Abstract
The proinflammatory state associated with diabetes mellitus (DM) remains poorly understood. We found patients with DM have 3- to 14-fold elevations of blood-borne microparticles (MPs) that bind phalloidin (Ph; Ph positive [+] MPs), indicating the presence of F-actin on their surface. We hypothesized that F-actin–coated MPs were an unrecognized cause for DM-associated proinflammatory status. Ph+MPs, but not Ph-negative MPs, activate human and murine (Mus musculus) neutrophils through biophysical attributes of F-actin and membrane expression of phosphatidylserine (PS). Neutrophils respond to Ph+MPs via a linked membrane array, including the receptor for advanced glycation end products and CD36, PS-binding membrane receptors. These proteins in conjunction with TLR4 are coupled to NO synthase 1 adaptor protein (NOS1AP). Neutrophil activation occurs because of Ph+MPs causing elevations of NF-κB and Src kinase (SrcK) via a concurrent increased association of NO synthase 2 and SrcK with NOS1AP, resulting in SrcK S-nitrosylation. We conclude that NOS1AP links PS-binding receptors with intracellular regulatory proteins. Ph+MPs are alarmins present in normal human plasma and are increased in those with DM and especially those with DM and a lower-extremity ulcer.
Introduction
A proinflammatory state is linked to acute and chronic adverse health outcomes among patients with diabetes mellitus (DM) (1–4). Patients with DM and a foot ulcer (DFU) are reported to have elevations of blood-borne microparticles (MPs) expressing F-actin on their membrane surface, which can be identified by phalloidin (Ph) binding (Ph positive [+] MPs) (5). As with most types of extracellular vesicles, MPs are found in all body fluids and increase in association with virtually every human disease and injury. They are 2- to 10-fold higher than normal in those with DM (6–9). Leukocyte-derived MPs have adverse effects on healing and play a role in endothelial injury and progression of atherosclerosis (8–10).
Ph+MPs were previously found to cause vascular injuries and to be produced by neutrophils in response to oxidative stress (11). Because hyperglycemia causes neutrophil oxidative stress that triggers MPs production, we questioned whether Ph+MPs were an unrecognized cause for DM-associated proinflammatory status (12). Although primarily an intracellular cytoskeletal protein, actin has been detected on the outer membrane surface of platelets, neutrophils, monocytes, lymphocytes, and endothelial cells (13–18). Macrophage MPs generation requires extracellular F-actin, which appears to influence caspase-1 activation at filopodia (19).
Study goals were to assess the prevalence of Ph+MPs and explore their proinflammatory mechanism. Previous studies have shown that in response to hyperglycemia, neutrophils produce MPs containing high concentrations of IL-1β (12). This process is catalyzed by NO synthase (NOS) 1 adaptor protein (NOS1AP) that shifts from a diffuse cytosolic localization to a dense submembranous site while colocalizing with inflammatory NOS2 to facilitate S-nitrosylation of F-actin and its turnover, resulting in formation of the nucleotide-binding domain leucine-rich repeat-like receptor (NLRP3) inflammasome. Variants of the NOS1AP gene are associated with peripheral neuropathy and lower-extremity amputations in those with DM, but not among those without DM (20). Several NOS1AP single-nucleotide polymorphisms are associated with impaired healing and amputations among patients with DM (21). Others have found associations between some NOS1AP gene variants and incidence of DM among users of some medications, as well as variations in therapeutic efficacy of several hyperglycemic medications (22–25).
We also investigated a role for the multiligand receptor for advanced glycation end products (RAGE), which mediates a variety of inflammatory responses in DM (4). Although glucose does not itself initiate cell signaling via RAGE, phosphatidylserine (PS) is among the known RAGE ligands, and it is highly expressed on the surface of MPs (7, 26). RAGE also plays a role in regulating another PS receptor, CD36 (27, 28). This occurs in conjunction with TLR4 (27). There is precedence for coordination of expression and responses involving CD36 and TLR4, typically through lipid rafts and caveolin-1 (27–29). Leukocytes and endothelium of patients with DM exhibit elevations of multiple receptors, including TLR4 and CD36, that contribute to the development of complications (30–32).
Materials and Methods
Experimental design
The study began with evaluating the presence of Ph+MPs in healthy humans and those with DM, then to investigate how they played a proinflammatory role. Murine neutrophils studies supplemented work with human cells to validate the role of specific proteins in neutrophil inflammatory responses.
Human subjects
All procedures were completed in accordance with the Declaration of Helsinki and approved by Ethical Committees of organizations involved with this investigation, and subjects signed informed consent before inclusion in the study. Those with DM were recruited as part of a multicenter study called the Diabetic Foot Ulcer Consortium (DFUC), which was designed to evaluate circulating cellular markers, circulating endothelial progenitor cells (CEPCs), and MPs as prognostic factors associated with the healing of DFU. The DFUC is composed of wound care centers at academic institutions, University of Miami, Icahn School of Medicine, and University of Pennsylvania, as well as the community-based MVS Wound Care in Maryland. All subjects were evaluated and treated by accomplished wound care specialists. All subjects were recruited by the local investigators, who had no knowledge of the laboratory methods or results.
Materials
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. BioAegis Therapeutics (North Brunswick, NJ) provided recombinant human gelsolin, and RAGE inhibitor (RAGE antagonist peptide) was from Tocris (catalog number [cat#] 6259). Abs and flow cytometry reagents are as follows: annexin V–FITC (cat# 556419; BD), Anti-actin (cat# A2066; Sigma-Aldrich, St. Louis, MO), anti-biotin (cat# A0185; Sigma), anti-CD66b, anti-Ly6G eFluor450 (cat#48-5931-82; eBioscience, San Diego, CA), anti–IL-1β (cat# ab9722; Abcam, Cambridge, MA), anti-Filamin Ab (cat# ab76289; Abcam), anti–IL-18 (cat# ab243091; Abcam), anti-myeloperoxidase (anti-MPO; cat# HM1051PE-100; Hycult Biotech, Plymouth Meeting, PA), anti-NOS1AP (cat# ab190686; Abcam), anti-NOS2 (cat# sc-7271; Santa Cruz), anti-p130CAS Ab (cat# PA5-83601; Invitrogen), anti-p65 subunit of NF-κB (cat# ab32536; Abcam), anti-phosphorylated at serine 536 p65 NF-κB (cat# 3031; Cell Signaling, Danvers, MA), anti–phosphorylated Src kinase (SrcK) Ab (cat# PA5-97364; Invitrogen), anti-RAGE (cat#: sc-365154; Santa Cruz), anti-SrcK Ab (cat# sc-5266; Santa Cruz), anti-TLR4 Ab (cat# 48-2300; Invitrogen), anti–TNF-α (cat# ab255275; Abcam), and N-(7-nitrobenz-2-oxa-1,3-thiazol-4-yl)-Ph (cat# N354; Life Technologies). Abs for flow cytometry and Western blots were specifically for that usage as documented by the manufacturers and used at the concentrations recommended. Verification that anti-actin recognizes β-actin was shown by Western blot and mass spectroscopy in a prior publication (11). Positive staining in flow cytometry was determined following the fluorescence-minus-one control test. Small inhibitory RNA (siRNA) sequences were purchased from Santa Cruz Biotechnology. These included a control, scrambled sequence siRNA that will not cause specific degradation of any known cellular mRNA and sequences for RAGE, TLR4, SrcK, and NOS1AP.
Animals
All study aspects were reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6J mice (Mus musculus) were purchased from Jackson Laboratories (Bar Harbor, ME). CD36 knockout (KO) mice purchased from Jackson Labs were raised in the university vivarium and housed in the animal facility with a 12/12-h light-dark cycle. Housing and all experiments were conducted at 22–24°C and 40–70% humidity. Mice received water ad libitum and were fed Laboratory Rodent Diet 5001 (PMI Nutritional, Brentwood, MO). Randomization of mice was performed by first collecting all mice to be used on a day into a single plastic cage and then randomly selected for use as control or in an intervention group. Studies were done over a span of 4 mo with acclimatized mice purchased in groups of 6–12 at biweekly intervals and used according to a block design. Data were scored and analyzed in a blinded manner. Data from all mice were included in data analysis.
Isolation of neutrophils
Blood from healthy human volunteers and mice was processed as previously described (11). Isolated neutrophils were suspended in PBS buffer (PBS + 1 mM CaCl2, 1.5 mM MgCl2 containing either 5.5 or 20 mM glucose). Where indicated, prior to studies, some murine cell suspensions were exposed in PBS buffer for 20 h at room temperature to 0.08 nM siRNA following manufacturer’s instructions. Protein reduction after these treatments was always >75% as assessed by Western blotting. Where indicated, inhibitors were present in cell suspensions as follows: 25 µmol sulfo-N-succinyloleate (SSO), 10 µmol RAGE inhibitor, 10 µmol TAK242 TLR4 inhibitor, 5 µmol PS, and 5–30 µmol phosphatidylcholine (PC). At the termination of each experiment, neutrophil viability was determined as trypan blue exclusion.
Standard procedures for MP isolation
MPs were isolated and prepared for analysis by flow cytometry as previously described (11). In brief, blood was centrifuged for 5 min at 1500 × g. EDTA was added to the supernatant to achieve 12.5 mM to prevent MPs aggregation, centrifuged at 15,000 × g for 30 min, and analyzed by flow cytometry on an eight-color, triple-laser MACSQuant Analyzer (Miltenyi Biotec Corp., Auburn, CA) using MACSQuantify software version 2.5 to analyze data. Analysis involved establishing true-negative controls by a fluorescence-minus-one analysis and by using isotype-matched irrelevant Abs at the same concentration and under the same conditions. Both forward scatter and sideward scatter were set at logarithmic gain. Microbeads of various diameters, 0.3 μm (Sigma), 1.0 μm (Spherotech, Lake Forest, IL), and 3.0 μm (Spherotech) were used for initial settings and before each experiment to measure MPs as an internal control. Samples were suspended in Annexin binding buffer solution and Abs as listed earlier. MPs were defined as annexin V–positive particles with diameters of 0.3–1 µm.
Separation of Ph+MPs was achieved by incubating MPs suspensions with Ph-biotin conjugates followed by streptavidin conjugated to 1-µm-diameter MagVigen magnetic nanoparticles (Nvigen, Sunnyvale, CA) and incubated for 12 h before separation using a magnet. Washing and magnetic bead separation followed the manufacturer’s recommended procedure. Suspensions of MPs remaining after magnetic extraction were centrifuged at 100,000 × g for 2 h to pellet particles for analysis.
Samples for Western blots were prepared following published procedures (12). NOS1AP immunoprecipitation was carried out by incubating cell lysates with anti-NOS1AP IgG that was then extracted using magnetic protein A/G nanoparticles (Nvigen, Sunnyvale, CA) as in prior publications (12). After Western blotting, protein band densities were quantified based on band pixel counts and normalized to the NOS1AP band in each lysate. Representative blots showing pixel counts for blots in this project are shown in Supplemental Figs. 1–4. The ratio of each protein relative to NOS1AP was compared with that calculated for the control sample in each experiment. Therefore, data are expressed as the fold change in band density normalized to the ratio observed in the control for each actin fraction.
Cell extract preparation and biotin-switch assay
Neutrophil suspensions were transferred to HEN buffer (250 mM Hepes [pH 7.7], 1 mM EDTA, 0.1 mM neocuproine), lysed, and subjected to the biotin-switch assay as previously described (12).
Electron microscopy
Preparation and imaging were done at the Electron Microscopy Core Imaging Facility, University of Maryland Dental School. MP samples were washed, immersed in low melting point agarose, and postfixed with 1% osmium tetroxide and 0.75% potassium ferrocyanide in 0.1 M PIPES (pH 7) for 1 h at 4°C. Specimens were washed in water, stained en bloc with 1% (w/v) uranyl acetate for 1 h, and dehydrated using 30, 50, 70, 90, and 100% ethanol, followed by two changes in 100% acetone. After dehydration, specimens were infiltrated and embedded in Araldite-Epoxy resin (Araldite, Embed 812; Electron Microscopy Sciences, Hatfield, PA) following the manufacturer’s recommendations. Sections were cut at ∼70 nm on a Leica UC6 ultramicrotome and collected onto 200 mesh copper grids. For negative staining, 10 µl purified MP suspensions were applied to Formvar-coated 400 mesh copper grids, stained with 2% uranyl acetate, and air dried. Samples were examined in a Tecnai T12 transmission electron microscope (Thermo Fisher Scientific) operated at 80 KeV. Digital images were acquired by using an AMT bottom-mount CCD camera (Advanced Microscopy Techniques, Woburn, MA) and AMT600 software.
Statistical analysis
Results are expressed as the mean ± SD for three or more independent experiments. Data were compared by ANOVA and Newman–Keuls post hoc test using SigmaStat (Jandel Scientific, San Jose, CA). For all studies, we deemed a result to be statistically significant if p < 0.05.
Ethics approval
As the oversight Ethics Organization for the entire project, the University of Maryland approval number was HCR-HP-00076048 with the protocol title, “NOS1AP and capon associated impaired healing in those with diabetic foot ulcers.” Animal use was performed with approval by the University of Maryland Institutional Animal Care and Use Committee protocol number 0119016.
Data availability
Data and materials from this project are available on request.
Results
Blood-borne MPs in human subjects
MPs were assayed in the blood of nondiabetic, healthy control subjects and patients with DM, some of whom also had a DFU (Tables I, II). Among controls, Ph+MPs represented ∼8% of all MPs, and values were insignificantly different between sexes and across ages from 18 to 70 y. Total numbers of MPs and Ph+MPs were significantly more numerous in those with DM and DM+DFU (p < 0.001, ANOVA). Hemoglobin A1C values for the two groups were 7.5 ± 1.9% (SD) for DM and 8.0 ± 2.5% for DM+DFU (NS).
Table I.
The distribution of MPs and % Ph+ (Ph+MPs) binding among age groups of healthy control subjects
| <35 y old (n = 50) | >35–45 y old (n = 32) | >45–60 y old (n = 29) | >60 y old (n = 13) | |
|---|---|---|---|---|
| Age [% male] | 25.8 (1.3) [56%] | 40.4 (1.1) [62%] | 54.0 (1.7) [62%] | 66.8 (2.4) [69%] |
| MPs/µl | 1954 (235) | 1765 (321) | 1765 (401) | 1813 (702) |
| % Ph+MPs | 12.4 (2.0) | 9.6 (2.7) | 9.6 (4.5) | 4.8 (2.7) |
MPs in human subjects as mean (CI).
Table II.
MPs number, % Ph (Ph+) binding and fractions of MPs expressing CD66b (neutrophil-specific protein), CD146 (endothelial cell–specific protein), CD41a (platelet-specific protein), and subsets of Ph+MPs expressing CD66b, CD146, or CD41a for controls, those with DM, and DM+DFU groups
| MPs/µl | % Ph+ | % CD66b | % CD146 | % CD41a | % Ph+ and CD66b | % Ph+ and CD146 | % Ph+ and CD41a | |
|---|---|---|---|---|---|---|---|---|
| Control (124) | 1874 (201) | 8.2 (3.9) | 8.1 (0.9) | 12.1 (3.1) | 23.7 (4.7)* | 3.6 (2.2) | 10.9 (2.2) | 5.2 (0.9) |
| DM (29) | 3201 (1049)* | 18.1 (8.8)* | 8.3 (5.3) | 13.0 (7.7) | 7.9 (4.5) | 3.9 (5.2) | 9.9 (3.7) | 3.4 (3.1) |
| DFU (181) | 8601 (2527)* | 32.0 (6.1)* | 28.2 (2.3)* | 17.9 (6.9) | 7.1 (4.7) | 27.7 (4.3)* | 17.9 (3.9) | 6.7 (1.6) |
MPs in human subjects as mean (CI).
p < 0.001 versus control, ANOVA.
Ph+MPs, but not Ph-negative MPs, stimulate neutrophils
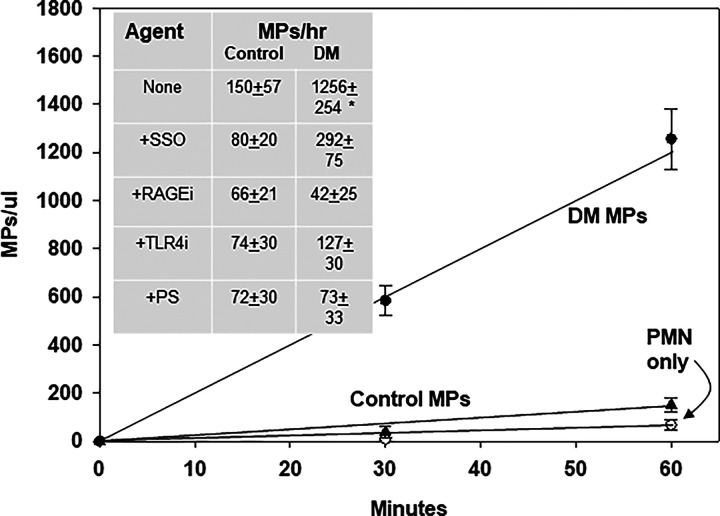
Some MPs will activate murine neutrophils to generate new MPs (11, 12). MPs isolated from patients with DM+DFU, but not control MPs, triggered new MP generation (Fig. 1). Coincubation with small chemical inhibitors of CD36, RAGE, or TLR4 inhibited new MPs generation (Fig. 1, inset). Because MPs express PS on the membrane, we assessed whether PS in suspension could antagonize the neutrophil response. MPs coincubation with 5 µmol PS, a concentration 40-fold lower than required to activate neutrophils via NADPH oxidase, inhibited MPs production, whereas inclusion of 5–30 µmol PC in the suspension had no effect (33).
FIGURE 1.
MPs production by murine neutrophils: cells were incubated with MPs from patients with DM+DFU or control subjects.
Data show MPs produced by 1.8 × 105 murine neutrophils incubated with 10,000 blood-borne MPs from four subjects who each had DM and a DFU (two men, two women, age 57 ± 7.8 [SD] years, DM duration 24 ± 7.8 y, hemoglobin A1C 8.1 ± 0.8%) and four healthy, nondiabetic subjects (2 men, 2 women, age 59 ± 9.4 y). Inset shows effects of inhibitors: 25 µmol SSO, a CD36 inhibitor; 10 µmol RAGE inhibitor (RAGE antagonist peptide; Tocris, Inc.), 10 µmol TAK242 (resatorvid), a TLR4 inhibitor; 5 µmol PS. Data are mean ± SD; n = 4 replicate studies; incubation of cells with inhibitors did not alter viability (data not shown).
Among the MPs sampled from DM+DFU patients in (Fig. 1, a greater percentage, 32.0 ± 3.1% (SD) was Ph+MPs, whereas for controls, only 8.2 ± 4.6% was Ph+MPs (p < 0.001). To test whether Ph+MPs from controls may activate neutrophils, Ph-positive and Ph-negative MPs were separated using magnetic beads (see Materials and Methods). When equal numbers of each type were tested, Ph+MPs activated neutrophils similarly whether from control or DM+DFU subjects, whereas Ph-negative MPs did not activate neutrophils (Table III). To assess possible cell origins of the circulating MPs, we assayed surface proteins expressed on particles that are specific to alternative cell types: CD66b for neutrophils, CD41a for platelets, and CD146 for endothelium. As shown in Tables I and II, MPs expressing CD66b were significantly more numerous in DM+DFU subjects, and virtually all of these MPs coexpressed Ph+ binding.
Table III.
MPs production in 60 min in response to additions of Ph+MPs or Ph-negative MPs from control or diabetic subjects to 1.8 × 105 murine neutrophils
| Addition | Control MPs | Diabetic MPs |
|---|---|---|
| 2500 Ph+MPs | 150 ± 56 | 139 ± 61 |
| 5000 Ph+MPs | 627 ± 57* | 582 ± 75* |
| 10,000 Ph+MPs | 947 ± 100*,† | 1441 ± 86*,† |
| 10,000 Ph-negative MPs | 153 ± 82 | 115 ± 31 |
| 10,000 Ph+MPs+gelsolin | 112 ± 33 | 162 ± 36 |
| 10,000 Ph+MPs+swinholide | 116 ± 17 | 172 ± 58 |
Where shown, samples included 1.25 µg/ml human recombinant gelsolin or 50 nmol swinholide A. Values are MPs produced × 10−3 as mean ± SD; n = 4–9/group.
p < 0.05, ANOVA versus neutrophils only (value was 157 ± 52, which is a carryover from blood during cell isolation).
p < 0.05, ANOVA versus all other groups in the column.
Because neither PS in the suspension nor Ph-negative MPs triggered MPs production, we assess whether surface F-actin was responsible for rendering Ph+MPs capable of activating neutrophils, because it would provide membrane rigidity. Human recombinant gelsolin severs surface F-actin and abrogates Ph+MPs injuries in a murine model (11). When Ph+MPs were incubated with 1.25 µg/ml (∼0.013 µmol) human recombinant gelsolin for 30 min and then added to neutrophil suspensions, no significant MPs production occurred (Table III). Flow cytometry studies showed that gelsolin addition decreased Ph+ binding on MPs by 25 ± 3% (SD, p < 0.05) in the 30 min before addition to neutrophils, but there was no significant MPs lysis (loss of 5.6 ± 3.7% [NS]). Because we could not rule out the possibility that gelsolin, a normal plasma protein, may directly influence neutrophils, we also performed studies with 50 nmol swinholide A, a marine toxin that also severs F-actin, although the process exhibits cooperativity and unlike gelsolin does not cap F-actin severed ends (34). As shown, both agents abrogated the stimulatory effect of Ph+MPs (Table III).
We next examined whether PS headgroups exposed on semirigid 3-µm commercial beads were capable of activating neutrophils. As shown in Table IV, beads displaying PS, but not PC, stimulated MPs generation, which was inhibited by adding free PS, but not PC, to the suspension, as well as inhibitors of RAGE, TLR4, or CD36.
Table IV.
MPs production in 60 min in response to additions of 10,000 semirigid beadsa displaying PS and PC to 1.8 × 105 neutrophils
| Addition | MPs × 10−3 |
|---|---|
| PC beads | 147 ± 28 |
| PS beads | 1318 ± 342* |
| PS beads + 5 µmol PS | 101 ± 55 |
| PS beads + 5 µmol PC | 1544 ± 115* |
| PS beads + 25 µmol SSO | 175 ± 62 |
| PS beads + 10 µmol RAGE inhibitor | 203 ± 42 |
| PS beads + 10 µmol TLR4 inhibitor | 160 ± 50 |
| Human neutrophils + PC beads | 33 ± 21 |
| Human neutrophils + PS beads | 1346 ± 154* |
Where shown, samples included inhibitors as described in (Fig. 1. Human rather than murine neutrophils were used in the last two rows. Data are MPs produced × 10−3 as mean ± SD; n = 4–9/group.
Beads from Echelon Bioscience (Salt Lake City, UT) contained 10 nmol lipid/2 × 107 beads.
p < 0.05, ANOVA versus PMN only.
Finally, we also examined whether MPs would trigger neutrophil activation assessed by flow cytometry. A 30-min incubation of 10,000 Ph-negative MPs with 180,000 murine neutrophils resulted in geometric mean fluorescence of 1.3 ± 0.4 (SD, n = 4) arbitrary units (AUs) for CD18 (a component of β2 integrins) and 1.3 ± 0.6 AUs for MPO. Incubation with 10,000 Ph+MPs resulted in CD18 expression of 3.9 ± 1.5 AUs (p < 0.05) and MPO expression of 5.4 ± 1.2 AUs (p < 0.05). Incubation with 10,000 PC-coated beads resulted in CD18 expression of 1.1 ± 1.0 AUs, and MPO expression was 1.4 ± 1.3 AUs, whereas incubation with PS-coated beads increased CD18 expression to 4.4 ± 2.5 AUs (p < 0.05) and MPO expression to 4.1 ± 2.2 AUs (p < 0.05).
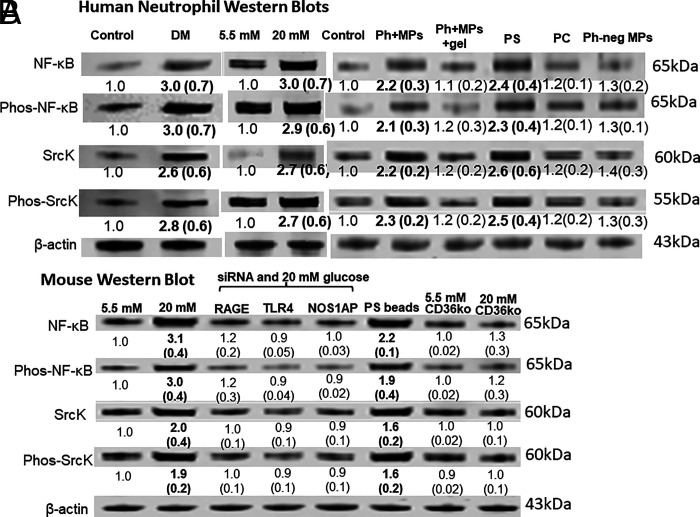
Neutrophils from diabetic subjects or exposed to Ph+MPs exhibit elevations of regulatory proteins
Neutrophils from DM+DFU subjects versus those from healthy control subjects exhibited elevations of the NF-κB p65 subunit, the activated serine 536 phosphorylated form of NF-κB p65, SrcK, and the activated tyrosine 416 phosphorylated form of SrcK (Fig. 2A). The same pattern of protein elevations occurred when control human neutrophils were incubated ex vivo with 20 mM glucose (a concentration that can be seen when DM is out of control), when incubated with human Ph+MPs, but not Ph-negative MPs, and with PS-expressing semirigid beads, but not PC-expressing beads. Because protein elevations did not occur if 10,000 Ph+MPs were incubated along with gelsolin, results are consistent with hyperglycemia activation due to Ph+MPs.
FIGURE 2.
Western blots of human and murine neutrophils.
Images are representative blots, and numbers beneath images are mean band densities and SD (n = 4 for human cell, 6 for murine), where for each study band densities were normalized to actin loading and then to the control value on individual blots. Bold numbers reflect those significantly different from control, p < 0.05, ANOVA. Human cells were obtained from control subjects or those with DM+DFU (labeled DM, left columns). Murine cells were obtained from wild type or CD36 KO mice. For ex vivo incubations, 1.8 × 105 human (A) or murine (B) neutrophils were incubated for 1 h with 5.5 or 20 mM glucose or, where indicated, 5.5 mM glucose with 10,000 Ph+MPs or Ph-negative MPs from patients with DM+DFU without or with recombinant human gelsolin (Ph+MPs+gel), or with 10,000 PS- or PC-expressing semirigid beads. Murine neutrophils were incubated for 20 h before study with siRNA, either a control that depletes no protein or sequences to specifically deplete RAGE, TLR4, or NOS1AP.
Membrane receptors are necessary for hyperglycemic neutrophil activation
The role of membrane receptors with neutrophil activation under hyperglycemic conditions was assessed using murine cells so that specific proteins could be depleted with siRNA. These studies could not be done with human neutrophils, because human cells do not survive the 20-h-long siRNA incubations. As shown in (Fig. 2B, murine neutrophil incubations with 20 mM glucose resulted in comparable protein elevations, as were seen with human neutrophils, and elevations did not occur in cells depleted of RAGE, TLR4, or NOS1AP. The role of CD36 was demonstrated using neutrophils from CD36 KO mice. MPs production by neutrophils following conditions as described in (Fig. 2 after incubation with a control siRNA (no protein depletion) and with 5.5 mM glucose was 139 ± 32 and 1693 ± 179 MPs/h (p < 0.05) with 20 mM glucose. With neutrophils depleted of RAGE, TLR4, or NOS1AP, MPs production was not significantly different from control siRNA incubated cells in the presence of 5.5 mM glucose (108 ± 31/h). When cells were incubated with 20 mM glucose, MPs production was, respectively, 121 ± 20, 112 ± 24, and 128 ± 234 MPs/h, values insignificantly different from after 5.5 mM glucose incubation. Neutrophils from CD36 KO mice were similarly unchanged with 5.5 versus 20 mM glucose incubations (81 ± 18 versus 112 ± 24/h). We conclude that hyperglycemia stimulates neutrophils as a result of production of Ph+MPs, and autoactivation occurs related to membrane receptor interactions with the MPs.
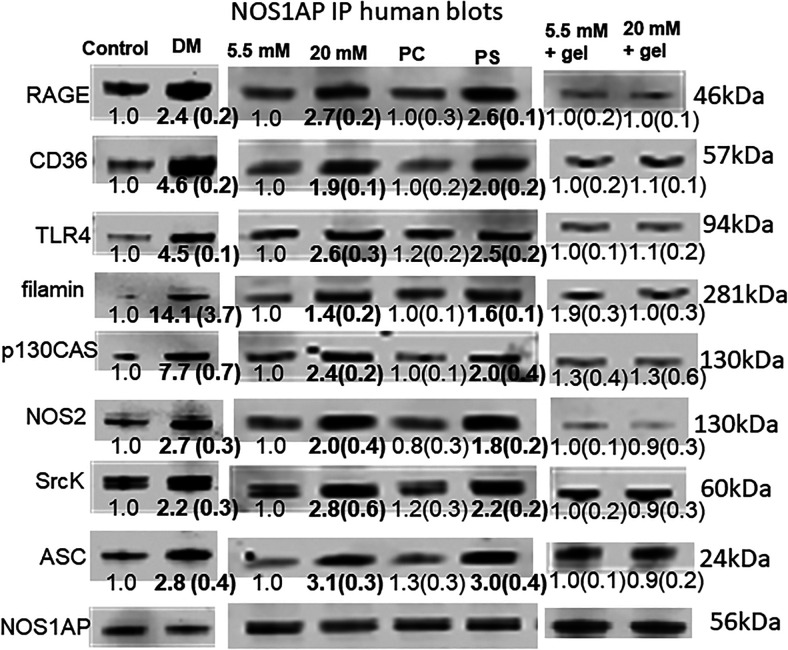
NOS1AP coprecipitates with membrane receptors and intracellular regulatory proteins
As discussed in the Introduction, we had interest in examining the role of NOS1AP in neutrophil responses. (Fig. 3 shows that immunoprecipitation of NOS1AP coprecipitated TLR4, RAGE and CD36, NOS2, SrcK, the NLRP3 inflammasome protein ASC (adaptor molecule apoptosis-associated speck-like protein containing a CARD), and the cytoskeletal components p130CAS and filamin. As shown, from 2- to 14-fold more proteins were coprecipitated using neutrophils from DM+DFU versus cells from healthy control subjects. The same pattern of responses occurred when control human neutrophils were incubated with PS-expressing, but not PC-expressing, semirigid beads and under hyperglycemic conditions, and coincubation with gelsolin abrogated protein changes seen with 20 mM glucose. Murine neutrophils exhibited a similarly enhanced coprecipitation pattern when incubated with 20 versus 5.5 mM glucose, and elevations did not occur when cells were depleted of RAGE or TLR4 and in cells from CD36 KO mice (Table V). These results indicate that NOS1AP serves as a scaffold linking the three membrane receptors, RAGE, CD36, and TLR4, as well as intracellular proteins NOS2, SrcK, ASC, the cytoskeletal proteins p130CAS, and filamin, and linkages are increased in response to stimulation by hyperglycemia and PS beads. Gelsolin inhibition supports Ph+MPs as the proximate cause for hyperglycemia-induced changes.
FIGURE 3.
Immunoprecipitation of human neutrophils using anti-NOS1AP Abs.
Images are representative blots, and numbers beneath images are mean band densities and SD normalized to NOS1AP loading and to the control value on individual blots (n = 4); bold numbers are significantly different from control, p < 0.05, ANOVA. The first two columns indicate cells obtained from control subjects or those with DM+DFU (labeled DM); the next two columns are control cells incubated for 2 h with 5.5 or 20 mM glucose, 10,000 PC- or PS-expressing beads; and the last two columns show blots from cells incubated with 5.5 or 20 mM glucose plus human recombinant gelsolin.
Table V.
Immunoprecipitation of murine neutrophils using anti-NOS1AP Abs
| RAGE | NOS2 | SrcK | ASC | TLR4 | CD36 | 130CAS | Filamin | |
|---|---|---|---|---|---|---|---|---|
| 20 mM | 2.6 ± 0.2 | 1.7 ± 0.2 | 2.8 ± 0.4 | 1.7 ± 0.3 | 2.1 ± 0.4 | 2.8 ± 0.5 | 2.2 ± 0.6 | 2.6 ± 0.4 |
| Small inhibitory RAGE 20 mM | 0.3 ± 0.2 | 0.9 ± 0.2 | 1.2 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.2 ± 0.3 |
| Small inhibitory TLR4 20 mM | 0.7 ± 0.2 | 0.8 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.3 | 0.3 ± 0.2 | 1.0 ± 0.4 | 0.8 ± 0.3 | 0.7 ± 0.1 |
| CD36KO 20 mM | 0.9 ± 0.2 | 0.8 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.4 | 0.1 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.1 |
Data are the fold change in mean band densities ± SD (n = 4 for all groups) for cells incubated in 20 mM glucose for 2 h normalized to NOS1AP loading and to the value of the protein band density of cells incubated at 5.5 mM glucose. Where indicated, cells had first been incubated overnight with siRNA to deplete RAGE or TLR4 or cells from CD36 KO mice. Bold numbers are significantly different from control p < 0.05 ANOVA.
SrcK S-nitrosylation
SrcKs were required for enhanced neutrophil MPs production by hyperglycemia. When murine neutrophils were depleted of SrcK using siRNA, MPs production was not increased with hyperglycemia (with 5.5 mM glucose, 106 ± 30 versus 108 ± 32/h [NS] with 20 mM glucose). The catalytic activity of SrcK can be enhanced by S-nitrosylation, and NOS1AP facilitates S-nitrosylation of intracellular proteins (35–37). Therefore, we examined S-nitrosylation of SrcK using murine neutrophils by the biotin-switch assay. SrcK S-nitrosylation was 3.0 ± 0.9-fold (SD, n = 4, p < 0.05 ANOVA) higher when cells were incubated for 2 h at 20 versus 5.5 mM glucose and 2.5 ± 0.7-fold (p < 0.05) higher when incubated with PS-coated semirigid beads (PC-coated beads had no significant effect). Incubation with Ph+MPs following conditions as described in (Fig. 3 increased SrcK S-nitrosylation by 2.5 ± 0.7 (n = 4, p < 0.05), whereas Ph-negative MPs had no significant effect. Depletion of NOS1AP using siRNA also abrogated enhanced S-nitrosylation seen in response to 20 mM glucose, Ph+MPs, and PS beads.
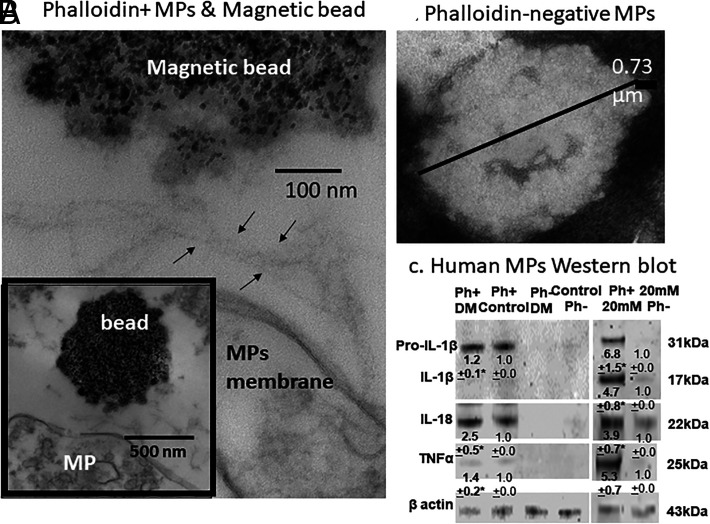
Characterization of Ph+MPs
Ph+MPs were isolated with Ph-coated magnetic beads and imaged in an electron microscope (Fig. 4). Filaments were always seen adjacent to the MPs and absent from Ph-negative MPs sedimented by ultracentrifugation (shown in a negatively stained image). We also probed Ph+MPs and Ph-negative MPs by Western blotting. As shown in (Fig. 4C, TNF-α, the proform of IL-1β and IL-18, was prominent in Ph+MPs from controls or diabetic patients, and these proteins, as well as the mature form of IL-1β, are present in Ph+MPs generated by neutrophils incubated with 20 mM glucose ex vivo, whereas scant levels were found in Ph-negative particles. Interestingly, we found that MPs expression of CD66b, a protein commonly used to indicate MPs of neutrophil origin, was present on just 4.9 ± 1.1% (SD, n = 15) of MPs generated ex vivo by human neutrophils incubated for 2 h in 5.5 mM glucose and on 5.4 ± 1.1% (NS) of MPs generated by neutrophils incubated in 20 mM glucose.
FIGURE 4.
Analyses of MPs.
(A) Representative images of a Ph+MP generated by human neutrophils (lower and higher magnification) incubated with 20 mM glucose extracted from a cell suspension using Ph-coated magnetic beads. The filamentous strands shown in (A) between arrows were always found between beads and MPs. (B) Negatively stained image of human MPs that remains in a suspension after Ph+MPs are removed using magnetic beads. MPs were pelleted by ultracentrifugation. (C) Representative Western blot of human MPs (140,000 MPs/lane) probed for the pro- and mature forms of IL-1β, IL-18, TNF-α, and actin. Numbers beneath images are mean ± SD (n = 4 samples; *p < 0.03) band densities versus control. The first two lanes depict results for Ph+MPs obtained from plasma of a patient with DM+DFU and a control healthy subject, the next two lanes Ph-negative MPs from the same individuals, and the last two lanes Ph-positive and Ph-negative MPs obtained from a suspension of human neutrophils incubated with 20 mM glucose for 2 h.
Discussion
We conclude that Ph+MPs are a normal blood component but present at an insufficient concentration in healthy subjects to trigger neutrophil activation. Ph+MPs are 2.2-fold higher in patients with DM and 4.0-fold higher in those who also have a foot ulcer. Ph+MPs interact with a receptor complex on the neutrophil membrane that includes RAGE, CD36, and TLR4, all of which associate with NOS1AP that serves as a scaffolding protein. This “outside-in” signaling triggers linkage of other proteins to NOS1AP that are known to play a role in cell activation, including SrcKs and NOS2, leading to progressive neutrophil activation and further MPs production (12, 38).
Neutrophil receptors bind to PS on the MPs membrane, but the proinflammatory stimulus requires a semirigid structure provided by surface F-actin. This conclusion is supported by elimination of the Ph+MPs responses either with PS in the suspension or with incubation with F-actin severing agents such as gelsolin. Because neutrophil activation responses were similar with Ph+MPs and PS-coated semirigid beads, we conclude that the cytokine cargo within Ph+MPs is not required. There is precedence for cell signaling by extracellular vesicles binding to target cell receptors, although most often activation occurs after discharge of luminal cargoes into the cytosol (7, 39).
Neutrophils from DM + DFU patients and those from controls incubated under hyperglycemic conditions, incubated with Ph+MPs and with PS-coated semirigid beads, all exhibited higher expression of NF-κB and SrcK. Abrogation of these responses with either RAGE, CD36, or TLR4 depletion or chemical inhibitors suggests that generation of Ph+MPs by neutrophils in hyperglycemic conditions sets off an autocatalytic activation process. Although the proinflammatory effects of hyperglycemia have been known for decades, Ph+MPs are an unrecognized element (4). Immunoprecipitation studies indicate that all three membrane receptors are linked to NOS1AP. Coprecipitation of SrcK and NOS2, along with enhanced SrcK S-nitrosylation, implicates NOS1AP as playing a key role in Ph+MPs stimulation, and thus hyperglycemia mediates inflammation.
The proinflammatory role of NOS1AP may explain why gene variants are associated with peripheral neuropathy, impaired healing, and lower-extremity amputations in those with DM (20, 21). Hemoglobin A1C values were not significantly different between the DM and DM+DFU groups in our study. However, hyperglycemia can trigger development of the protein complex surrounding NOS1AP and MPs production by neutrophils over minutes to hours. The MPs production rate increases progressively with short-term increases in glucose concentration from 5.5 to 20 mM (12). This may explain why an index of glycemic control over months is not reflected in differences found with Ph+MPs number and particle characteristics between subjects with DM versus DM+DFU. All subjects were being treated in an outpatient setting, and none exhibited evidence of systemic infections or sepsis. Approximately one third of all MPs in the DM+DFU group express CD66b, considered to be a neutrophil-specific protein, and virtually all of these MPs bound Ph, whereas in controls and those with DM, but no ulcer, only 8% of MPs expressed CD66b and half (4%) bound Ph (40).
Protein linkages to NOS1AP offer insight into the mechanism for neutrophil activation by Ph+MPs. RAGE and CD36 are receptors for PS, and there is precedence for coordination of their expression and function, along with TLR4 (7, 26–29). All three proteins coprecipitate with NOS1AP. The proximity of NOS2 to SrcK linked to NOS1AP triggers S-nitrosylation that will cause kinase activation that can be perpetuated by autophosphorylation (41). Ligand engagement by CD36 is known to trigger a 5- to 6-fold increase in adhesion as a result of receptor clustering because of SrcK-mediated phosphorylation of p130CAS and actin recruitment (42). Thus, linkages of these proteins to NOS1AP provide an attractive explanation for why leukocytes of patients with DM exhibit elevations of these membrane receptors and, through multiple pathways, these receptors contribute to the development and complications of DM (30–32).
We found that plasma gelsolin inhibits neutrophil activation initiated by Ph+MPs and hyperglycemia. This observation may have clinical pertinence because plasma gelsolin levels are reported to decrease by nearly 50% in patients with type 2 DM and in mice rendered diabetic by streptozocin treatment (43). These findings are consistent with observed elevations of Ph+MPs in those with DM (Tables I, II). We have previously reported that when Ph+MPs are increased in humans and in mice, there is a reciprocal decrease in plasma gelsolin due to gelsolin binding to the MPs expressing F-actin (11). Repletion of gelsolin improves glucose tolerance in the mouse streptozocin model, which could be caused by Ph+MPs lysis and a variety of other anti-inflammatory actions (43, 44). Note that in this study, gelsolin incubations of just 1.25 µg/ml were used to remove F-actin without causing marked MPs lysis. The normal level of plasma gelsolin is ∼170 µg/ml, a concentration that not only removes F-actin but also causes extensive Ph+MPs lysis (11).
The inflammatory role of NOS1AP provides mechanistic insight into our recent study of 207 DM patients with DFUs (5). Although NOS1AP expression in leukocytes was variable, the ratio of NOS1AP/actin in leukocytes was inversely associated with the number of CEPCs characterized as CD34+CD45dim with a linear regression coefficient of −0.38 (95% confidence interval −0.74, −0.02; p = 0.034) (5). DFUs are more likely to heal if patients have more CEPCs (5, 45). Because excessive inflammation has adverse effects on stem/progenitor cells, the role of NOS1AP in inflammation may explain why natural variations in the NOS1AP gene, as well as variations in CEPC numbers, bear relationships to healing success of DFUs and to lower-extremity amputations (20).
Our observations raise new questions about MPs production mechanisms. There is a linkage of cytoskeletal components involved with MPs production and the formation of the NLRP3 inflammasome when neutrophils respond to hyperglycemia (12). Therefore, finding that IL-1β content of Ph+MPs differed markedly from the Ph-negative particles was surprising. We also do not yet understand the mechanism for extracellular F-actin polymerization. More generally, characterization of MPs remains imprecise, and F-actin is yet another facet. Surface protein expression is only an approximation for the cells producing MPs. Particles appear to interact in vivo because they can express multiple cell-specific proteins so that when totaling all blood-borne subgroups, values often exceed 100% (11, 46). In this regard, we were surprised to find that a minority of MPs generated by neutrophils ex vivo express CD66b. Therefore, using membrane expression of this protein to identify neutrophil-derived MPs in vivo, as is a common practice, may underestimate neutrophil-derived MPs involvement in pathophysiological events.
Supplementary Material
Acknowledgments
The authors are grateful for the voluntary participation of the study subjects. We also are indebted to the late Dr. Thomas P. Stossel for postulating a possible connection between MPs and gelsolin because of the involvement of F-actin.
Footnotes
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes for Health (R01-DK116199 to multiple principal investigators D.J.M. and S.R.T.); National Institute of Neurological Disorders and Stroke, National Institutes for Health (R01-NS122855 to S.R.T.); and the U.S. Office of Naval Research (N00014-20-1-2641 to S.R.T.).
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
S.R.T. conceived ideas, oversaw the research program, performed laboratory studies, analyzed data, and wrote the manuscript. V.M.B., A.K.A., D.R., and A.R.B. performed laboratory studies, reviewed the manuscript, and provided advice. N.M. and O.H. analyzed data, reviewed the manuscript, and provided advice. D.S.M., Z.K.M., J.C.L., H.A.L.-T., and R.S.K. aided with study subject recruitment, collected blood, reviewed the manuscript, and provided advice. R.-C.H. aided with sample preparation and electron microscopic imaging, reviewed the manuscript, and provided advice. S.L.L. and M.J.D. provided human recombinant gelsolin, reviewed the manuscript, and provided advice. D.J.M. conceived ideas, oversaw portions of the work, analyzed data, reviewed the manuscript, and provided advice. S.R.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The online version of this article contains supplemental material.
- ASC
- adaptor molecule apoptosis-associated speck-like protein containing a CARD
- AU
- arbitrary unit
- cat#
- catalog number
- CEPC
- circulating endothelial progenitor cell
- DFU
- diabetes mellitus and a foot ulcer
- DFUC
- Diabetic Foot Ulcer Consortium
- DM
- diabetes mellitus
- KO
- knockout
- MP
- microparticle
- MPO
- myeloperoxidase
- NLRP3
- nucleotide-binding domain leucine-rich repeat-like receptor
- NOS
- NO synthase
- NOS1AP
- NO synthase 1 adaptor protein
- PC
- phosphatidylcholine
- Ph
- phalloidin
- Ph+
- phalloidin positive
- PS
- phosphatidylserine
- RAGE
- receptor for advanced glycation end products
- siRNA
- small inhibitory RNA
- SrcK
- Src kinase
- SSO
- sulfo-N-succinyloleate
Disclosures
S.L.L. and M.J.D. are chief executive officer and chief medical officer, respectively, of BioAegis Therapeutics, which is developing human recombinant gelsolin for clinical use. The other authors have no financial conflicts of interest.
References
- 1. Zhang W., Li C., Xu Y., He B., Hu M., Cao G., Li L., Wu S., Wang X., Zhang C., et al. . 2021. Hyperglycemia and correlated high levels of inflammation have a positive relationship with the severity of coronavirus disease 2019. Mediators Inflamm. 2021: 8812304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paolisso P., Foà A., Bergamaschi L., Donati F., Fabrizio M., Chiti C., Angeli F., Toniolo S., Stefanizzi A., Armillotta M., et al. . 2021. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc. Diabetol. 20: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Esposito K., Nappo F., Marfella R., Giugliano G., Giugliano F., Ciotola M., Quagliaro L., Ceriello A., Giugliano D.. 2002. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106: 2067–2072. [DOI] [PubMed] [Google Scholar]
- 4. Ramasamy R., Yan S. F., Schmidt A. M.. 2012. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul. Pharmacol. 57: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margolis D. J., Mitra N., Hoffstad O., Malay D. S., Mirza Z. K., Lantis J. C., Lev-Tov H. A., Kirsner R. S., Ruhela D., Bhopale V. M., Thom S. R.. 2022. Circulating endothelial precursor cells are associated with a healed diabetic foot ulcer evaluated in a prospective cohort study. Wound Repair Regen. DOI: 10.1093/cid/ciab454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li S., Wei J., Zhang C., Li X., Meng W., Mo X., Zhang Q., Liu Q., Ren K., Du R., et al. . 2016. Cell-derived microparticles in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Cell. Physiol. Biochem. 39: 2439–2450. [DOI] [PubMed] [Google Scholar]
- 7. Meldolesi J. 2018. Exosomes and ectosomes in intercellular communication. Curr. Biol. 28: R435–R444. [DOI] [PubMed] [Google Scholar]
- 8. Slater T. W., Finkielsztein A., Mascarenhas L. A., Mehl L. C., Butin-Israeli V., Sumagin R.. 2017. Neutrophil microparticles deliver active myeloperoxidase to injured mucosa to inhibit epithelial wound healing. J. Immunol. 198: 2886–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogata N., Nomura S., Shouzu A., Imaizumi M., Arichi M., Matsumura M.. 2006. Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 73: 241–248. [DOI] [PubMed] [Google Scholar]
- 10. Butin-Israeli V., Houser M. C., Feng M., Thorp E. B., Nusrat A., Parkos C. A., Sumagin R.. 2016. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 30: 4007–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhopale V. M., Ruhela D., Brett K. D., Nugent N. Z., Fraser N. K., Levinson S. L., DiNubile M. J., Thom S. R.. 2021. Plasma gelsolin modulates the production and fate of IL-1β-containing microparticles following high-pressure exposure and decompression. J Appl Physiol (1985) 130: 1604–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thom S. R., Bhopale V. M., Yu K., Huang W., Kane M. A., Margolis D. J.. 2017. Neutrophil microparticle production and inflammasome activation by hyperglycemia due to cytoskeletal instability. J. Biol. Chem. 292: 18312–18324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu L., Han L., Xie C., Li W., Lin L., Pan S., Zhou Y., Li Z., Jin M., Zhang A.. 2017. Identification of extracellular actin as a ligand for triggering receptor expressed on myeloid cells-1 signaling. Front. Immunol. 8: 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dudani A. K., Ganz P. R.. 1996. Endothelial cell surface actin serves as a binding site for plasminogen, tissue plasminogen activator and lipoprotein(a). Br. J. Haematol. 95: 168–178. [DOI] [PubMed] [Google Scholar]
- 15. Miles L. A., Andronicos N. M., Baik N., Parmer R. J.. 2006. Cell-surface actin binds plasminogen and modulates neurotransmitter release from catecholaminergic cells. J. Neurosci. 26: 13017–13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pardridge W. M., Nowlin D. M., Choi T. B., Yang J., Calaycay J., Shively J. E.. 1989. Brain capillary 46,000 dalton protein is cytoplasmic actin and is localized to endothelial plasma membrane. J. Cereb. Blood Flow Metab. 9: 675–680. [DOI] [PubMed] [Google Scholar]
- 17. Por S. B., Cooley M. A., Breit S. N., Penny R., French P. W.. 1991. Antibodies to tubulin and actin bind to the surface of a human monocytic cell line, U937. J. Histochem. Cytochem. 39: 981–985. [DOI] [PubMed] [Google Scholar]
- 18. Smalheiser N. R. 1996. Proteins in unexpected locations. Mol. Biol. Cell 7: 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothmeier A. S., Marchese P., Petrich B. G., Furlan-Freguia C., Ginsberg M. H., Ruggeri Z. M., Ruf W.. 2015. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J. Clin. Invest. 125: 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Margolis D. J., Gupta J., Thom S. R., Townsend R. R., Kanetsky P. A., Hoffstad O., Papdopoulos M., Fischer M., Schelling J. R., Mitra N.. 2013. Diabetes, lower extremity amputation, loss of protective sensation, and neuronal nitric oxide synthase associated protein in the chronic renal insufficiency cohort study. Wound Repair Regen. 21: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Margolis D. J., Hampton M., Hoffstad O., Mala D. S., Mirza Z., Woltereck D., Shannon S., Troiano M. A., Mitra N., Yang M., et al. . 2017. NOS1AP genetic variation is associated with impaired healing of diabetic foot ulcers and diminished response to healing of circulating stem/progenitor cells. Wound Repair Regen. 25: 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Becker M. L., Aarnoudse A. J., Newton-Cheh C., Hofman A., Witteman J. C., Uitterlinden A. G., Visser L. E., Stricker B. H.. 2008. Common variation in the NOS1AP gene is associated with reduced glucose-lowering effect and with increased mortality in users of sulfonylurea. Pharmacogenet. Genomics 18: 591–597. [DOI] [PubMed] [Google Scholar]
- 23. Chu A. Y., Coresh J., Arking D. E., Pankow J. S., Tomaselli G. F., Chakravarti A., Post W. S., Spooner P. H., Boerwinkle E., Kao W. H.. 2010. NOS1AP variant associated with incidence of type 2 diabetes in calcium channel blocker users in the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 53: 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin W., Zhang R., Hu C., Wang C. R., Lu J. Y., Yu W. H., Bao Y. Q., Xiang K. S., Jia W. P.; International Type 2 Diabetes 1q Consortium . 2010. A variation in NOS1AP gene is associated with repaglinide efficacy on insulin resistance in type 2 diabetes of Chinese. Acta Pharmacol. Sin. 31: 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang T., Wang Y., Lv D. M., Song J. F., Lu Q., Gao X., Zhang F., Guo H., Li W., Yin X. X.. 2014. Effects of NOS1AP rs12742393 polymorphism on repaglinide response in Chinese patients with type 2 diabetes mellitus. Pharmacotherapy 34: 131–139. [DOI] [PubMed] [Google Scholar]
- 26. Sparvero L. J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A. A., Zeh H. J., Lotze M. T.. 2009. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 7: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xanthis A., Hatzitolios A., Fidani S., Befani C., Giannakoulas G., Koliakos G.. 2009. Receptor of advanced glycation end products (RAGE) positively regulates CD36 expression and reactive oxygen species production in human monocytes in diabetes. Angiology 60: 772–779. [DOI] [PubMed] [Google Scholar]
- 28. Ryeom S. W., Silverstein R. L., Scotto A., Sparrow J. R.. 1996. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J. Biol. Chem. 271: 20536–20539. [DOI] [PubMed] [Google Scholar]
- 29. Płóciennikowska A., Hromada-Judycka A., Borzęcka K., Kwiatkowska K.. 2015. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72: 557–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dasu M. R., Devaraj S., Zhao L., Hwang D. H., Jialal I.. 2008. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta S., Maratha A., Siednienko J., Natarajan A., Gajanayake T., Hoashi S., Miggin S.. 2017. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. [Published erratum appears in 2018 Sci. Rep. 8: 5768.] Sci. Rep. 7: 7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puchałowicz K., Rać M. E.. 2020. The multifunctionality of CD36 in diabetes mellitus and its complications: update in pathogenesis, treatment and monitoring. Cells 9: 1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamura M., Tamura T., Tyagi S. R., Lambeth J. D.. 1988. The superoxide-generating respiratory burst oxidase of human neutrophil plasma membrane. Phosphatidylserine as an effector of the activated enzyme. J. Biol. Chem. 263: 17621–17626. [PubMed] [Google Scholar]
- 34. Allingham J. S., Zampella A., D’Auria M. V., Rayment I.. 2005. Structures of microfilament destabilizing toxins bound to actin provide insight into toxin design and activity. Proc. Natl. Acad. Sci. USA 102: 14527–14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaffrey S. R., Snowman A. M., Eliasson M. J., Cohen N. A., Snyder S. H.. 1998. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 20: 115–124. [DOI] [PubMed] [Google Scholar]
- 36. Jaffrey S. R., Benfenati F., Snowman A. M., Czernik A. J., Snyder S. H.. 2002. Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. Proc. Natl. Acad. Sci. USA 99: 3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernandez K., Swiatkowski P., Patel M. V., Liang C., Dudzinski N. R., Brzustowicz L. M., Firestein B. L.. 2016. Overexpression of isoforms of nitric oxide synthase 1 adaptor protein, encoded by a risk gene for schizophrenia, alters actin dynamics and synaptic function. Front. Cell. Neurosci. 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rohwedder I., Kurz A. R. M., Pruenster M., Immler R., Pick R., Eggersmann T., Klapproth S., Johnson J. L., Alsina S. M., Lowell C. A., et al. . 2020. Src family kinase-mediated vesicle trafficking is critical for neutrophil basement membrane penetration. Haematologica 105: 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mulcahy L. A., Pink R. C., Carter D. R. F.. 2014. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3: 24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Skubitz K. M., Ducker T. P., Goueli S. A.. 1992. CD66 monoclonal antibodies recognize a phosphotyrosine-containing protein bearing a carcinoembryonic antigen cross-reacting antigen on the surface of human neutrophils. J. Immunol. 148: 852–860. [PubMed] [Google Scholar]
- 41. Andre F. R., dos Santos P. F., Rando D. G.. 2016. Theoretical studies of the role of C-terminal cysteines in the process of S-nitrosylation of human Src kinases. J. Mol. Model. 22: 23. [DOI] [PubMed] [Google Scholar]
- 42. Davis S. P., Amrein M., Gillrie M. R., Lee K., Muruve D. A., Ho M.. 2012. Plasmodium falciparum-induced CD36 clustering rapidly strengthens cytoadherence via p130CAS-mediated actin cytoskeletal rearrangement. FASEB J. 26: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khatri N., Sagar A., Peddada N., Choudhary V., Chopra B. S., Garg V., Garg R., Ashish. 2014. Plasma gelsolin levels decrease in diabetic state and increase upon treatment with F-actin depolymerizing versions of gelsolin. J. Diabetes Res. 2014: 152075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piktel E., Levental I., Durnaś B., Janmey P. A., Bucki R.. 2018. Plasma gelsolin: indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 19: 2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thom S. R., Hampton M., Troiano M. A., Mirza Z., Malay D. S., Shannon S., Jennato N. B., Donohue C. M., Hoffstad O., Woltereck D., et al. . 2016. Measurements of CD34+/CD45-dim stem cells predict healing of diabetic neuropathic wounds. Diabetes 65: 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thom S. R., Yang M., Bhopale V. M., Huang S., Milovanova T. N.. 2011. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol (1985) 110: 340–351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials from this project are available on request.