Abstract
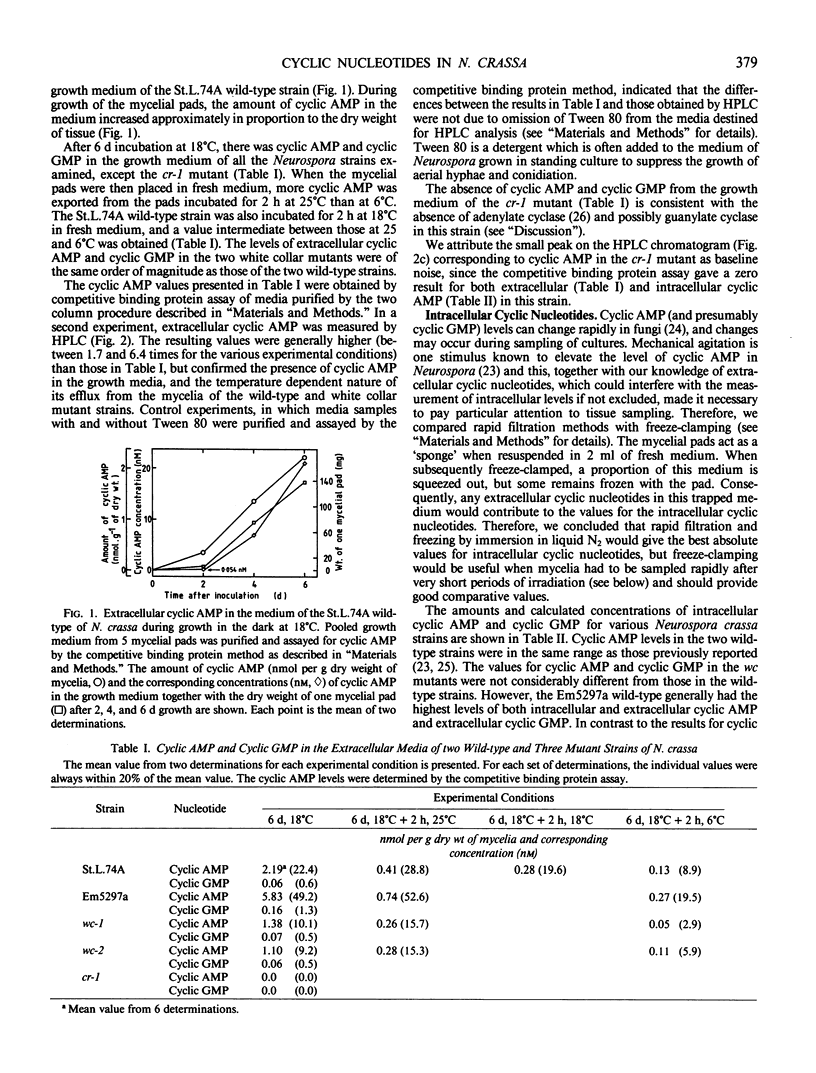
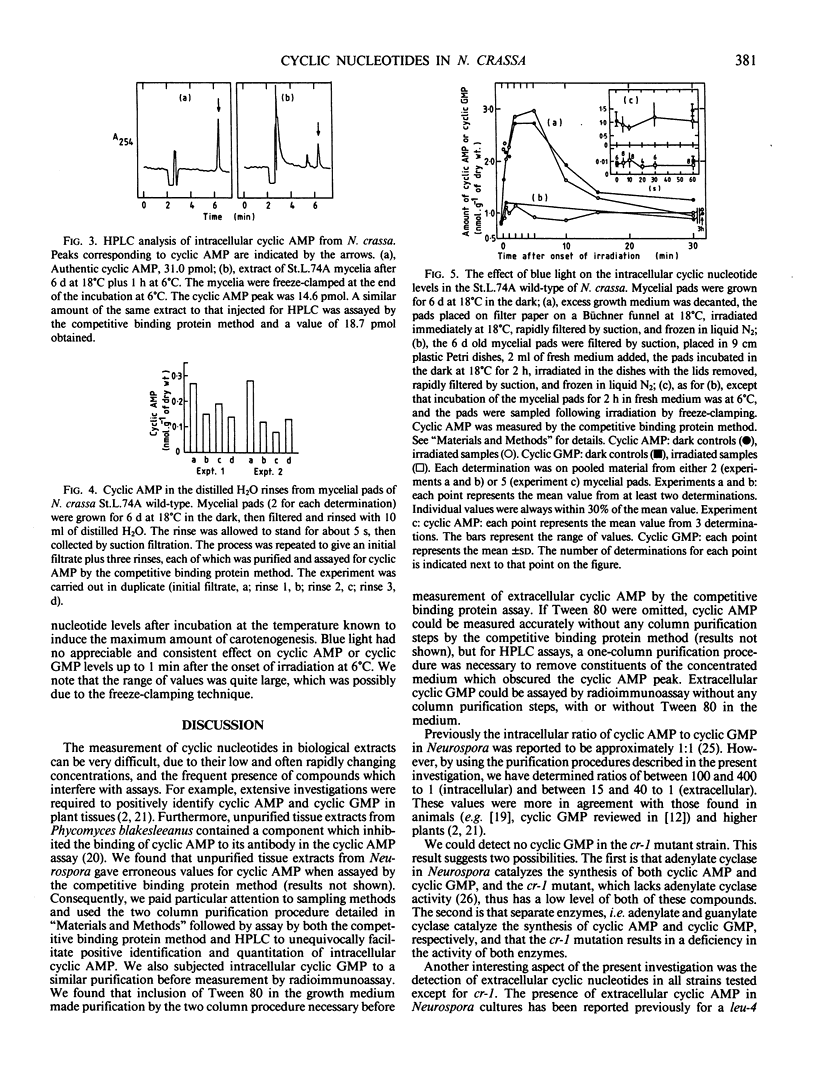
Cyclic AMP and cyclic GMP were released into the growth medium of mycelia of Neurospora crassa wild-type strains St.L.74A and Em5297a and by white collar-1 and white collar-2 mutant strains. After growth for 6 days at 18°C, there were 2.19 (St.L.74A), 5.83 (Em5297a), 1.38 (white collar-1), and 1.10 (white collar-2) nanomoles of cyclic AMP per gram dry weight of mycelia in the growth medium. These values corresponded to concentrations of cyclic AMP of between approximately 10 and 50 nanomolar. The corresponding values for extracellular cyclic GMP were typically less than 6% of the values for cyclic AMP. Following transfer to fresh medium, cyclic AMP efflux was demonstrated for each of the strains, and the amount of cyclic AMP exported into the fresh medium was greater at 25°C than 6°C. Intracellular cyclic AMP and cyclic GMP were also measured in each of the strains. The values for cyclic AMP were in the same range as those in the literature (approximately 0.5 to 1.5 nanomoles per gram dry weight of mycelia). However, the corresponding intracellular cyclic GMP values were less than 1% of the cyclic AMP values, i.e. more than 50 times lower than the value previously reported for the St.L.74A wild-type. Transfer of mycelia after 6 days at 18°C to fresh media and incubation for 2 hours at 25°C or 6°C did not consistently affect the intracellular level of cyclic AMP or cyclic GMP in the strains examined. We could detect no change in intracellular cyclic AMP when mycelia of the St.L.74A wild-type strain were irradiated with blue light for periods of up to 3.0 hours at 18°C, or in cyclic AMP and cyclic GMP for irradiation times of up to 1 minute at 6°C. We propose that the plasma membrane of Neurospora crassa is permeable to cyclic nucleotides, and the export of cyclic nucleotides into the growth medium may be a means of regulating intracellular levels. We conclude that three factors that affect carotenogenesis in Neurospora crassa (blue light, temperature, and the white collar mutations) have no appreciable effect on the total measurable intracellular cyclic nucleotides in this organism. There was no extracellular or intracellular cyclic AMP or cyclic GMP in the crisp-1 mutant strain, which suggested either that adenylate cyclase (which is absent in crisp-1) catalyzes the synthesis of both cyclic AMP and cyclic GMP or that the crisp-1 mutation somehow results in a deficiency of two enzymes (adenylate and guanylate cyclase).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S. C., Brown P. R., Rosie D. M. Extraction procedures for use prior to HPLC nucleotide analysis using microparticle chemically bonded packings. J Chromatogr Sci. 1977 Jun;15(6):218–221. doi: 10.1093/chromsci/15.6.218. [DOI] [PubMed] [Google Scholar]
- Cohen R. J., Atkinson M. M. Activation of Phycomyces adenosine 3', 5'-monophosphate phosphodiesterase by blue light. Biochem Biophys Res Commun. 1978 Jul 28;83(2):616–621. doi: 10.1016/0006-291x(78)91034-3. [DOI] [PubMed] [Google Scholar]
- Cohen R. J. Cyclic AMP levels in Phycomyces during a response to light. Nature. 1974 Sep 13;251(5471):144–146. doi: 10.1038/251144a0. [DOI] [PubMed] [Google Scholar]
- DeCarlo R. R., Somberg E. W. The effect of essential amino acid deprivation on macromolecular synthesis and nucleotide pool sizes in Neurospora crassa. Arch Biochem Biophys. 1974 Nov;165(1):201–212. doi: 10.1016/0003-9861(74)90156-8. [DOI] [PubMed] [Google Scholar]
- Degli-Innocenti F., Russo V. E. Isolation of new white collar mutants of Neurospora crassa and studies on their behavior in the blue light-induced formation of protoperithecia. J Bacteriol. 1984 Aug;159(2):757–761. doi: 10.1128/jb.159.2.757-761.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Melles S. Genetic Analysis of Phototropism of Neurospora crassa Perithecial Beaks Using White Collar and Albino Mutants. Plant Physiol. 1983 Aug;72(4):996–1000. doi: 10.1104/pp.72.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. W. The Effect of Temperature on Photo-induced Carotenoid Biosynthesis in Neurospora crassa. Plant Physiol. 1974 Aug;54(2):142–147. doi: 10.1104/pp.54.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. W., Turner R. V. Photoregulation of the Carotenoid Biosynthetic Pathway in Albino and White Collar Mutants of Neurospora crassa. Plant Physiol. 1981 Sep;68(3):745–749. doi: 10.1104/pp.68.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasunuma K. Repressible extracellular phosphodiesterases showing cyclic 2',3'- and cyclic 3',5'-nucleotide phosphodiesterase activities in Neurospora crassa. J Bacteriol. 1983 Oct;156(1):291–300. doi: 10.1128/jb.156.1.291-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Ham E. A., Zanetti M. E., Sanford C. H., Nicol S. E., Goldberg N. D. Estrogen-related increases in uterine guanosine 3':5'-cyclic momophosphate levels. Proc Natl Acad Sci U S A. 1974 May;71(5):1866–1870. doi: 10.1073/pnas.71.5.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwiler L. S., Brandt M. Absence of significant light-induced changes in cAMP levels in sporangiophores of Phycomyces blakesleeanus. J Bacteriol. 1983 Jan;153(1):555–557. doi: 10.1128/jb.153.1.555-557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Cyclic AMP and the plasma membrane potential in Neurospora crassa. J Biol Chem. 1977 Oct 25;252(20):7146–7150. [PubMed] [Google Scholar]
- Rosenberg G. B., Pall M. L. Reconstitution of adenylate cyclase in Neurospora from two components of the enzyme. Arch Biochem Biophys. 1983 Feb 15;221(1):254–260. doi: 10.1016/0003-9861(83)90142-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg G., Pall M. L. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1140–1144. doi: 10.1128/jb.137.3.1140-1144.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYMAN C. W., TATUM E. L. POTASSIUM TRANSPORT IN NEUROSPORA. I. INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS, AND CATION REQUIREMENTS FOR GROWTH. Biochim Biophys Acta. 1964 Nov 29;88:578–592. [PubMed] [Google Scholar]
- Terenzi H. F., Flawia M. M., Tellez-Inon M. T., Torres H. N. Control of Neurospora crassa morphology by cyclic adenosine 3', 5'-monophosphate and dibutyryl cyclic adenosine 3', 5'-monophosphate. J Bacteriol. 1976 Apr;126(1):91–99. doi: 10.1128/jb.126.1.91-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


