Abstract
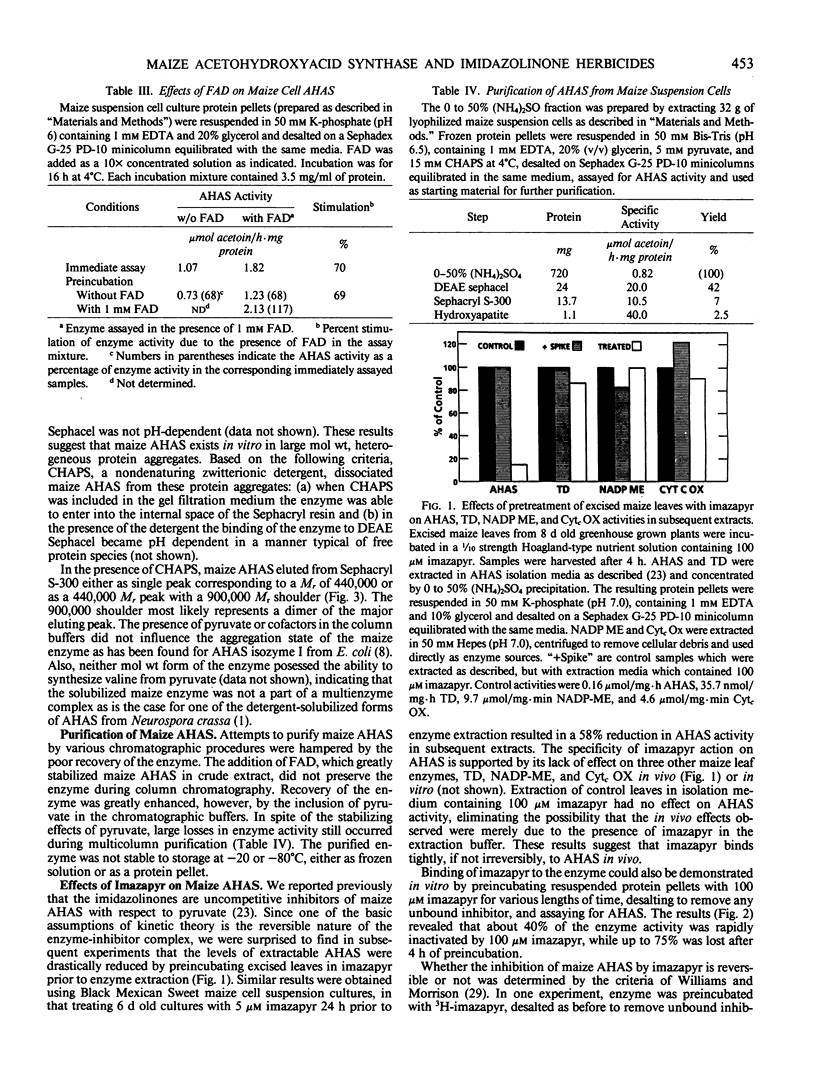
Acetohydroxyacid synthase has been purified from maize (Zea mays, var Black Mexican Sweet) suspension culture cells 49-fold by a combination of ion exchange chromatography, gel filtration, and hydroxyapatite chromatography. Use of the nondenaturing, zwitterionic detergent 3-([3-cholamidopropyl]dimethyl-ammonio)-1-propanesulfonate was necessary to dissociate the enzyme from the heterogeneous, high molecular weight aggregates in which it appears to reside in vitro. The solubilized maize acetohydroxyacid synthase had a relative molecular mass of 440,000. The purified enzyme was highly unstable. Acetohydroxyacid synthase activities in crude extracts of excised maize leaves and suspension cultured cells were reduced 85 and 58%, respectively, by incubation of the tissue with 100 micromolar (excised leaves) and 5 micromolar (suspension cultures) of the imidazolinone imazapyr prior to enzyme extraction, suggesting that the inhibitor binds tightly to the enzyme in vivo. Binding of imazapyr to maize acetohydroxyacid synthase could also be demonstrated in vitro. Evidence is presented which suggests that the interaction between imazapyr and the enzyme is reversible. Imazapyr also exhibited slow-binding properties when incubated with maize cell acetohydroxyacid synthase in extended time course experiments. Initial and final Ki values for the inhibition were 15 and 0.9 micromolar, respectively. The results suggest that imazapyr is a slow, tight-binding inhibitor of acetohydroxyacid synthase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergquist A., Eakin E. A., Murali D. K., Wagner R. P. A pyruvate-valine enzyme complex that is dependent upon the metabolic state of the mitochondria. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4352–4355. doi: 10.1073/pnas.71.11.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E. Acetolactate and Acetoin Synthesis in Ripening Peas. Plant Physiol. 1964 Jan;39(1):53–59. doi: 10.1104/pp.39.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S. Genetic analysis of mutants of Saccharomyces cerevisiae resistant to the herbicide sulfometuron methyl. Genetics. 1985 Jan;109(1):21–35. doi: 10.1093/genetics/109.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatzer L., Eakin E., Wagner R. P. Acetohydroxy acid synthetase with a pH optimum of 7.5 from Neurospora crassa mitochondria: characterization and partial purification. J Bacteriol. 1972 Oct;112(1):453–464. doi: 10.1128/jb.112.1.453-464.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F. R., McFadden B. A. Enzyme Profiles in Seedling Development and the Effect of Itaconate, an Isocitrate Lyase-directed Reagent. Plant Physiol. 1979 Aug;64(2):228–231. doi: 10.1104/pp.64.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRossa R. A., Schloss J. V. The sulfonylurea herbicide sulfometuron methyl is an extremely potent and selective inhibitor of acetolactate synthase in Salmonella typhimurium. J Biol Chem. 1984 Jul 25;259(14):8753–8757. [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter E. H., LaBrie D. A., Bergquist A., Wagner R. P. In vitro mitochondrial complementation in Neurospora crassa. Biochem Genet. 1971 Dec;5(6):549–561. doi: 10.1007/BF00485673. [DOI] [PubMed] [Google Scholar]
- Miflin B. J. Cooperative feedback control of barley acetohydroxyacid synthetase by leucine, isoleucine, and valine. Arch Biochem Biophys. 1971 Oct;146(2):542–550. doi: 10.1016/0003-9861(71)90159-7. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Cleland W. W. Inhibition of isocitrate lyase by 3-nitropropionate, a reaction-intermediate analogue. Biochemistry. 1982 Aug 31;21(18):4420–4427. doi: 10.1021/bi00261a035. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Shaner D. L., Anderson P. C., Stidham M. A. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984 Oct;76(2):545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. J., Mazumder R. Purification, properties, and feedback control of L-threonine dehydratase from spinach. J Biol Chem. 1970 Jun 10;245(11):3008–3014. [PubMed] [Google Scholar]
- Simonds W. F., Koski G., Streaty R. A., Hjelmeland L. M., Klee W. A. Solubilization of active opiate receptors. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4623–4627. doi: 10.1073/pnas.77.8.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Kuwana H. A basal unit of valine-sensitive acetolactate synthase of Neurospora crassa. Biochem Biophys Res Commun. 1984 Sep 17;123(2):418–423. doi: 10.1016/0006-291x(84)90246-8. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]
- Winter K., Foster J. G., Edwards G. E., Holtum J. A. Intracellular Localization of Enzymes of Carbon Metabolism in Mesembryanthemum crystallinum Exhibiting C(3) Photosynthetic Characteristics or Performing Crassulacean Acid Metabolism. Plant Physiol. 1982 Feb;69(2):300–307. doi: 10.1104/pp.69.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]


