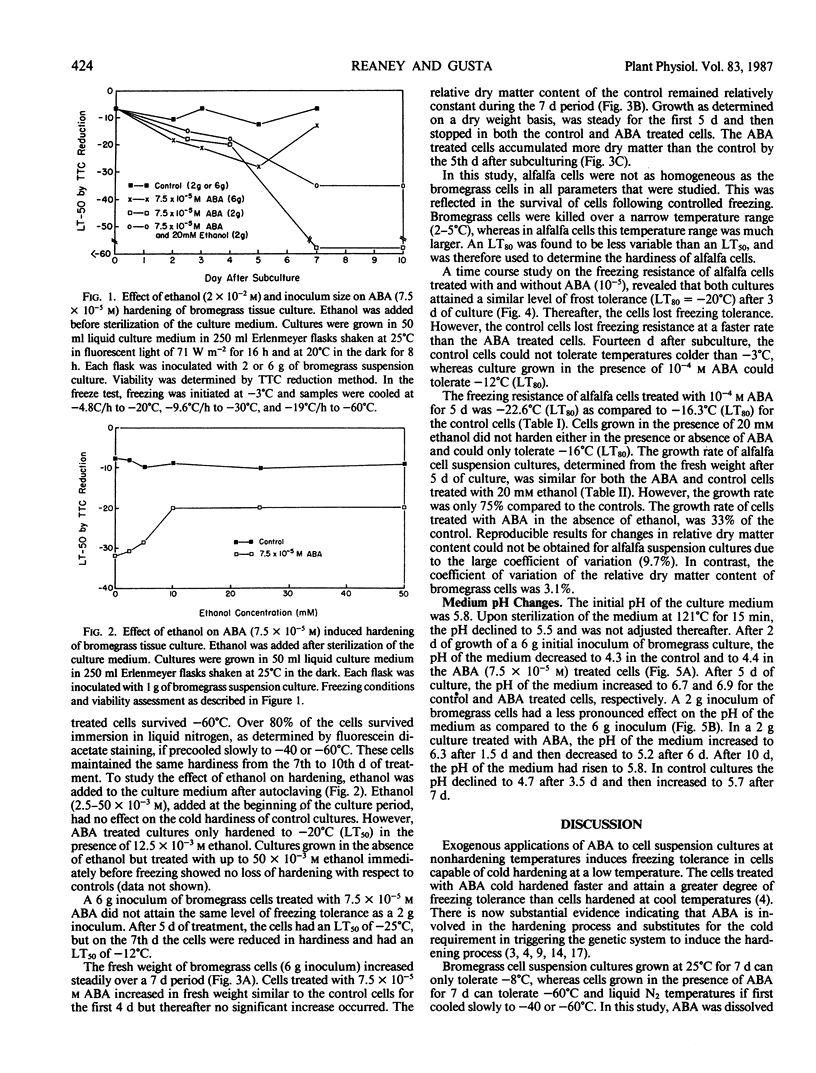
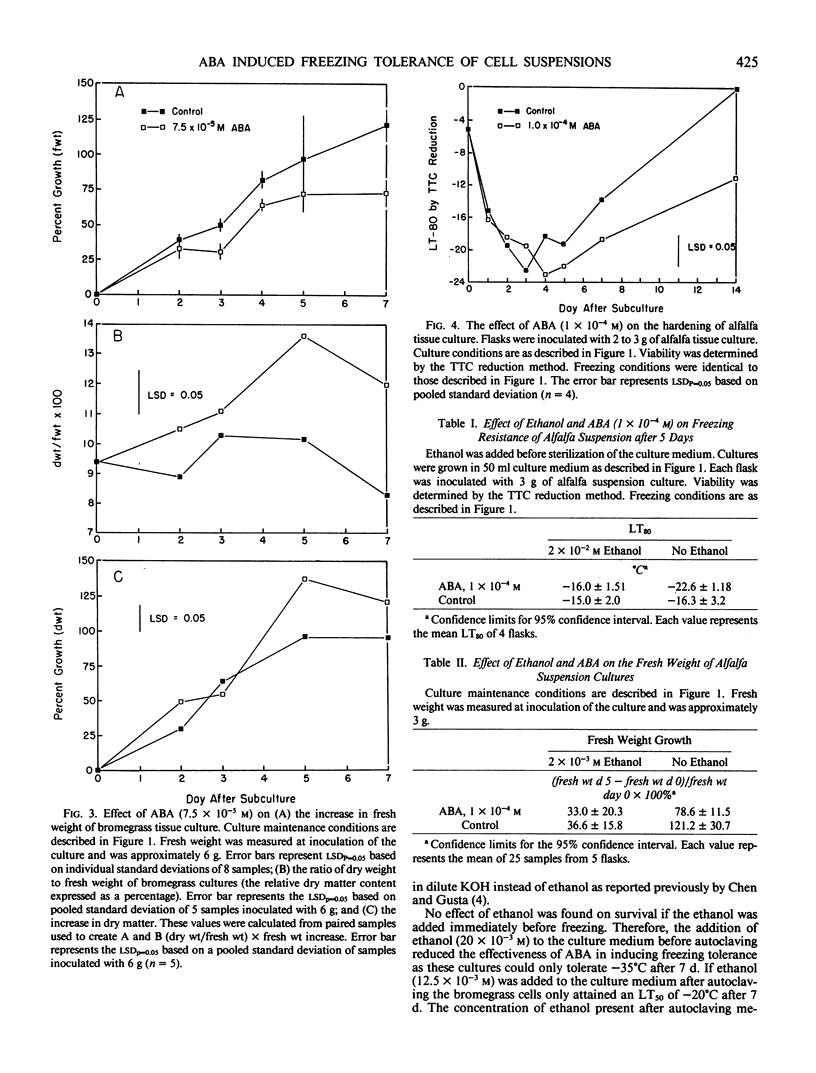
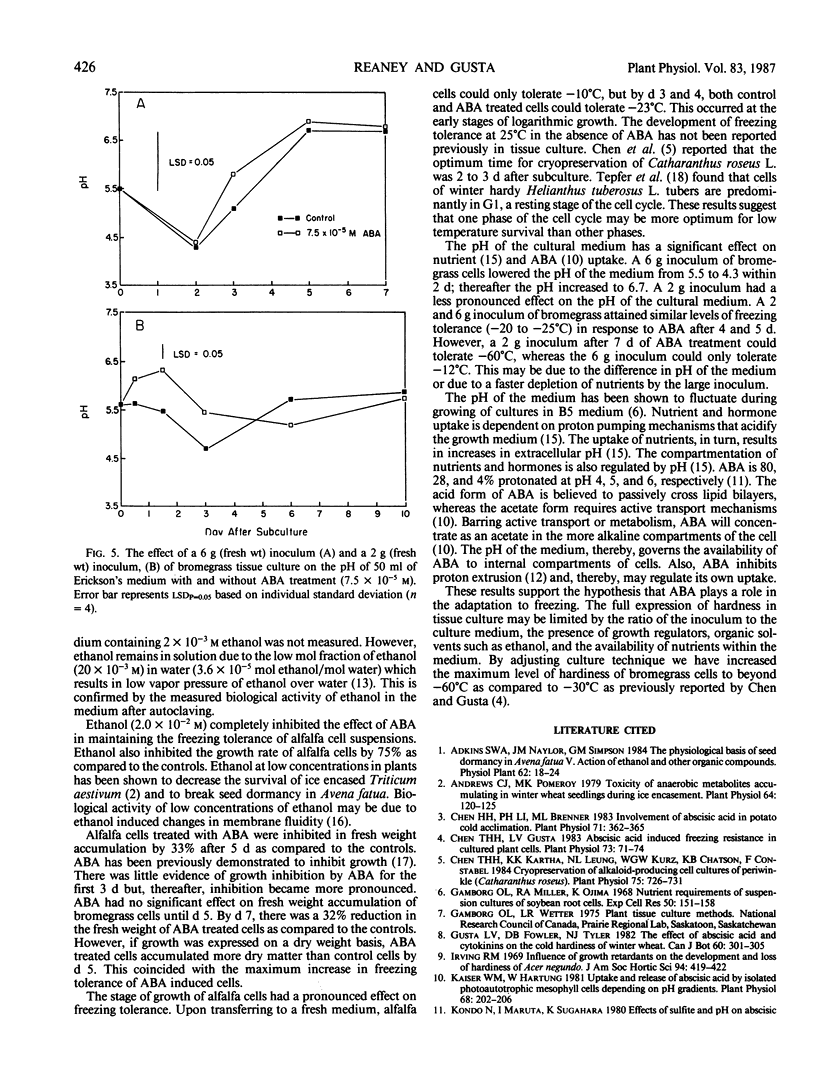
Abstract
A 2-gram fresh weight inoculum of bromegrass (Bromus inermis Leyss. culture BG970) cell suspension culture treated with 7.5 × 10−5 molar abscisic acid (ABA) for 7 days at 25°C survived slow cooling to −60°C. Over 80% of the cells in ABA treated cultures survived immersion in liquid N2 after slow cooling to −40 or −60°C. In contrast, a 6-gram fresh weight inoculum only attained a hardiness level of −28°C after 5 days of ABA treatment. Ethanol (2 × 10−2 molar) added to the culture medium at the time of ABA addition, inhibited the freezing tolerance of bromegrass cells by 25°C. A 6-gram inoculum of both control and ABA treated bromegrass cells altered the pH of the medium more than a 2-gram inoculum. ABA inhibited the increase in fresh weight of bromegrass by 20% after 4 days. Both control and ABA (10−4 molar) treated alfalfa cells (Medicago sativa L.) grown at 25°C hardened from an initial LT50 of −5°C to an LT50 of −23°C by the third to fifth day after subculture. Thereafter, the cells dehardened but the ABA treated cells did not deharden to the same level as the control cells. ABA inhibited the increase in fresh weight of alfalfa by 50% after 5 days.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Pomeroy M. K. Toxicity of Anaerobic Metabolites Accumulating in Winter Wheat Seedlings during Ice Encasement. Plant Physiol. 1979 Jul;64(1):120–125. doi: 10.1104/pp.64.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. H., Li P. H., Brenner M. L. Involvement of abscisic Acid in potato cold acclimation. Plant Physiol. 1983 Feb;71(2):362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Gusta L. V. Abscisic Acid-induced freezing resistance in cultured plant cells. Plant Physiol. 1983 Sep;73(1):71–75. doi: 10.1104/pp.73.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Kartha K. K., Leung N. L., Kurz W. G., Chatson K. B., Constabel F. Cryopreservation of Alkaloid-Producing Cell Cultures of Periwinkle (Catharanthus roseus). Plant Physiol. 1984 Jul;75(3):726–731. doi: 10.1104/pp.75.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Kaiser W. M., Hartung W. Uptake and Release of Abscisic Acid by Isolated Photoautotrophic Mesophyll Cells, Depending on pH Gradients. Plant Physiol. 1981 Jul;68(1):202–206. doi: 10.1104/pp.68.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]


