Abstract
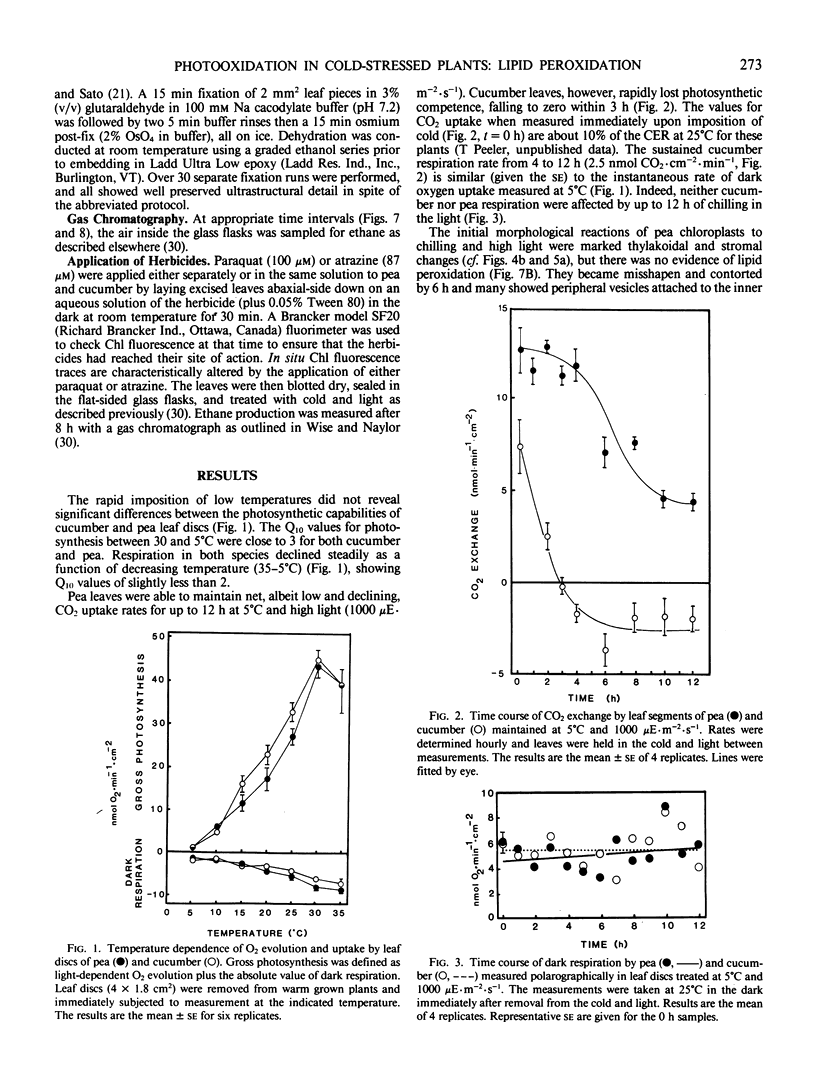
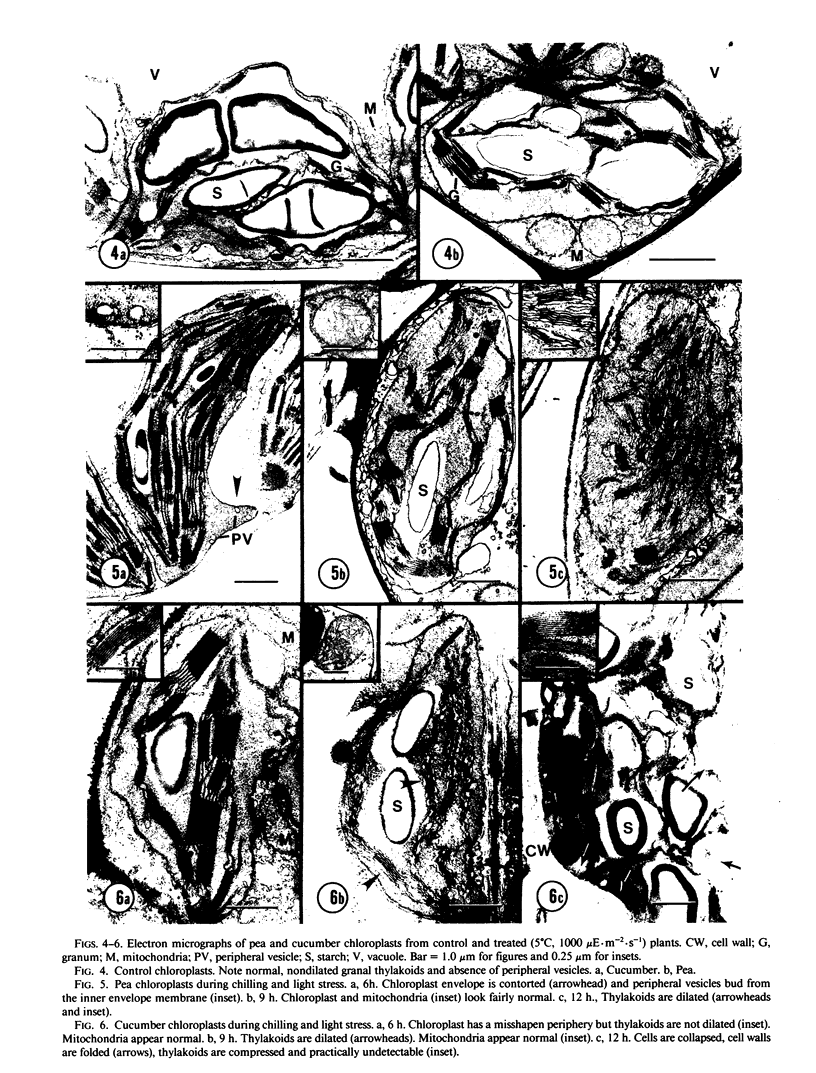
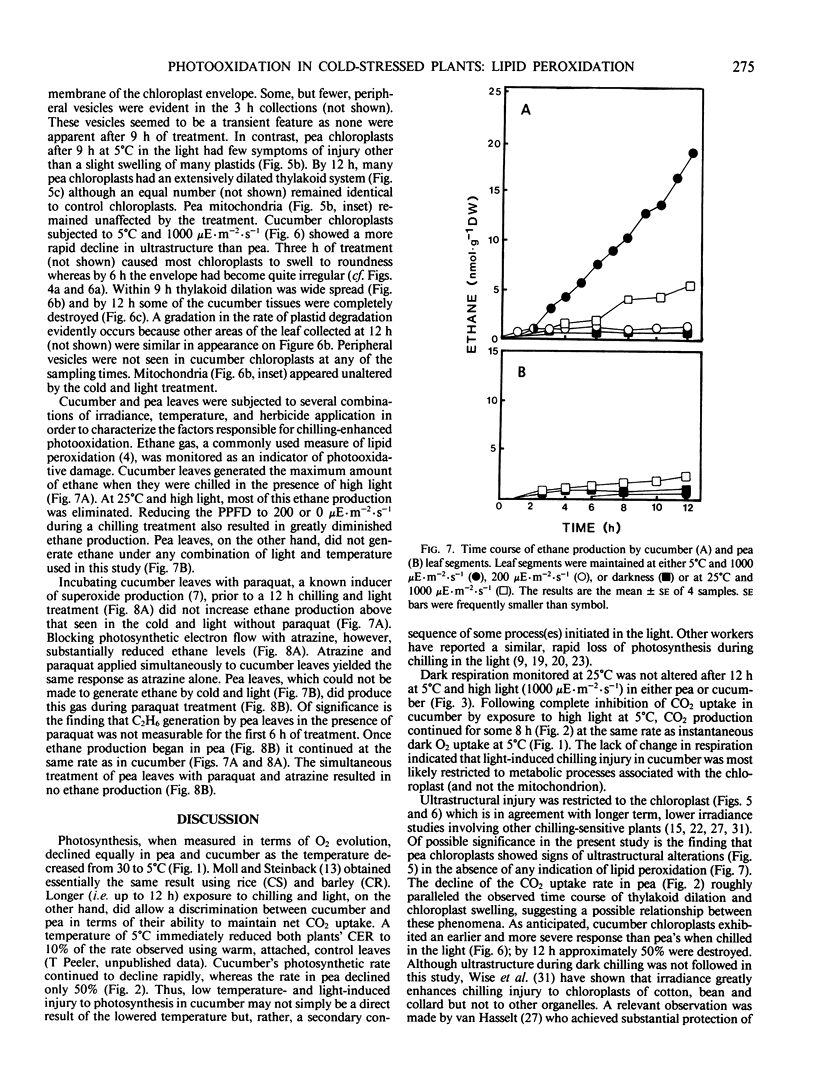
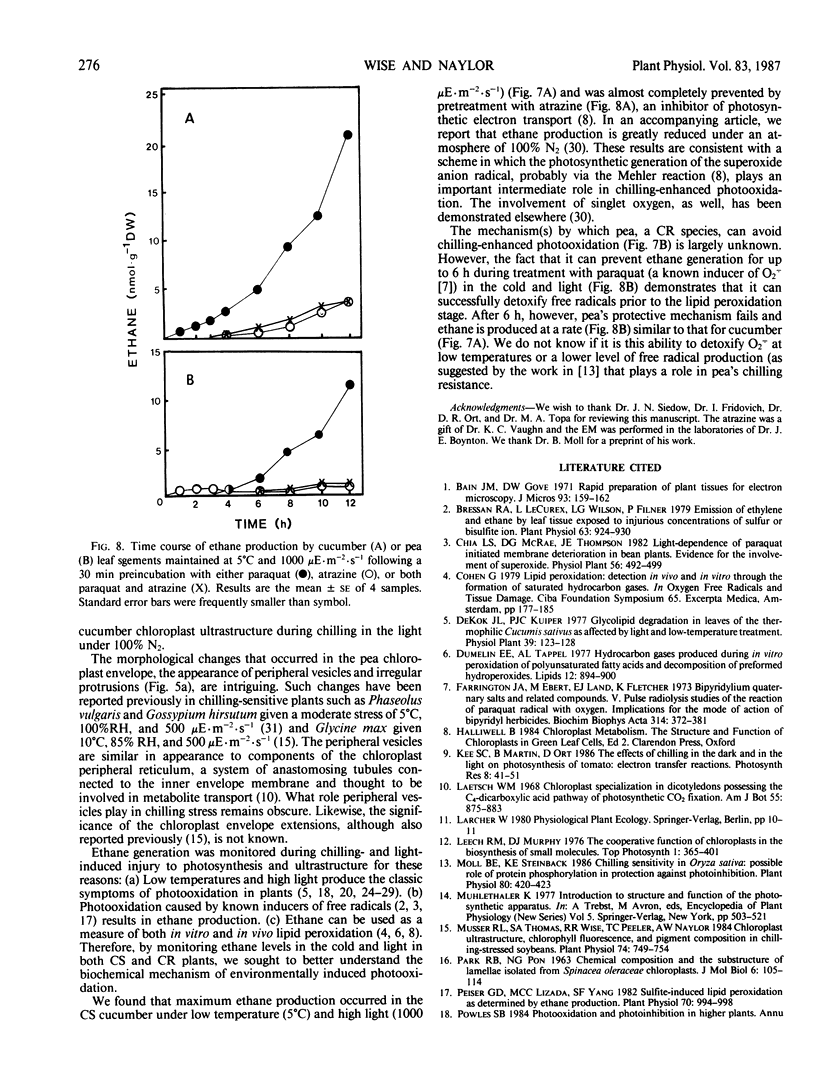
Chilling-induced photooxidation was studied in detached leaves of chilling-sensitive (CS) cucumber (Cucumis sativus L.) and chilling resistant (CR) pea (Pisum sativum L.). The rates of photosynthesis and respiration, measured as O2 exchange, were found to be comparable in the two species over a temperature range of 5 to 35°C. Chilling at 5°C for 12 hours in high light (1000 microeinsteins per square meter per second) decreased CO2 uptake 75% in detached pea leaves whereas CO2 uptake by cucumber was reduced to zero within 2 hours. Respiration was unaffected in either species by the chilling and light treatment. Although ultrastructural alterations were apparent in chloroplasts of both species, cucumber's were affected sooner and more severely. The mechanism of photooxidative lipid peroxidation was investigated by following the production of ethane gas under a variety of conditions. Maximum ethane production occurred in the CS cucumber at low temperature (5°C) and high light (1000 microeinsteins per square meter per second). Atrazine, an inhibitor of photosynthetic electron transport, almost completely halted this chilling- and light-induced ethane production. These data, taken with those reported in an accompanying article (RR Wise, AW Naylor 1986 Plant Physiol 83: 278-282) suggest that the superoxide anion radical is generated in cucumber chloroplasts (probably via a Mehler-type reaction) during chilling-enhanced photooxidation. Parallel experiments were conducted on pea, a CR species. Detached pea leaves could only be made to generate ethane in the cold and light if they were pretreated with the herbicide parquat, a known effector of O2− production. Even so, pea showed no lipid peroxidation for 6 hours, at which time ethane production began and was at a rate equal to that for the chilled and irradiated cucumber leaves. The results indicate that pea has an endogenous mechanism(s) for the removal of toxic oxygen species prior to lipid peroxidation. This mechanism breaks down in pea after 6 hours in the cold, light, and the presence of paraquat.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bressan R. A., Lecureux L., Wilson L. G., Filner P. Emission of ethylene and ethane by leaf tissue exposed to injurious concentrations of sulfur dioxide or bisulfite ion. Plant Physiol. 1979 May;63(5):924–930. doi: 10.1104/pp.63.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumelin E. E., Tappel A. L. Hydrocarbon gases produced during in vitro peroxidation of polyunsaturated fatty acids and decomposition of preformed hydroperoxides. Lipids. 1977 Nov;12(11):894–900. doi: 10.1007/BF02533308. [DOI] [PubMed] [Google Scholar]
- Farrington J. A., Ebert M., Land E. J., Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973 Sep 26;314(3):372–381. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- Moll B. A., Steinback K. E. Chilling Sensitivity in Oryza sativa: The Role of Protein Phosphorylation in Protection against Photoinhibition. Plant Physiol. 1986 Feb;80(2):420–423. doi: 10.1104/pp.80.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser R. L., Thomas S. A., Wise R. R., Peeler T. C., Naylor A. W. Chloroplast ultrastructure, chlorophyll fluorescence, and pigment composition in chilling-stressed soybeans. Plant Physiol. 1984 Apr;74(4):749–754. doi: 10.1104/pp.74.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK R. B., PON N. G. Chemical composition and the substructure of lamellae isolated from Spinacea oleracea chloroplasts. J Mol Biol. 1963 Feb;6:105–114. doi: 10.1016/s0022-2836(63)80126-6. [DOI] [PubMed] [Google Scholar]
- Peiser G. D., Lizada M. C., Yang S. F. Sulfite-induced lipid peroxidation in chloroplasts as determined by ethane production. Plant Physiol. 1982 Oct;70(4):994–998. doi: 10.1104/pp.70.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Craig A. S. Plants under Climatic Stress: II. Low Temperature, High Light Effects on Chloroplast Ultrastructure. Plant Physiol. 1971 May;47(5):719–725. doi: 10.1104/pp.47.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Rowley J. A. Plants under Climatic Stress: I. Low Temperature, High Light Effects on Photosynthesis. Plant Physiol. 1971 May;47(5):713–718. doi: 10.1104/pp.47.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. R., Naylor A. W. Calibration and use of a Clark-type oxygen electrode from 5 to 45 degrees C. Anal Biochem. 1985 Apr;146(1):260–264. doi: 10.1016/0003-2697(85)90424-5. [DOI] [PubMed] [Google Scholar]
- Wise R. R., Naylor A. W. Chilling-enhanced photooxidation : evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol. 1987 Feb;83(2):278–282. doi: 10.1104/pp.83.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]





