 10.1136/thoraxjnl-2019-213571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cheuk YC, Niu X, Mao Y, Li J, Wang J, Xu S, et al. Integration of transcriptomics and metabolomics reveals pathways involved in MDSC supernatant attenuation of TGF-β1-induced myofibroblastic differentiation of mesenchymal stem cells. Cell Tissue Res. 2022;390(3):465–489. doi: 10.1007/s00441-022-03681-2. [DOI] [PubMed] [Google Scholar]
- 165.Zou GL, Zhang XR, Ma YL, Lu Q, Zhao R, Zhu YZ, et al. The role of Nrf2/PIWIL2/purine metabolism axis in controlling radiation-induced lung fibrosis. Am J Cancer Res. 2020;10(9):2752–2767. [PMC free article] [PubMed] [Google Scholar]
- 166.Wei X, Hou Y, Long M, Jiang L, Du Y. Advances in energy metabolism in renal fibrosis. Life Sci. 2023;312:121033. doi: 10.1016/j.lfs.2022.121033. [DOI] [PubMed] [Google Scholar]
- 167.Hu S, He W, Wu G. Hydroxyproline in animal metabolism, nutrition, and cell signaling. Amino Acids. 2022;54(4):513–528. doi: 10.1007/s00726-021-03056-x. [DOI] [PubMed] [Google Scholar]
- 168.Wang S, Li X, Ma Q, Wang Q, Wu J, Yu H, et al. Glutamine metabolism is required for alveolar regeneration during lung injury. Biomolecules. 2022;12(5):728. doi: 10.3390/biom12050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ge J, Cui H, Xie N, Banerjee S, Guo S, Dubey S, et al. Glutaminolysis promotes collagen translation and stability via α-ketoglutarate-mediated mTOR activation and proline hydroxylation. Am J Respir Cell Mol Biol. 2018;58(3):378–390. doi: 10.1165/rcmb.2017-0238OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li J, Yang Y, Li Q, Wei S, Zhou Y, Yu W, et al. STAT6 contributes to renal fibrosis by modulating PPARα-mediated tubular fatty acid oxidation. Cell Death Dis. 2022;13(1):66. doi: 10.1038/s41419-022-04515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bates J, Vijayakumar A, Ghoshal S, Marchand B, Yi S, Kornyeyev D, et al. Acetyl-CoA carboxylase inhibition disrupts metabolic reprogramming during hepatic stellate cell activation. J Hepatol. 2020;73(4):896–905. doi: 10.1016/j.jhep.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 172.Yang X, Chen J, Wang J, Ma S, Feng W, Wu Z, et al. Very-low-density lipoprotein receptor-enhanced lipid metabolism in pancreatic stellate cells promotes pancreatic fibrosis. Immunity. 2022;55(7):1185–99.e8. doi: 10.1016/j.immuni.2022.06.001. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y, He W, He C, Wan J, Lin X, Zheng X, et al. Large triglyceride-rich lipoproteins in hypertriglyceridemia are associated with the severity of acute pancreatitis in experimental mice. Cell Death Dis. 2019;10(10):728. doi: 10.1038/s41419-019-1969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang N, Zhang Y, Qian H, Wu S, Cao L, Sun Y. Selective targeting of ubiquitination and degradation of PARP1 by E3 ubiquitin ligase WWP2 regulates isoproterenol-induced cardiac remodeling. Cell Death Differ. 2020;27(9):2605–2619. doi: 10.1038/s41418-020-0523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zeng W, Gu S, Yu Y, Feng Y, Xiao M, Feng XH. ZNF451 stabilizes TWIST2 through SUMOylation and promotes epithelial-mesenchymal transition. Am J Cancer Res. 2021;11(3):898–915. [PMC free article] [PubMed] [Google Scholar]
- 176.Tsubouchi K, Araya J, Minagawa S, Hara H, Ichikawa A, Saito N, et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy. 2017;13(8):1420–1434. doi: 10.1080/15548627.2017.1328348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sawa-Aihara A, Hattori K, Nagao G, Yamada Y, Ishida T. Potential efficacy of proteasome inhibitor, delanzomib, for the treatment of renal fibrosis. Biol Pharm Bull. 2023;46(2):279–285. doi: 10.1248/bpb.b22-00713. [DOI] [PubMed] [Google Scholar]
- 178.Wang W, Luo J, Sheng W, Xue J, Li M, Ji J, et al. Proteomic profiling of radiation-induced skin fibrosis in rats: targeting the ubiquitin-proteasome system. Int J Radiat Oncol Biol Phys. 2016;95(2):751–760. doi: 10.1016/j.ijrobp.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 179.Liu N, Chai R, Liu B, Zhang Z, Zhang S, Zhang J, et al. Ubiquitin-specific protease 14 regulates cardiac hypertrophy progression by increasing GSK-3β phosphorylation. Biochem Biophys Res Commun. 2016;478(3):1236–1241. doi: 10.1016/j.bbrc.2016.08.100. [DOI] [PubMed] [Google Scholar]
- 180.Wei L, Zhang Y, Qi X, Sun X, Li Y, Xu Y. Ubiquitin-proteasomes are the dominant mediators of the regulatory effect of microRNA-1 on cardiac remodeling after myocardial infarction. Int J Mol Med. 2019;44(5):1899–1907. doi: 10.3892/ijmm.2019.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Semren N, Welk V, Korfei M, Keller IE, Fernandez IE, Adler H, et al. Regulation of 26S proteasome activity in pulmonary fibrosis. Am J Respir Crit Care Med. 2015;192(9):1089–1101. doi: 10.1164/rccm.201412-2270OC. [DOI] [PubMed] [Google Scholar]
- 182.Chen C, Li X, Zhou T, Su Y, Yu B, Jin J, et al. Ubiquitin like protein FAT10 repressed cardiac fibrosis after myocardial ischemic via mediating degradation of Smad3 dependent on FAT10-proteasome system. Int J Biol Sci. 2023;19(3):881–896. doi: 10.7150/ijbs.77677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang J, Ruan Y, Wang D, Fan J, Luo N, Chen H, et al. VHL-recruiting PROTAC attenuates renal fibrosis and preserves renal function via simultaneous degradation of Smad3 and stabilization of HIF-2α. Cell Biosci. 2022;12(1):203. doi: 10.1186/s13578-022-00936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Y, Liu Q, Ning J, Jiang T, Kang A, Li L, et al. The proteasome-dependent degradation of ALKBH5 regulates ECM deposition in PM(2.5) exposure-induced pulmonary fibrosis of mice. J Hazard Mater. 2022;432:128655. doi: 10.1016/j.jhazmat.2022.128655. [DOI] [PubMed] [Google Scholar]
- 185.Pankova D, Jiang Y, Chatzifrangkeskou M, Vendrell I, Buzzelli J, Ryan A, et al. RASSF1A controls tissue stiffness and cancer stem-like cells in lung adenocarcinoma. Embo J. 2019;38(13):e100532. doi: 10.15252/embj.2018100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu N, Guo Z, Xia X, Liao Y, Zhang F, Huang C, et al. Auranofin lethality to prostate cancer includes inhibition of proteasomal deubiquitinases and disrupted androgen receptor signaling. Eur J Pharmacol. 2019;846:1–11. doi: 10.1016/j.ejphar.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 187.Xu Q, Liu M, Zhang F, Liu X, Ling S, Chen X, et al. Ubiquitin-specific protease 2 regulates Ang II-induced cardiac fibroblasts activation by up-regulating cyclin D1 and stabilizing β-catenin in vitro. J Cell Mol Med. 2021;25(2):1001–1011. doi: 10.1111/jcmm.16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9(2):134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 190.Wang X, Sun X, Mu L, Chen W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition in endometrial cancer cells by regulating pituitary tumor transforming gene. Cancer Invest. 2019;37(3):134–143. doi: 10.1080/07357907.2019.1575969. [DOI] [PubMed] [Google Scholar]
- 191.Nagaraja SS, Subramanian U, Nagarajan D. Radiation-induced H3K9 methylation on E-cadherin promoter mediated by ROS/Snail axis: role of G9a signaling during lung epithelial-mesenchymal transition. Toxicol In Vitro. 2021;70:105037. doi: 10.1016/j.tiv.2020.105037. [DOI] [PubMed] [Google Scholar]
- 192.Kim JY, Jeon S, Yoo YJ, Jin H, Won HY, Yoon K, et al. The Hsp27-mediated IkBα-NFκB signaling axis promotes radiation-induced lung fibrosis. Clin Cancer Res. 2019;25(17):5364–5375. doi: 10.1158/1078-0432.CCR-18-3900. [DOI] [PubMed] [Google Scholar]
- 193.Balli D, Ustiyan V, Zhang Y, Wang IC, Masino AJ, Ren X, et al. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. Embo j. 2013;32(2):231–244. doi: 10.1038/emboj.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liang X, Yan Z, Wang P, Liu Y, Ao X, Liu Z, et al. Irradiation activates MZF1 to inhibit miR-541-5p expression and promote epithelial-mesenchymal transition (EMT) in radiation-induced pulmonary fibrosis (RIPF) by upregulating slug. Int J Mol Sci. 2021;22(21):11309. doi: 10.3390/ijms222111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yan Z, Ao X, Liang X, Chen Z, Liu Y, Wang P, et al. Transcriptional inhibition of miR-486-3p by BCL6 upregulates Snail and induces epithelial-mesenchymal transition during radiation-induced pulmonary fibrosis. Respir Res. 2022;23(1):104. doi: 10.1186/s12931-022-02024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang D, Liu Z, Yan Z, Liang X, Liu X, Liu Y, et al. MiRNA-155-5p inhibits epithelium-to-mesenchymal transition (EMT) by targeting GSK-3β during radiation-induced pulmonary fibrosis. Arch Biochem Biophys. 2021;697:108699. doi: 10.1016/j.abb.2020.108699. [DOI] [PubMed] [Google Scholar]
- 197.Gong L, Wu X, Li X, Ni X, Gu W, Wang X, et al. S1PR3 deficiency alleviates radiation-induced pulmonary fibrosis through the regulation of epithelial–mesenchymal transition by targeting miR-495-3p. J Cell Physiol. 2020;235(3):2310–2324. doi: 10.1002/jcp.29138. [DOI] [PubMed] [Google Scholar]
- 198.Guo T, Zou L, Ni J, Zhou Y, Ye L, Yang X, et al. Regulatory T cells: an emerging player in radiation-induced lung injury. Front Immunol. 2020;11:1769. doi: 10.3389/fimmu.2020.01769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA. 2007;104(49):19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Park HR, Jo SK, Jung U. Ionizing radiation promotes epithelial-to-mesenchymal transition in lung epithelial cells by TGF-β-producing M2 macrophages. In Vivo. 2019;33(6):1773–1784. doi: 10.21873/invivo.11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li Q, Cheng Y, Zhang Z, Bi Z, Ma X, Wei Y, et al. Inhibition of ROCK ameliorates pulmonary fibrosis by suppressing M2 macrophage polarisation through phosphorylation of STAT3. Clin Transl Med. 2022;12(10):e1036. doi: 10.1002/ctm2.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chung EJ, Kwon S, Reedy JL, White AO, Song JS, Hwang I, et al. IGF-1 receptor signaling regulates type II pneumocyte senescence and resulting macrophage polarization in lung fibrosis. Int J Radiat Oncol Biol Phys. 2021;110(2):526–538. doi: 10.1016/j.ijrobp.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nagano H, Hashimoto N, Nakayama A, Suzuki S, Miyabayashi Y, Yamato A, et al. p53-inducible DPYSL4 associates with mitochondrial supercomplexes and regulates energy metabolism in adipocytes and cancer cells. Proc Natl Acad Sci USA. 2018;115(33):8370–8375. doi: 10.1073/pnas.1804243115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Judge JL, Owens KM, Pollock SJ, Woeller CF, Thatcher TH, Williams JP, et al. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L879–L887. doi: 10.1152/ajplung.00153.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Herskind C, Sticht C, Sami A, Giordano FA, Wenz F. Gene expression profiles reveal extracellular matrix and inflammatory signaling in radiation-induced premature differentiation of human fibroblast in vitro. Front Cell Dev Biol. 2021;9:539893. doi: 10.3389/fcell.2021.539893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Müller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35(4 Suppl 1):96–104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 207.de Andrade CBV, Ramos IPR, de Moraes ACN, Nascimento do ALR, Salata C, Goldenberg R, et al. Radiotherapy-induced skin reactions induce fibrosis mediated by TGF-β1 cytokine. Dose Response. 2017;15(2):1559325817705019. doi: 10.1177/1559325817705019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cao J, Zhu W, Yu D, Pan L, Zhong L, Xiao Y, et al. The involvement of SDF-1α/CXCR4 axis in radiation-induced acute injury and fibrosis of skin. Radiat Res. 2019;192(4):410–421. doi: 10.1667/RR15384.1. [DOI] [PubMed] [Google Scholar]
- 209.Horton JA, Chung EJ, Hudak KE, Sowers A, Thetford A, White AO, et al. Inhibition of radiation-induced skin fibrosis with imatinib. Int J Radiat Biol. 2013;89(3):162–170. doi: 10.3109/09553002.2013.741281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hsu PH, Chen YH, Huang PI, Hwang PA. Skin proteomic profiling of irradiation-induced fibrosis and its modulation by low molecular weight fucoidan via tight junction pathway. Biomed Pharmacother. 2022;153:113417. doi: 10.1016/j.biopha.2022.113417. [DOI] [PubMed] [Google Scholar]
- 211.Qiu Y, Gao Y, Yu D, Zhong L, Cai W, Ji J, et al. Genome-wide analysis reveals zinc transporter ZIP9 regulated by DNA methylation promotes radiation-induced skin fibrosis via the TGF-β signaling pathway. J Invest Dermatol. 2020;140(1):94–102.e7. doi: 10.1016/j.jid.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 212.Weigel C, Veldwijk MR, Oakes CC, Seibold P, Slynko A, Liesenfeld DB, et al. Epigenetic regulation of diacylglycerol kinase alpha promotes radiation-induced fibrosis. Nat Commun. 2016;7:10893. doi: 10.1038/ncomms10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Liu C-S, Toth R, Bakr A, Goyal A, Islam MS, Breuer K, et al. Epigenetic modulation of radiation-induced diacylglycerol kinase alpha expression prevents pro-fibrotic fibroblast response. Cancers. 2021;13(10):2455. doi: 10.3390/cancers13102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Valinciute G, Weigel C, Veldwijk MR, Oakes CC, Herskind C, Wenz F, et al. BET-bromodomain inhibitors modulate epigenetic patterns at the diacylglycerol kinase alpha enhancer associated with radiation-induced fibrosis. Radiother Oncol. 2017;125(1):168–174. doi: 10.1016/j.radonc.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 215.Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med. 2017;49(7):e359. doi: 10.1038/emm.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hu Z, Qin F, Gao S, Zhen Y, Huang D, Dong L. Paeoniflorin exerts protective effect on radiation-induced hepatic fibrosis in rats via TGF-β1/Smads signaling pathway. Am J Transl Res. 2018;10(3):1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 217.Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernández M, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49(6):965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 218.Latella G, Vetuschi A, Sferra R, Catitti V, D'Angelo A, Zanninelli G, et al. Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int. 2009;29(7):997–1009. doi: 10.1111/j.1478-3231.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- 219.Du SS, Qiang M, Zeng ZC, Zhou J, Tan YS, Zhang ZY, et al. Radiation-induced liver fibrosis is mitigated by gene therapy inhibiting transforming growth factor-β signaling in the rat. Int J Radiat Oncol Biol Phys. 2010;78(5):1513–1523. doi: 10.1016/j.ijrobp.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 220.Wang S, Lee Y, Kim J, Hyun J, Lee K, Kim Y, et al. Potential role of Hedgehog pathway in liver response to radiation. PLoS ONE. 2013;8(9):e74141. doi: 10.1371/journal.pone.0074141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Melin N, Yarahmadov T, Sanchez-Taltavull D, Birrer FE, Brodie TM, Petit B, et al. A new mouse model of radiation-induced liver disease reveals mitochondrial dysfunction as an underlying fibrotic stimulus. JHEP Rep. 2022;4(7):100508. doi: 10.1016/j.jhepr.2022.100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zheng J, Dong P, Mao Y, Chen S, Wu X, Li G, et al. lincRNA-p21 inhibits hepatic stellate cell activation and liver fibrogenesis via p21. Febs j. 2015;282(24):4810–4821. doi: 10.1111/febs.13544. [DOI] [PubMed] [Google Scholar]
- 223.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56(3):1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen Y, Zhou P, Deng Y, Cai X, Sun M, Sun Y, et al. ALKBH5-mediated m(6) A demethylation of TIRAP mRNA promotes radiation-induced liver fibrosis and decreases radiosensitivity of hepatocellular carcinoma. Clin Transl Med. 2023;13(2):e1198. doi: 10.1002/ctm2.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gu CY, Lee TKW. CircTUBD1: a novel circular RNA molecule as a therapeutic target in radiation-induced liver fibrosis. J Clin Transl Hepatol. 2022;10(4):571–573. doi: 10.14218/JCTH.2022.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Niu H, Zhang L, Wang B, Zhang GC, Liu J, Wu ZF, et al. CircTUBD1 regulates radiation-induced liver fibrosis response via a circTUBD1/micro-203a-3p/Smad3 positive feedback loop. J Clin Transl Hepatol. 2022;10(4):680–691. doi: 10.14218/JCTH.2021.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Niu H, Zhang L, Chen YH, Yuan BY, Wu ZF, Cheng JC, et al. Circular RNA TUBD1 acts as the miR-146a-5p sponge to affect the viability and pro-inflammatory cytokine production of LX-2 cells through the TLR4 pathway. Radiat Res. 2020;193(4):383–393. doi: 10.1667/RR15550.1. [DOI] [PubMed] [Google Scholar]
- 228.Yuan BY, Chen YH, Wu ZF, Zhuang Y, Chen GW, Zhang L, et al. MicroRNA-146a-5p attenuates fibrosis-related molecules in irradiated and TGF-beta1-treated human hepatic stellate cells by regulating PTPRA-SRC signaling. Radiat Res. 2019;192(6):621–629. doi: 10.1667/RR15401.1. [DOI] [PubMed] [Google Scholar]
- 229.Czubryt MP. Cardiac fibroblast to myofibroblast phenotype conversion-an unexploited therapeutic target. J Cardiovasc Dev Dis. 2019;6(3):28. doi: 10.3390/jcdd6030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang J, He X, Bai X, Sun Y, Jiang P, Wang X, et al. Protective effect of trimetazidine in radiation-induced cardiac fibrosis in mice. J Radiat Res. 2020;61(5):657–665. doi: 10.1093/jrr/rraa043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang K, He X, Zhou Y, Gao L, Qi Z, Chen J, et al. Atorvastatin ameliorates radiation-induced cardiac fibrosis in rats. Radiat Res. 2015;184(6):611–620. doi: 10.1667/RR14075.1. [DOI] [PubMed] [Google Scholar]
- 232.Azimzadeh O, Sievert W, Sarioglu H, Yentrapalli R, Barjaktarovic Z, Sriharshan A, et al. PPAR alpha: a novel radiation target in locally exposed Mus musculus heart revealed by quantitative proteomics. J Proteome Res. 2013;12(6):2700–2714. doi: 10.1021/pr400071g. [DOI] [PubMed] [Google Scholar]
- 233.Subramanian V, Seemann I, Merl-Pham J, Hauck SM, Stewart FA, Atkinson MJ, et al. Role of TGF beta and PPAR alpha signaling pathways in radiation response of locally exposed heart: integrated global transcriptomics and proteomics analysis. J Proteome Res. 2017;16(1):307–318. doi: 10.1021/acs.jproteome.6b00795. [DOI] [PubMed] [Google Scholar]
- 234.Subramanian V, Borchard S, Azimzadeh O, Sievert W, Merl-Pham J, Mancuso M, et al. PPARα is necessary for radiation-induced activation of noncanonical TGFβ signaling in the heart. J Proteome Res. 2018;17(4):1677–1689. doi: 10.1021/acs.jproteome.8b00001. [DOI] [PubMed] [Google Scholar]
- 235.Bansal T, Chatterjee E, Singh J, Ray A, Kundu B, Thankamani V, et al. Arjunolic acid, a peroxisome proliferator-activated receptor α agonist, regresses cardiac fibrosis by inhibiting non-canonical TGF-β signaling. J Biol Chem. 2017;292(40):16440–16462. doi: 10.1074/jbc.M117.788299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Guo H, Zhao X, Li H, Liu K, Jiang H, Zeng X, et al. GDF15 promotes cardiac fibrosis and proliferation of cardiac fibroblasts via the MAPK/ERK1/2 pathway after irradiation in rats. Radiat Res. 2021;196(2):183–191. doi: 10.1667/RADE-20-00206.1. [DOI] [PubMed] [Google Scholar]
- 237.Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–346. doi: 10.1016/j.redox.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li X, Ding D, Chen W, Liu Y, Pan H, Hu J. Growth differentiation factor 11 mitigates cardiac radiotoxicity via activating AMPKα. Free Radic Res. 2021;55(2):176–185. doi: 10.1080/10715762.2021.1885653. [DOI] [PubMed] [Google Scholar]
- 239.Li B, Wang Z, He Y, Chen T, Zhang Y, Yuan X, et al. Adropin improves radiation-induced myocardial injury via VEGFR2/PI3K/Akt Pathway. Oxid Med Cell Longev. 2022;2022:8230214. doi: 10.1155/2022/8230214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wei J, Wang B, Wang H, Meng L, Zhao Q, Li X, et al. Radiation-induced normal tissue damage: oxidative stress and epigenetic mechanisms. Oxid Med Cell Longev. 2019;2019:3010342. doi: 10.1155/2019/3010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mortezaee K, Goradel NH, Amini P, Shabeeb D, Musa AE, Najafi M, et al. NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr Mol Pharmacol. 2019;12(1):50–60. doi: 10.2174/1874467211666181010154709. [DOI] [PubMed] [Google Scholar]
- 242.Kolivand S, Amini P, Saffar H, Rezapoor S, Najafi M, Motevaseli E, et al. Selenium-L-methionine modulates radiation injury and Duox1 and Duox2 upregulation in rat's heart tissues. J Cardiovasc Thorac Res. 2019;11(2):121–126. doi: 10.15171/jcvtr.2019.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ameziane-El-Hassani R, Talbot M, de Souza dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, et al. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci USA. 2015;112(16):5051–5056. doi: 10.1073/pnas.1420707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ping Z, Peng Y, Lang H, Xinyong C, Zhiyi Z, Xiaocheng W, et al. Oxidative stress in radiation-induced cardiotoxicity. Oxid Med Cell Longev. 2020;2020:3579143. doi: 10.1155/2020/3579143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang Z, Yang B, Chen X, Zhou Q, Li H, Chen S, et al. Nobiletin regulates ROS/ADMA/DDAHII/eNOS/NO pathway and alleviates vascular endothelium injury by iron overload. Biol Trace Elem Res. 2020;198(1):87–97. doi: 10.1007/s12011-020-02038-6. [DOI] [PubMed] [Google Scholar]
- 246.Maroney SA, Siebert AE, Martinez ND, Rasmussen M, Peterson JA, Weiler H, et al. Platelet tissue factor pathway inhibitor-α dampens cardiac thrombosis and associated fibrosis in mice. J Thromb Haemost. 2023;21(3):639–651. doi: 10.1016/j.jtha.2022.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Braga TT, Brandao WN, Azevedo H, Terra FF, Melo ACL, Pereira FV, et al. NLRP3 gain-of-function in CD4(+) T lymphocytes ameliorates experimental autoimmune encephalomyelitis. Clin Sci (Lond) 2019;133(17):1901–1916. doi: 10.1042/CS20190506. [DOI] [PubMed] [Google Scholar]
- 248.Guo H, Zhao X, Su H, Ma C, Liu K, Kong S, et al. miR-21 is upregulated, promoting fibrosis and blocking G2/M in irradiated rat cardiac fibroblasts. PeerJ. 2020;8:e10502. doi: 10.7717/peerj.10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kura B, Kalocayova B, LeBaron TW, Frimmel K, Buday J, Surovy J, et al. Regulation of microRNAs by molecular hydrogen contributes to the prevention of radiation-induced damage in the rat myocardium. Mol Cell Biochem. 2019;457(1–2):61–72. doi: 10.1007/s11010-019-03512-z. [DOI] [PubMed] [Google Scholar]
- 250.Sridharan V, Tripathi P, Sharma S, Moros EG, Zheng J, Hauer-Jensen M, et al. Roles of sensory nerves in the regulation of radiation-induced structural and functional changes in the heart. Int J Radiat Oncol Biol Phys. 2014;88(1):167–174. doi: 10.1016/j.ijrobp.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Klaus R, Niyazi M, Lange-Sperandio B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol. 2021;16(1):43. doi: 10.1186/s13014-021-01764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xu D, Li H, Katsube T, Huang G, Liu J, Wang B, et al. Effects of concurrent exposure to chronic restraint-induced stress and total-body iron ion radiation on induction of kidney injury in mice. Int J Mol Sci. 2022;23(9):4866. doi: 10.3390/ijms23094866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ki Y, Kim W, Kim YH, Kim D, Bae JS, Park D, et al. Effect of coenzyme Q10 on radiation nephropathy in rats. J Korean Med Sci. 2017;32(5):757–763. doi: 10.3346/jkms.2017.32.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saadat I, Shakibaie M, Jomehzadeh A, Salimi A, Rahimi HR, Torabizadeh SA. Radioprotective effect of zinc nanoparticles on ionizing radiation-induced nephrotoxicity in mice. Pharmacology. 2023;108(1):101–110. doi: 10.1159/000526776. [DOI] [PubMed] [Google Scholar]
- 255.Scharpfenecker M, Floot B, Russell NS, Stewart FA. The TGF-β co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother Oncol. 2012;105(3):313–320. doi: 10.1016/j.radonc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 256.Chaves-Pérez A, Yilmaz M, Perna C, de la Rosa S, Djouder N. URI is required to maintain intestinal architecture during ionizing radiation. Science. 2019;364(6443):eaaq1165. doi: 10.1126/science.aaq1165. [DOI] [PubMed] [Google Scholar]
- 257.Huang S, Huang Y, Lin W, Wang L, Yang Y, Li P, et al. Sitagliptin alleviates radiation-induced intestinal injury by activating NRF2-antioxidant axis, mitigating NLRP3 inf–lammasome activation, and reversing gut microbiota disorder. Oxid Med Cell Longev. 2022;2022:2586305. doi: 10.1155/2022/2586305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rieder F, Kessler S, Sans M, Fiocchi C. Animal models of intestinal fibrosis: new tools for the understanding of pathogenesis and therapy of human disease. Am J Physiol Gastrointest Liver Physiol. 2012;303(7):G786–801. doi: 10.1152/ajpgi.00059.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Vozenin-Brotons MC, Milliat F, Sabourin JC, de Gouville AC, François A, Lasser P, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56(2):561–572. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 260.Zhao X, Ji K, Zhang M, Huang H, Wang F, Liu Y, et al. NMN alleviates radiation-induced intestinal fibrosis by modulating gut microbiota. Int J Radiat Biol. 2023;99(5):823–834. doi: 10.1080/09553002.2023.2145029. [DOI] [PubMed] [Google Scholar]
- 261.Yeh MH, Chang YH, Tsai YC, Chen SL, Huang TS, Chiu JF, et al. Bone marrow derived macrophages fuse with intestine stromal cells and contribute to chronic fibrosis after radiation. Radiother Oncol. 2016;119(2):250–258. doi: 10.1016/j.radonc.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 262.Takemura N, Kurashima Y, Mori Y, Okada K, Ogino T, Osawa H, et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med. 2018;10(429):eaan0333. doi: 10.1126/scitranslmed.aan0333. [DOI] [PubMed] [Google Scholar]
- 263.Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016;7(9):e2387. doi: 10.1038/cddis.2016.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Berchtold L, Friedli I, Vallée JP, Moll S, Martin PY, de Seigneux S. Diagnosis and assessment of renal fibrosis: the state of the art. Swiss Med Wkly. 2017;147:w14442. doi: 10.4414/smw.2017.14442. [DOI] [PubMed] [Google Scholar]
- 265.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 266.Kumar V, Mitchell MD, Umscheid CA, Berns JS, Hogan JJ. Risk of complications with use of aspirin during renal biopsy: a systematic review. Clin Nephrol. 2018;89(2):67–76. doi: 10.5414/CN109274. [DOI] [PubMed] [Google Scholar]
- 267.Patel K, Bedossa P, Castera L. Diagnosis of liver fibrosis: present and future. Semin Liver Dis. 2015;35(2):166–183. doi: 10.1055/s-0035-1550059. [DOI] [PubMed] [Google Scholar]
- 268.Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763–772. doi: 10.1002/hep.29797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Amano Y, Kitamura M, Takano H, Yanagisawa F, Tachi M, Suzuki Y, et al. Cardiac MR imaging of hypertrophic cardiomyopathy: techniques, findings, and clinical relevance. Magn Reson Med Sci. 2018;17(2):120–131. doi: 10.2463/mrms.rev.2017-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ohkubo H, Nakagawa H, Niimi A. Computer-based quantitative computed tomography image analysis in idiopathic pulmonary fibrosis: a mini review. Respir Investig. 2018;56(1):5–13. doi: 10.1016/j.resinv.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 271.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mah K, Van Dyk J, Keane T, Poon PY. Acute radiation-induced pulmonary damage: a clinical study on the response to fractionated radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13(2):179–188. doi: 10.1016/0360-3016(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 273.Bell J, McGivern D, Bullimore J, Hill J, Davies ER, Goddard P. Diagnostic imaging of post-irradiation changes in the chest. Clin Radiol. 1988;39(2):109–119. doi: 10.1016/s0009-9260(88)80003-5. [DOI] [PubMed] [Google Scholar]
- 274.EASL-ALEH Clinical Practice Guidelines Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 275.Ni S, Song M, Guo W, Guo T, Shen Q, Peng H. Biomarkers and their potential functions in idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2020;14(6):593–602. doi: 10.1080/17476348.2020.1745066. [DOI] [PubMed] [Google Scholar]
- 276.Mansour SG, Puthumana J, Coca SG, Gentry M, Parikh CR. Biomarkers for the detection of renal fibrosis and prediction of renal outcomes: a systematic review. BMC Nephrol. 2017;18(1):72. doi: 10.1186/s12882-017-0490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 278.Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, et al. The good and the bad collagens of fibrosis - their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 279.Baues M, Dasgupta A, Ehling J, Prakash J, Boor P, Tacke F, et al. Fibrosis imaging: current concepts and future directions. Adv Drug Deliv Rev. 2017;121:9–26. doi: 10.1016/j.addr.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Barton AK, Tzolos E, Bing R, Singh T, Weber W, Schwaiger M, et al. Emerging molecular imaging targets and tools for myocardial fibrosis detection. Eur Heart J Cardiovasc Imaging. 2023;24(3):261–275. doi: 10.1093/ehjci/jeac242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jenkins WS, Vesey AT, Stirrat C, Connell M, Lucatelli C, Neale A, et al. Cardiac α(V)β(3) integrin expression following acute myocardial infarction in humans. Heart. 2017;103(8):607–615. doi: 10.1136/heartjnl-2016-310115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Toms J, Kogler J, Maschauer S, Daniel C, Schmidkonz C, Kuwert T, et al. Targeting fibroblast activation protein: radiosynthesis and preclinical evaluation of an (18)F-labeled FAP inhibitor. J Nucl Med. 2020;61(12):1806–1813. doi: 10.2967/jnumed.120.242958. [DOI] [PubMed] [Google Scholar]
- 283.Acharya PS, Zukas A, Chandan V, Katzenstein AL, Puré E. Fibroblast activation protein: a serine protease expressed at the remodeling interface in idiopathic pulmonary fibrosis. Hum Pathol. 2006;37(3):352–360. doi: 10.1016/j.humpath.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 284.Syed M, Flechsig P, Liermann J, Windisch P, Staudinger F, Akbaba S, et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur J Nucl Med Mol Imaging. 2020;47(12):2836–2845. doi: 10.1007/s00259-020-04859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Chen L, Zhong X, Li L, Li X, Liu Y, Guo C, et al. [(68)Ga]Ga-FAPI-04 PET/CT on assessing Crohn’s disease intestinal lesions. Eur J Nucl Med Mol Imaging. 2023;50(5):1360–1370. doi: 10.1007/s00259-023-06107-5. [DOI] [PubMed] [Google Scholar]
- 286.Rosenkrans ZT, Massey CF, Bernau K, Ferreira CA, Jeffery JJ, Schulte JJ, et al. [(68) Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur J Nucl Med Mol Imaging. 2022;49(11):3705–3716. doi: 10.1007/s00259-022-05814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang G, Yang Q, Wu S, Xu X, Li X, Liang S, et al. Molecular imaging of fibroblast activity in pressure overload heart failure using [(68) Ga]Ga-FAPI-04 PET/CT. Eur J Nucl Med Mol Imaging. 2023;50(2):465–474. doi: 10.1007/s00259-022-05984-6. [DOI] [PubMed] [Google Scholar]
- 288.Zhao L, Gu J, Fu K, Lin Q, Chen H. 68Ga-FAPI PET/CT in assessment of liver nodules in a cirrhotic patient. Clin Nucl Med. 2020;45(10):e430–e432. doi: 10.1097/RLU.0000000000003015. [DOI] [PubMed] [Google Scholar]
- 289.Phan THG, Paliogiannis P, Nasrallah GK, Giordo R, Eid AH, Fois AG, et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol Life Sci. 2021;78(5):2031–2057. doi: 10.1007/s00018-020-03693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Scharitzer M, Macher-Beer A, Mang T, Unger LW, Haug A, Reinisch W, et al. Evaluation of intestinal fibrosis with (68)Ga-FAPI PET/MR enterography in Crohn disease. Radiology. 2023;307(3):e222389. doi: 10.1148/radiol.222389. [DOI] [PubMed] [Google Scholar]
- 291.Wilson BN, Shah R, Menzer C, Aleisa A, Sun MD, Kwong BY, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187(6):1054–1056. doi: 10.1111/bjd.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pratson CL, Larkins MC, Karimian BH, Curtis CM, Lepera PA, Brodish BN, et al. The impact of smoking, alcohol use, recurrent disease, and age on the development of neck fibrosis in head and neck cancer patients following radiation therapy. Front Oncol. 2021;11:707418. doi: 10.3389/fonc.2021.707418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rosa AC, Bruni N, Meineri G, Corsi D, Cavi N, Gastaldi D, et al. Strategies to expand the therapeutic potential of superoxide dismutase by exploiting delivery approaches. Int J Biol Macromol. 2021;168:846–865. doi: 10.1016/j.ijbiomac.2020.11.149. [DOI] [PubMed] [Google Scholar]
- 294.Johnke RM, Sattler JA, Allison RR. Radioprotective agents for radiation therapy: future trends. Future Oncol. 2014;10(15):2345–2357. doi: 10.2217/fon.14.175. [DOI] [PubMed] [Google Scholar]
- 295.Landeen KC, Spanos WC, Gromer L. Topical superoxide dismutase in posttreatment fibrosis in patients with head and neck cancer. Head Neck. 2018;40(7):1400–1405. doi: 10.1002/hed.25119. [DOI] [PubMed] [Google Scholar]
- 296.Pan J, Su Y, Hou X, He H, Liu S, Wu J, et al. Protective effect of recombinant protein SOD-TAT on radiation-induced lung injury in mice. Life Sci. 2012;91(3–4):89–93. doi: 10.1016/j.lfs.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 297.Pan J, He H, Su Y, Zheng G, Wu J, Liu S, et al. In vivo radioprotective activity of cell-permeable bifunctional antioxidant enzyme GST-TAT-SOD against whole-body ionizing irradiation in mice. Oxid Med Cell Longev. 2017;2017:2689051. doi: 10.1155/2017/2689051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 299.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gauter-Fleckenstein B, Reboucas JS, Fleckenstein K, Tovmasyan A, Owzar K, Jiang C, et al. Robust rat pulmonary radioprotection by a lipophilic Mn N-alkylpyridylporphyrin, MnTnHex-2-PyP(5+) Redox Biol. 2014;2:400–410. doi: 10.1016/j.redox.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48(8):1034–1043. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Archambeau JO, Tovmasyan A, Pearlstein RD, Crapo JD, Batinic-Haberle I. Superoxide dismutase mimic, MnTE-2-PyP(5+) ameliorates acute and chronic proctitis following focal proton irradiation of the rat rectum. Redox Biol. 2013;1(1):599–607. doi: 10.1016/j.redox.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Batinic-Haberle I, Tome ME. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019;25:101139. doi: 10.1016/j.redox.2019.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Shrishrimal S, Chatterjee A, Kosmacek EA, Davis PJ, McDonald JT, Oberley-Deegan RE. Manganese porphyrin, MnTE-2-PyP, treatment protects the prostate from radiation-induced fibrosis (RIF) by activating the NRF2 signaling pathway and enhancing SOD2 and sirtuin activity. Free Radic Biol Med. 2020;152:255–270. doi: 10.1016/j.freeradbiomed.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.MacVittie TJ, Gibbs A, Farese AM, Barrow K, Bennett A, Taylor-Howell C, et al. AEOL 10150 mitigates radiation-induced lung injury in the nonhuman primate: morbidity and mortality are administration schedule-dependent. Radiat Res. 2017;187(3):298–318. doi: 10.1667/RR4413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, et al. Melatonin as an adjuvant in radiotherapy for radioprotection and radiosensitization. Clin Transl Oncol. 2019;21(3):268–279. doi: 10.1007/s12094-018-1934-0. [DOI] [PubMed] [Google Scholar]
- 307.Sheikholeslami S, Aryafar T, Abedi-Firouzjah R, Banaei A, Dorri-Giv M, Zamani H, et al. The role of melatonin on radiation-induced pneumonitis and lung fibrosis: a systematic review. Life Sci. 2021;281:119721. doi: 10.1016/j.lfs.2021.119721. [DOI] [PubMed] [Google Scholar]
- 308.Yahyapour R, Amini P, Saffar H, Motevaseli E, Farhood B, Pooladvand V, et al. Protective effect of metformin, resveratrol and alpha-lipoic acid on radiation- induced pneumonitis and fibrosis: a histopathological study. Curr Drug Res Rev. 2019;11(2):111–117. doi: 10.2174/2589977511666191018180758. [DOI] [PubMed] [Google Scholar]
- 309.Kim JH, Kim KM, Jung MH, Jung JH, Kang KM, Jeong BK, et al. Protective effects of alpha lipoic acid on radiation-induced salivary gland injury in rats. Oncotarget. 2016;7(20):29143–29153. doi: 10.18632/oncotarget.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ryu SH, Park EY, Kwak S, Heo SH, Ryu JW, Park JH, et al. Protective effect of alpha-lipoic acid against radiation-induced fibrosis in mice. Oncotarget. 2016;7(13):15554–15565. doi: 10.18632/oncotarget.6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mohamed HA, Said RS. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-kB/TGF-beta/MMP-9 pathways. Int Immunopharmacol. 2021;92:107347. doi: 10.1016/j.intimp.2020.107347. [DOI] [PubMed] [Google Scholar]
- 312.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97(7):481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 313.Bairati I, Meyer F, Gelinas M, Fortin A, Nabid A, Brochet F, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23(24):5805–5813. doi: 10.1200/JCO.2005.05.514. [DOI] [PubMed] [Google Scholar]
- 314.Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17(10):3283–3290. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 315.Haddad P, Kalaghchi B, Amouzegar-Hashemi F. Pentoxifylline and vitamin E combination for superficial radiation-induced fibrosis: a phase II clinical trial. Radiother Oncol. 2005;77(3):324–326. doi: 10.1016/j.radonc.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 316.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23(34):8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 317.Gothard L, Cornes P, Earl J, Hall E, MacLaren J, Mortimer P, et al. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiother Oncol. 2004;73(2):133–139. doi: 10.1016/j.radonc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 318.Cook M, Johnson N, Zegzula HD, Schray M, Glissmeyer M, Sorenson L. Prophylactic use of pentoxifylline (Trental) and vitamin E to prevent capsular contracture after implant reconstruction in patients requiring adjuvant radiation. Am J Surg. 2016;211(5):854–859. doi: 10.1016/j.amjsurg.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 319.Gou S, Del Rio-Sancho S, Laubach HJ, Kalia YN. Erbium:YAG fractional laser ablation improves cutaneous delivery of pentoxifylline from different topical dosage forms. Int J Pharm. 2022;628:122259. doi: 10.1016/j.ijpharm.2022.122259. [DOI] [PubMed] [Google Scholar]
- 320.Gou S, Del Rio-Sancho S, Singhal M, Laubach HJ, Kalia YN. Er:YAG fractional laser ablation for cutaneous co-delivery of pentoxifylline and d-alpha-tocopherol succinate: a new approach for topical treatment of radiation-induced skin fibrosis. Eur J Pharm Sci. 2019;135:22–31. doi: 10.1016/j.ejps.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 321.Chen C, Yang S, Zhang M, Zhang Z, Hong J, Han D, et al. Triptolide mitigates radiation-induced pulmonary fibrosis via inhibition of axis of alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biol Ther. 2016;17(4):381–389. doi: 10.1080/15384047.2016.1139229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yang S, Zhang M, Chen C, Cao Y, Tian Y, Guo Y, et al. Triptolide mitigates radiation-induced pulmonary fibrosis. Radiat Res. 2015;184(5):509–517. doi: 10.1667/RR13831.1. [DOI] [PubMed] [Google Scholar]
- 323.Chen C, Yang S, Zhang M, Zhang Z, Zhang SB, Wu B, et al. Triptolide mitigates radiation-induced pneumonitis via inhibition of alveolar macrophages and related inflammatory molecules. Oncotarget. 2017;8(28):45133–45142. doi: 10.18632/oncotarget.16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Guo K, Chen J, Chen Z, Luo G, Yang S, Zhang M, et al. Triptolide alleviates radiation-induced pulmonary fibrosis via inhibiting IKKbeta stimulated LOX production. Biochem Biophys Res Commun. 2020;527(1):283–288. doi: 10.1016/j.bbrc.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 325.Park JH, Ryu SH, Choi EK, Ahn SD, Park E, Choi KC, et al. SKI2162, an inhibitor of the TGF-beta type I receptor (ALK5), inhibits radiation-induced fibrosis in mice. Oncotarget. 2015;6(6):4171–4179. doi: 10.18632/oncotarget.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Park J, Choi J, Cho I, Sheen YY. Radiotherapy-induced oxidative stress and fibrosis in breast cancer are suppressed by vactosertib, a novel, orally bioavailable TGF-beta/ALK5 inhibitor. Sci Rep. 2022;12(1):16104. doi: 10.1038/s41598-022-20050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Dadrich M, Nicolay NH, Flechsig P, Bickelhaupt S, Hoeltgen L, Roeder F, et al. Combined inhibition of TGFbeta and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. 2016;5(5):e1123366. doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18(13):3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 329.Cruz-Morande S, Dotor J, San-Julian M. P144 a transforming growth factor beta inhibitor peptide, generates antifibrogenic effects in a radiotherapy induced fibrosis model. Curr Oncol. 2022;29(4):2650–2661. doi: 10.3390/curroncol29040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Li M, Abdollahi A, Grone HJ, Lipson KE, Belka C, Huber PE. Late treatment with imatinib mesylate ameliorates radiation-induced lung fibrosis in a mouse model. Radiat Oncol. 2009;4:66. doi: 10.1186/1748-717X-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Richeldi L, Fernandez Perez ER, Costabel U, Albera C, Lederer DJ, Flaherty KR, et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2020;8(1):25–33. doi: 10.1016/S2213-2600(19)30262-0. [DOI] [PubMed] [Google Scholar]
- 333.Eberlein M, Heusinger-Ribeiro J, Goppelt-Struebe M. Rho-dependent inhibition of the induction of connective tissue growth factor (CTGF) by HMG CoA reductase inhibitors (statins) Br J Pharmacol. 2001;133(7):1172–1180. doi: 10.1038/sj.bjp.0704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Haydont V, Mathe D, Bourgier C, Abdelali J, Aigueperse J, Bourhis J, et al. Induction of CTGF by TGF-beta1 in normal and radiation enteritis human smooth muscle cells: Smad/Rho balance and therapeutic perspectives. Radiother Oncol. 2005;76(2):219–225. doi: 10.1016/j.radonc.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 335.Ostrau C, Hulsenbeck J, Herzog M, Schad A, Torzewski M, Lackner KJ, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92(3):492–499. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 336.Williams JP, Hernady E, Johnston CJ, Reed CM, Fenton B, Okunieff P, et al. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat Res. 2004;161(5):560–567. doi: 10.1667/rr3168. [DOI] [PubMed] [Google Scholar]
- 337.Haydont V, Bourgier C, Pocard M, Lusinchi A, Aigueperse J, Mathe D, et al. Pravastatin Inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13(18 Pt 1):5331–5340. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]
- 338.Bourgier C, Auperin A, Rivera S, Boisselier P, Petit B, Lang P, et al. Pravastatin reverses established radiation-induced cutaneous and subcutaneous fibrosis in patients with head and neck cancer: results of the biology-driven phase 2 clinical trial pravacur. Int J Radiat Oncol Biol Phys. 2019;104(2):365–373. doi: 10.1016/j.ijrobp.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 339.Wang C, Zhu H, Sun Z, Xiang Z, Ge Y, Ni C, et al. Inhibition of Wnt/beta-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am J Physiol Cell Physiol. 2014;307(3):C234–C244. doi: 10.1152/ajpcell.00366.2013. [DOI] [PubMed] [Google Scholar]
- 340.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, et al. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1(5):369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(12):1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ghebremariam YT, Cooke JP, Gerhart W, Griego C, Brower JB, Doyle-Eisele M, et al. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J Transl Med. 2015;13:249. doi: 10.1186/s12967-015-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ghebre Y, Raghu G. Proton pump inhibitors in IPF: beyond mere suppression of gastric acidity. QJM. 2016;109(9):577–579. doi: 10.1093/qjmed/hcw115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ebrahimpour A, Wang M, Li L, Jegga AG, Bonnen MD, Eissa NT, et al. Esomeprazole attenuates inflammatory and fibrotic response in lung cells through the MAPK/Nrf2/HO1 pathway. J Inflamm (Lond) 2021;18(1):17. doi: 10.1186/s12950-021-00284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pham N, Ludwig MS, Wang M, Ebrahimpour A, Bonnen MD, Diwan AH, et al. Topical esomeprazole mitigates radiation-induced dermal inflammation and fibrosis. Radiat Res. 2019;192(5):473–482. doi: 10.1667/RR15398.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Ebrahimpour A, Ahir M, Wang M, Jegga AG, Bonnen MD, Eissa NT, et al. Combination of esomeprazole and pirfenidone enhances antifibrotic efficacy in vitro and in a mouse model of TGFbeta-induced lung fibrosis. Sci Rep. 2022;12(1):20668. doi: 10.1038/s41598-022-24985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tan WQ, Fang QQ, Shen XZ, Giani JF, Zhao TV, Shi P, et al. Angiotensin-converting enzyme inhibitor works as a scar formation inhibitor by down-regulating Smad and TGF-beta-activated kinase 1 (TAK1) pathways in mice. Br J Pharmacol. 2018;175(22):4239–4252. doi: 10.1111/bph.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Couluris M, Kinder BW, Xu P, Gross-King M, Krischer J, Panos RJ. Treatment of idiopathic pulmonary fibrosis with losartan: a pilot project. Lung. 2012;190(5):523–527. doi: 10.1007/s00408-012-9410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Hedayatyanfard K, Ziai SA, Niazi F, Habibi I, Habibi B, Moravvej H. Losartan ointment relieves hypertrophic scars and keloid: a pilot study. Wound Repair Regen. 2018;26(4):340–343. doi: 10.1111/wrr.12648. [DOI] [PubMed] [Google Scholar]
- 350.Kovacs MG, Kovacs ZZA, Varga Z, Szucs G, Freiwan M, Farkas K, et al. Investigation of the antihypertrophic and antifibrotic effects of losartan in a rat model of radiation-induced heart disease. Int J Mol Sci. 2021;22(23):12963. doi: 10.3390/ijms222312963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res. 2013;180(5):546–552. doi: 10.1667/RR13350.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Kma L, Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Angiotensin converting enzyme inhibitors mitigate collagen synthesis induced by a single dose of radiation to the whole thorax. J Radiat Res. 2012;53(1):10–17. doi: 10.1269/jrr.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Erpolat OP, Senturk E, Saribas S, Pasinlioglu B, Gulbahar O, Tuncer S, et al. Angiotensin-converting enzyme inhibitor reduces radiation-induced periprosthetic capsular fibrosis. J Surg Res. 2021;263:167–175. doi: 10.1016/j.jss.2021.01.033. [DOI] [PubMed] [Google Scholar]
- 354.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(1):238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 355.Farberg AS, Jing XL, Monson LA, Donneys A, Tchanque-Fossuo CN, Deshpande SS, et al. Deferoxamine reverses radiation induced hypovascularity during bone regeneration and repair in the murine mandible. Bone. 2012;50(5):1184–1187. doi: 10.1016/j.bone.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Mericli AF, Das A, Best R, Rodeheaver P, Rodeheaver G, Lin KY. Deferoxamine mitigates radiation-induced tissue injury in a rat irradiated TRAM flap model. Plast Reconstr Surg. 2015;135(1):124e–e134. doi: 10.1097/PRS.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 357.Dassoulas KR, Mericli AF, Wang JS, Lei SS, Kim T, Cottler PS, et al. Treatment with topical deferoxamine improves cutaneous vascularity and tissue pliability in an irradiated animal model of tissue expander-based breast reconstruction. Ann Plast Surg. 2019;82(1):104–109. doi: 10.1097/SAP.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 358.Shen AH, Borrelli MR, Adem S, Deleon NMD, Patel RA, Mascharak S, et al. Prophylactic treatment with transdermal deferoxamine mitigates radiation-induced skin fibrosis. Sci Rep. 2020;10(1):12346. doi: 10.1038/s41598-020-69293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Kim J-H, Nam J-K, Kim A-R, Park M-S, Lee H-J, Park J, et al. 2-Methoxyestradiol inhibits radiation-induced skin injuries. Int J Mol Sci. 2022;23(8):4171. doi: 10.3390/ijms23084171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Elzayat MA, Bayoumi AMA, Abdel-Bakky MS, Mansour AM, Kamel M, Abo-Saif A, et al. Ameliorative effect of 2-methoxyestradiol on radiation-induced lung injury. Life Sci. 2020;255:117743. doi: 10.1016/j.lfs.2020.117743. [DOI] [PubMed] [Google Scholar]
- 361.Nam JK, Kim AR, Choi SH, Kim JH, Han SC, Park S, et al. Pharmacologic inhibition of HIF-1alpha attenuates radiation-induced pulmonary fibrosis in a preclinical image guided radiation therapy. Int J Radiat Oncol Biol Phys. 2021;109(2):553–566. doi: 10.1016/j.ijrobp.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 362.Li W, Jiang L, Lu X, Liu X, Ling M. Curcumin protects radiation-induced liver damage in rats through the NF-kappaB signaling pathway. BMC Complement Med Ther. 2021;21(1):10. doi: 10.1186/s12906-020-03182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kolivand S, Amini P, Saffar H, Rezapoor S, Motevaseli E, Najafi M, et al. Evaluating the radioprotective effect of curcumin on rat's heart tissues. Curr Radiopharm. 2019;12(1):23–28. doi: 10.2174/1874471011666180831101459. [DOI] [PubMed] [Google Scholar]
- 364.Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173(5):590–601. doi: 10.1667/RR1522.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Shi HS, Gao X, Li D, Zhang QW, Wang YS, Zheng Y, et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int J Nanomedicine. 2012;7:2601–2611. doi: 10.2147/IJN.S31439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Shabeeb D, Musa AE, Abd Ali HS, Najafi M. Curcumin protects against radiotherapy-induced oxidative injury to the skin. Drug Des Devel Ther. 2020;14:3159–3163. doi: 10.2147/DDDT.S265228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shah S, Rath H, Sharma G, Senapati SN, Mishra E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: a triple-blind, pilot randomised controlled trial. Indian J Dent Res. 2020;31(5):718–727. doi: 10.4103/ijdr.IJDR_822_18. [DOI] [PubMed] [Google Scholar]
- 368.Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, et al. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013;180(1):34–43. doi: 10.1667/RR3255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ryan Wolf J, Heckler CE, Guido JJ, Peoples AR, Gewandter JS, Ling M, et al. Oral curcumin for radiation dermatitis: a URCC NCORP study of 686 breast cancer patients. Support Care Cancer. 2018;26(5):1543–1552. doi: 10.1007/s00520-017-3957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Zhang T, Liu C, Ma S, Gao Y, Wang R. Protective effect and mechanism of action of rosmarinic acid on radiation-induced parotid gland injury in rats. Dose Response. 2020;18(1):1559325820907782. doi: 10.1177/1559325820907782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Zhang T, Ma S, Liu C, Hu K, Xu M, Wang R. Rosmarinic acid prevents radiation-induced pulmonary fibrosis through attenuation of ROS/MYPT1/TGFbeta1 signaling via miR-19b-3p. Dose Response. 2020;18(4):1559325820968413. doi: 10.1177/1559325820968413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Huang Y, Liu W, Liu H, Yang Y, Cui J, Zhang P, et al. Grape seed pro-anthocyanidins ameliorates radiation-induced lung injury. J Cell Mol Med. 2014;18(7):1267–1277. doi: 10.1111/jcmm.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Huang Y, Zhao H, Cao K, Sun D, Yang Y, Liu C, et al. Radioprotective effect of grape seed proanthocyanidins in vitro and in vivo. Oxid Med Cell Longev. 2016;2016:5706751. doi: 10.1155/2016/5706751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yang X, Liu T, Chen B, Wang F, Yang Q, Chen X. Grape seed proanthocyanidins prevent irradiation-induced differentiation of human lung fibroblasts by ameliorating mitochondrial dysfunction. Sci Rep. 2017;7(1):62. doi: 10.1038/s41598-017-00108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Xu Y, Huang Y, Chen Y, Cao K, Liu Z, Wan Z, et al. Grape Seed proanthocyanidins play the roles of radioprotection on normal lung and radiosensitization on lung cancer via differential regulation of the MAPK signaling pathway. J Cancer. 2021;12(10):2844–2854. doi: 10.7150/jca.49987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Shen H, Han J, Liu C, Cao F, Huang Y. Grape seed proanthocyanidins exert a radioprotective effect on the testes and intestines through antioxidant effects and inhibition of MAPK signal pathways. Front Med (Lausanne) 2021;8:836528. doi: 10.3389/fmed.2021.836528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Brooker S, Martin S, Pearson A, Bagchi D, Earl J, Gothard L, et al. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother Oncol. 2006;79(1):45–51. doi: 10.1016/j.radonc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 378.Horton JA, Li F, Chung EJ, Hudak K, White A, Krausz K, et al. Quercetin inhibits radiation-induced skin fibrosis. Radiat Res. 2013;180(2):205–215. doi: 10.1667/RR3237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Verma S, Dutta A, Dahiya A, Kalra N. Quercetin-3-Rutinoside alleviates radiation-induced lung inflammation and fibrosis via regulation of NF-kappaB/TGF-beta1 signaling. Phytomedicine. 2022;99:154004. doi: 10.1016/j.phymed.2022.154004. [DOI] [PubMed] [Google Scholar]
- 380.Zhang C, Zeng W, Yao Y, Xu B, Wei X, Wang L, et al. Naringenin ameliorates radiation-induced lung injury by lowering IL-1beta level. J Pharmacol Exp Ther. 2018;366(2):341–348. doi: 10.1124/jpet.118.248807. [DOI] [PubMed] [Google Scholar]
- 381.Komorowska D, Radzik T, Kalenik S, Rodacka A. Natural radiosensitizers in radiotherapy: cancer treatment by combining ionizing radiation with resveratrol. Int J Mol Sci. 2022;23(18):10627. doi: 10.3390/ijms231810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Rangarajan S, Locy ML, Luckhardt TR, Thannickal VJ. Targeted therapy for idiopathic pulmonary fibrosis: where to now? Drugs. 2016;76(3):291–300. doi: 10.1007/s40265-015-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Qin W, Liu B, Yi M, Li L, Tang Y, Wu B, et al. Antifibrotic agent pirfenidone protects against development of radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2018;190(4):396–403. doi: 10.1667/RR15017.1. [DOI] [PubMed] [Google Scholar]
- 384.Ying H, Fang M, Hang QQ, Chen Y, Qian X, Chen M. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-beta1/Smad3 pathway. J Cell Mol Med. 2021;25(18):8662–8675. doi: 10.1111/jcmm.16821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Simone NL, Soule BP, Gerber L, Augustine E, Smith S, Altemus RM, et al. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol. 2007;2:19. doi: 10.1186/1748-717X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.De Ruysscher D, Granton PV, Lieuwes NG, van Hoof S, Wollin L, Weynand B, et al. Nintedanib reduces radiation-induced microscopic lung fibrosis but this cannot be monitored by CT imaging: a preclinical study with a high precision image-guided irradiator. Radiother Oncol. 2017;124(3):482–487. doi: 10.1016/j.radonc.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 387.Takahashi S, Endo M, Fukuda Y, Ogawa K, Nakamura M, Okada K, et al. Nintedanib-induced delayed mucosal healing after adjuvant radiation in a case of oropharyngeal carcinoma. Case Rep Oncol. 2022;15(2):776–782. doi: 10.1159/000526077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Mortezaee K, Shabeeb D, Musa AE, Najafi M, Farhood B. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol. 2019;14(1):41–53. doi: 10.2174/1574884713666181025141559. [DOI] [PubMed] [Google Scholar]
- 389.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond) 2012;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Wang J, Wang Y, Han J, Mei H, Yu D, Ding Q, et al. Metformin attenuates radiation-induced pulmonary fibrosis in a murine model. Radiat Res. 2017;188(1):105–113. doi: 10.1667/RR14708.1. [DOI] [PubMed] [Google Scholar]
- 391.Azmoonfar R, Amini P, Saffar H, Rezapoor S, Motevaseli E, Cheki M, et al. Metformin protects against radiation-induced pneumonitis and fibrosis and attenuates upregulation of dual oxidase genes expression. Adv Pharm Bull. 2018;8(4):697–704. doi: 10.15171/apb.2018.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Farhood B, Aliasgharzadeh A, Amini P, Rezaeyan A, Tavassoli A, Motevaseli E, et al. Mitigation of radiation-induced lung pneumonitis and fibrosis using metformin and melatonin: a histopathological study. Medicina. 2019;55(8):417. doi: 10.3390/medicina55080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Yahyapour R, Amini P, Saffar H, Rezapoor S, Motevaseli E, Cheki M, et al. Metformin protects against radiation-induced heart injury and attenuates the upregulation of dual oxidase genes following rat's chest irradiation. Int J Mol Cell Med. 2018;7(3):193–202. doi: 10.22088/IJMCM.BUMS.7.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Kim JM, Yoo H, Kim JY, Oh SH, Kang JW, Yoo BR, et al. Metformin alleviates radiation-induced skin fibrosis via the downregulation of FOXO3. Cell Physiol Biochem. 2018;48(3):959–970. doi: 10.1159/000491964. [DOI] [PubMed] [Google Scholar]
- 395.Usunier B, Brossard C, L’Homme B, Linard C, Benderitter M, Milliat F, et al. HGF and TSG-6 released by mesenchymal stem cells attenuate colon radiation-induced fibrosis. Int J Mol Sci. 2021;22(4):1790. doi: 10.3390/ijms22041790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Dong LH, Jiang YY, Liu YJ, Cui S, Xia CC, Qu C, et al. The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep. 2015;5:8713. doi: 10.1038/srep08713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Zhang Y, Jiang X, Ren L. Optimization of the adipose-derived mesenchymal stem cell delivery time for radiation-induced lung fibrosis treatment in rats. Sci Rep. 2019;9(1):5589. doi: 10.1038/s41598-019-41576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Liu D, Kong F, Yuan Y, Seth P, Xu W, Wang H, et al. Decorin-modified umbilical cord mesenchymal stem cells (MSCs) attenuate radiation-induced lung injuries via regulating inflammation, fibrotic factors, and immune responses. Int J Radiat Oncol Biol Phys. 2018;101(4):945–956. doi: 10.1016/j.ijrobp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 399.Wei L, Zhang J, Yang ZL, You H. Extracellular superoxide dismutase increased the therapeutic potential of human mesenchymal stromal cells in radiation pulmonary fibrosis. Cytotherapy. 2017;19(5):586–602. doi: 10.1016/j.jcyt.2017.02.359. [DOI] [PubMed] [Google Scholar]
- 400.Gronhoj C, Jensen DH, Glovinski PV, Jensen SB, Bardow A, Oliveri RS, et al. First-in-man mesenchymal stem cells for radiation-induced xerostomia (MESRIX): study protocol for a randomized controlled trial. Trials. 2017;18(1):108. doi: 10.1186/s13063-017-1856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]