Abstract
Purpose of Review
This article provides an overview of the diagnosis and management of primary and secondary headaches that may occur during pregnancy and postpartum. Headache presenting in pregnancy is of significant concern to the affected woman. Quick and correct diagnosis leads to the optimal management, minimizing risks to the pregnancy.
Recent Findings
Several strategies have been developed to distinguish secondary headaches that need urgent assessment and management from benign primary and secondary headaches and to minimize the risk of misdiagnosis. Recent guidelines for the drug treatment of headaches are considered in the context of updated information on the safety of drugs in pregnancy and lactation.
Summary
Primary headaches are common and typically improve during pregnancy. Management during pregnancy and lactation is similar to management in the nonpregnant state, with a few exceptions. Secondary causes of headache that are more likely to occur during pregnancy include cerebral venous thrombosis, posterior reversible encephalopathy syndrome resulting from eclampsia, post–dural puncture headache, stroke, and pituitary apoplexy.
INTRODUCTION
Headaches are common during the reproductive years and are just as likely to occur in pregnancy as in nonpregnant women. Most headaches will be benign with no impact on the pregnancy. However, the potential effects of drugs on the fetus and the increased risk of certain secondary headaches during pregnancy call for careful assessment and management of the pregnant woman presenting with headaches.
Approximately half of the pregnancies in the United States are unplanned; of these, over 40% continue to birth. Many women take medication during pregnancy, with each woman taking an average of four to five different medications.1 Drugs and other teratogens exert their greatest effects on the fetus during the second and third months of gestation, so health care providers need to advise women on safe and effective treatment of headaches during pregnancy and lactation.
CLASSIFICATION OF HEADACHES
The International Classification of Headache Disorders categorizes primary and secondary headaches.2 Primary headaches account for the majority of headaches during pregnancy. Of the primary headaches, tension-type headache and migraine generally improve during pregnancy. The effect of pregnancy on cluster headache is limited because of the rarity of the condition, and the data are conflicting. A common cause of daily headache in a patient with a history of primary headache is medication overuse headache. Secondary causes of headache that are more likely to occur during pregnancy include cerebral venous thrombosis, posterior reversible encephalopathy syndrome (PRES) resulting from eclampsia, post–dural puncture headache, stroke, and pituitary apoplexy (Table 7-1). In addition, headaches can be symptomatic of emotional stress.3
Table 7-1.
International Classification of Headache Disorders, 3rd Edition (Beta Version)a

ASSESSING THE PREGNANT WOMAN WITH HEADACHE
The history should focus on establishing the likelihood that the headache has some secondary cause or defining it among the primary headache disorders. Specific questions can help to evaluate secondary causes of headache that may need urgent assessment (Table 7-2). Because secondary headaches can occur in a patient with a long-standing history of primary headache, it is important to elicit new symptoms. This approach can separate those who need further investigation from those with benign secondary headaches or typical histories of primary headaches. The latter can be reassured, treated, and followed by the neurologist or primary care physician as appropriate.
Table 7-2.
Warning Signs and Symptoms for Secondary Headaches
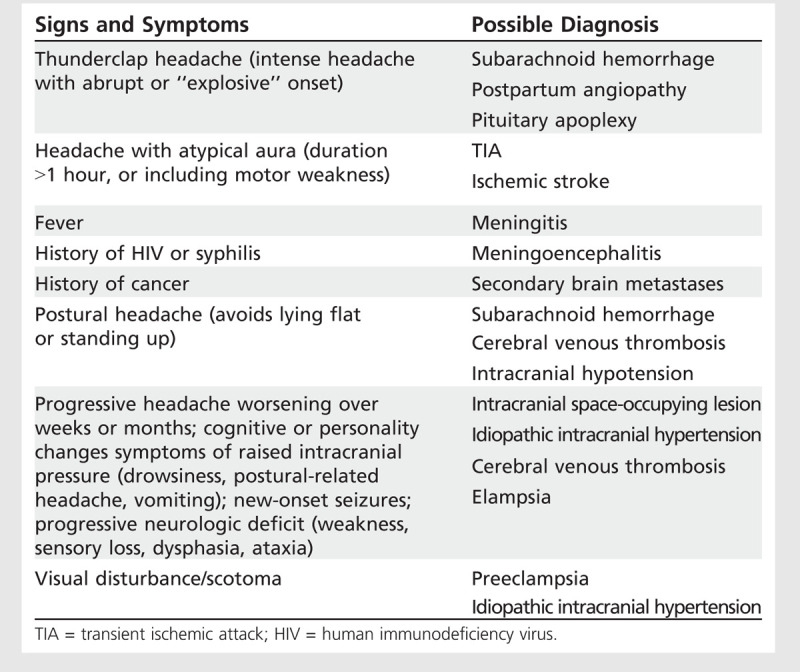
Risk factors for pregnancy-related stroke include diabetes mellitus, migraine with aura, preexisting hypertension, hypertensive disorders of pregnancy, sickle cell disease, structural heart disease, and thrombophilia. Complications of pregnancy and the puerperium associated with increased risk of stroke include anemia, hyperemesis gravidarum, thrombocytopenia, postpartum hemorrhage, and infection. Depressive disorders often present with frequent prepregnancy headache, which is a strong predictor of poor general and emotional health during pregnancy.3,4
Examination should focus on assessment for signs of concerning diagnoses such as infection or hemorrhage; severe hypertension; and neurologic signs including papilledema or hemorrhages on funduscopy, neck stiffness, altered consciousness, or weakness.
Symptoms and Signs of Secondary Headaches in Pregnancy
Although stroke can occur during pregnancy, it is more likely to occur in the 6 weeks postpartum. Focal neurologic symptoms and signs with or without alterations in consciousness are typical of stroke. Headache accompanies ischemic stroke in 17% to 34% of cases and is usually nonspecific in quality and of moderate intensity.
Subarachnoid hemorrhage typically presents as an abrupt-onset thunderclap headache that is intense and incapacitating. It is often unilateral and accompanied by nausea, vomiting, neck stiffness, and fluctuating consciousness. If neuroimaging is negative, lumbar puncture is imperative. Sentinel headache associated with a leaking cerebral aneurysm can precede subarachnoid hemorrhage in up to 50% of cases, typically occurring a couple of weeks before the aneurysm ruptures. An acute third nerve palsy, with retro-orbital pain and a dilated pupil, indicates impending rupture of aposterior communicating cerebral artery aneurysm.
A nonspecific unremitting headache is a common symptom of cerebral venous thrombosis, as seen in Case 7-1. Cerebral venous thrombosis is estimated to occur in 1 per 2500 to 10,000 pregnancies and is most likely in women with hypercoagulability. Headache may be the only finding, but often it is associated with seizures, focal neurologic symptoms, and raised intracranial pressure.
The combination of hypertension and proteinuria in a pregnant woman suggests preeclampsia and needs urgent management to reduce blood pressure and prevent eclampsia. Headache is a sentinel symptom of eclamptic seizure in around three-quarters of women with preeclampsia. The headache is progressive and refractory to analgesia. Many patients with preeclampsia present with headache due to hypertensive encephalopathy (ie, PRES) without seizures. Blurred vision, scotoma, and flashing lights in such patients can be misdiagnosed as migraine aura. Within the spectrum of preeclampsia/eclampsia, while some patients will present with PRES, others will present with the reversible cerebral vasoconstriction syndrome (RCVS), called postpartum angiopathy when it occurs in the postpartum period. This is a cerebral vasoconstriction syndrome that can be complicated by ischemic stroke. It usually occurs in the week after an uncomplicated pregnancy and delivery. It can mimic subarachnoid hemorrhage, presenting as a thunderclap headache associated with fluctuating neurologic deficits and sometimes seizures. Angiography reveals a classic “string of beads” appearance with areas of stenosis and dilation in multiple intracranial vessels. There is strong evidence for the benefit of magnesium sulfate for preeclampsia/eclampsia. The authors of the article “Cerebrovascular Disorders Complicating Pregnancy” in this issue of CONTINUUM recommend this therapy for preeclampsia/eclampsia in all of its forms, along with prompt control of blood pressure. For further details regarding PRES and RCVS, refer to “Cerebrovascular Disorders Complicating Pregnancy” by Drs Steven K. Feske and Aneesh B. Singhal in this issue of CONTINUUM
Pituitary apoplexy, resulting from spontaneous hemorrhagic infarction of the pituitary gland, is rare but life threatening. It presents with a retro-orbital, frontal, or diffuse thunderclap headache associated with nausea and vomiting, fluctuating consciousness, hypotension, and visual loss. If MRI is not available, an urgent pituitary CT scan is indicated.
Idiopathic intracranial hypertension presents with a diffuse, nonthrobbing, daily headache aggravated by coughing and straining. Signs include papilledema, an enlarged blind spot, visual field defect, or sixth nerve palsy. Lumbar puncture (performed after brain imaging excludes an intracranial mass lesion) reveals increased CSF pressure with normal CSF chemistry. Headache improves with reduction in CSF pressure.
Post–dural puncture headache affects one-third of patients after lumbar puncture with onset typically within 5 days of the procedure. The headache is postural, worsening on standing and improving when lying flat. Associated symptoms include neck stiffness, tinnitus, hyperacusia, photophobia, and nausea. In the majority of cases, the headache resolves within a week without further intervention. If conservative methods fail, an epidural blood patch should be considered.
Case 7-1
A 26-year-old woman who was 25 weeks pregnant with her first child was admitted after having a generalized tonic-clonic seizure. During the 8 days before admission, she had a constant, nonthrobbing headache associated with nausea and vomiting. She had taken the combined oral contraceptive pill from 18 to 25 years of age and had no problems other than headaches in the hormone-free interval, which resolved when she took the pill continuously, without a break; she stopped taking the pill when she was 25 years old because she wished to become pregnant and conceived 6 months later. Apart from nausea and vomiting during early pregnancy, she had been well.
On examination, blood pressure was normal, temperature was 36.9°C, and body mass index was 28.4 kg/m2. Remaining physical and neurologic assessments, including optic funduscopy, were normal.
Laboratory tests were all within normal ranges. A noncontrast brain MRI scan revealed a superior sagittal sinus thrombosis. She was treated with low-molecular-weight heparin for the remainder of the pregnancy and for 6 weeks post partum. Her headache resolved within 3 days of starting treatment, and the remainder of her pregnancy was uneventful, with a spontaneous normal delivery of a healthy boy at 39 weeks.
Comment. This pregnant migraineur presented with a new headache. While it is more common in the peripartum, cerebral venous thrombosis should be excluded in patients with progressive headache with seizure during pregnancy. Focal neurologic deficits and coma can also occur. Funduscopy may show signs of raised intracranial pressure, but brain imaging is indicated even if normal. This woman was fortunate to receive an early diagnosis and management, which led to a full recovery without compromising the pregnancy. Delayed diagnosis can result in permanent dysfunction and death.
Symptoms and Signs of Primary Headaches in Pregnancy
Each primary headache has a specific pattern of symptoms in the absence of clinical signs (Figure 7-1). The history is diagnostic, and investigations are only required to rule out a suspected secondary headache.
Figure 7-1.
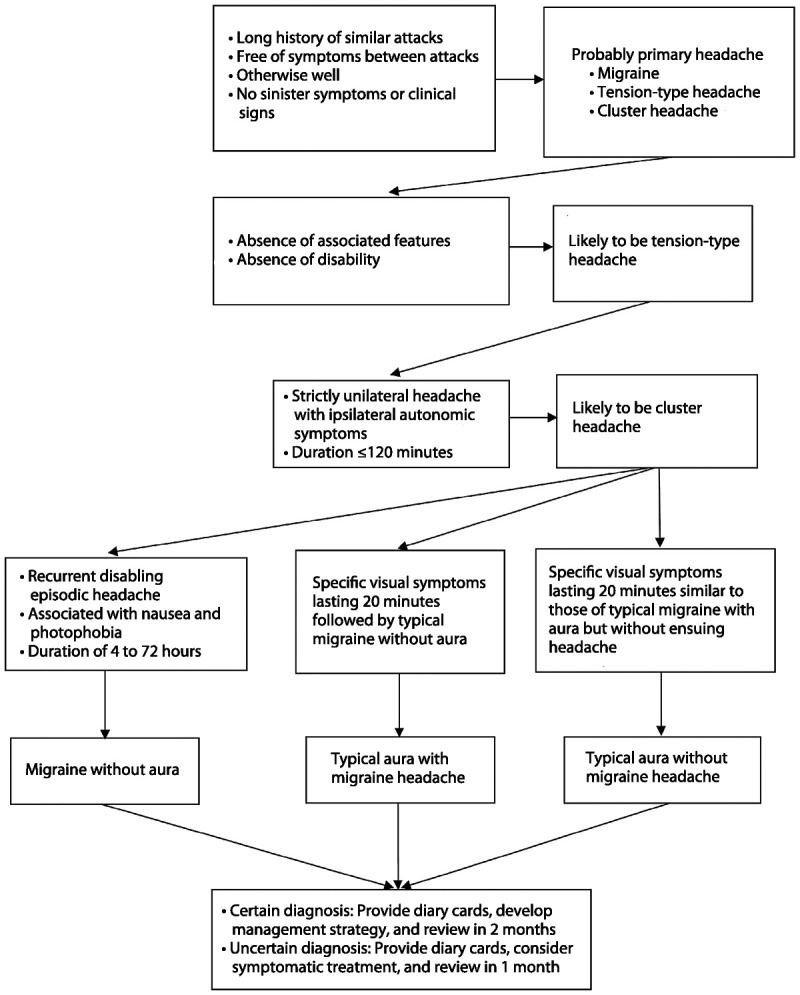
Algorithm for the diagnosis of primary headaches. Adapted with permission from MacGregor A, Wiley Blackwell.39 © 2008, John Wiley and Sons.
Tension-type headache. Headaches that lack associated symptoms, in an otherwise well person who is not overusing medication, are likely to be tension-type headaches.
In the majority of women, tension-type headache will improve during pregnancy.
Migraine. Recurrent episodic headaches that last between 4 and 72 hours and are associated with photophobia, nausea, and disability in an otherwise well person are typical features of migraine.
Up to 60% to 70% of women with preexisting migraine report fewer migraine attacks during pregnancy. Women with a history of menstrual or menstrually related migraine without aura are more likely to report improvement than for those with no evidence of a menstrual association.6 Migraine is likely to continue throughout pregnancy and postpartum if it does not improve by the end of the first trimester. Breast-feeding is encouraged because it maintains the protective effect of pregnancy on migraine headache during the postpartum period.
Women who have preexisting migraine with aura do not experience the same benefit and are more likely to continue to have attacks during pregnancy. As seen in Case 7-2, women may develop aura for the first time during pregnancy, irrespective of whether they have a past history of migraine without aura.7
Women can be reassured that thereis evidence that migraine does not adversely affect the outcome of pregnancy in otherwise healthy women.8 However, migraine during pregnancy is associated with increased risk of arterial and venous thrombosis, preeclampsia, and gestational hypertension.9,10,11,12,13,14,15,16,17 Pregnant women with migraine should be monitored for these conditions—for example, rising systolic or diastolic blood pressure should prompt a check for proteinuria.
Disorders such as thrombocytopenia, cerebral venous sinus thrombosis, or imminent eclampsia may mimic aura and should be excluded in women presenting with their first attack of aura during pregnancy.
Cluster headache. Cluster headache affects fewer than 1 in 500 people, and in contrast to migraine and tension-type headache, it is much more common in men than in women. It is frequently misdiagnosed as migraine despite stereotypical symptoms of strictly unilateral headache and autonomic symptoms lasting up to 2 hours in clusters typically lasting 6 to 8 weeks. Data on cluster headache during pregnancy are limited, but it has been noted that women who have their first cluster headache before their first pregnancy have fewer children than those who already have children at the time of their first attack.18,19 While the possibility of hypofertility has been raised, the more likely explanation is that women choose not to conceive as they are concerned about the effects of medication for cluster headache on the outcome of pregnancy.19
Medication overuse headache. Medication overuse headache affects people with a history of a primary headache that has become more frequent (Case 7-3). Medication overuse headache should always be excluded in anyone using symptomatic treatments for headache more often than 2 to 3 days a week or taking frequent caffeine in any form. It is an avoidable cause of treatment failure, as headache becomes resistant to all lines of management until symptomatic drugs are withdrawn. Drug withdrawal typically resolves headache frequency, and residual episodic headache can be treated appropriately.
Case 7-2
A 34-year-old woman was 32 weeks pregnant with her first child. While at work she developed blurred vision to the right, difficulty with speech, and tingling, numbness, and weakness of her right arm lasting 20 minutes. This was followed by nausea and a left temporal headache lasting 5 hours. By the time she was seen in the emergency department, all symptoms had resolved apart from the headache. She had a history of migraine without aura since the age of 15 years but no other history of note. She took no regular medication other than pregnancy supplements. On examination, her blood pressure was 110/70 mm Hg, temperature was 36.8°C, and body mass index was 23.6 kg/m2. Remaining physical and neurologic assessments, including optic funduscopy, were normal.
Laboratory tests, thrombophilia screen, EEG, echocardiogram, and noncontrast brain MRI were normal. By the time the investigations were complete, the headache had resolved, and the patient felt “back to normal.” Migraine with aura was considered the most likely diagnosis. She was discharged with advice to return if her condition deteriorated at all and with an analgesic/antiemetic combination to take at the onset of symptoms if they recurred. She had three further attacks during the remainder of the otherwise uneventful pregnancy, all of which responded to medication.
Comment. Although migraine without aura typically improves during pregnancy, migraine with aura can occur for the first time in women with or without previous attacks of migraine without aura. While migraine itself, with or without aura, poses no risk to the pregnancy, migraine in pregnancy is a risk factor for hypertensive disorders of pregnancy, so these women should be monitored carefully.
Case 7-3
A 27-year-old woman presented at 17 weeks of pregnancy with daily headaches. She had had migraine without aura since she was 11 years old. One year earlier, she had developed daily headaches and was diagnosed with medication overuse headache as a consequence of frequent use of triptans. She stopped this medication at that time and was started on topiramate. She had a very severe headache for a week, after which symptoms resolved. The pattern of headache settled to one migraine attack every 4 to 6 weeks, which responded to a triptan, and she was free of symptoms between attacks.
At the current presentation, her diaries showed a fluctuating pattern of headache over the preceding months, with a gradual increase in headache frequency beginning when she stopped topiramate 3 months earlier to become pregnant. After she realized she was pregnant, she used only acetaminophen with codeine, which she had been taking most days, as she was worried that she would develop a severe migraine and be unable to get to work. Vital signs were within normal range, and physical and neurologic examination was unremarkable.
Medication overuse headache was diagnosed, and she chose to stop analgesics without additional support. She was advised that a severe migraine could be treated with IV magnesium sulfate, if necessary. At follow-up 2 weeks later, she reported that she had been unwell for the first week since stopping analgesics but felt much better in the last week and had not had any further headaches.
Comment. Medication overuse headache is a common cause of daily headaches in patients with a history of primary headaches. The key finding is the use of symptomatic drugs for headache on more days than not in the absence of any red flags in the history and in the presence of a normal examination.
INVESTIGATIONS DURING PREGNANCY AND LACTATION
Investigations are indicated only to exclude suspected secondary headache resulting from underlying pathology. For a discussion of brain imaging during pregnancy, see the article “Neuroradiology in Women of Childbearing Age” by Drs Riley Bove and Joshua Klein in this issue of CONTINUUM
MANAGEMENT OF PRIMARY HEADACHES IN PREGNANCY
Nonpharmacologic
Nonpharmacologic treatment should be considered as the initial step in the management of tension-type headache or migraine but is ineffective for cluster headache, which can only be controlled with medication.
The most frequently reported triggers for tension-type headache and migraine are stress (mental or physical), irregular or inappropriate meals, high intake or withdrawal of coffee and other caffeine-containing drinks, dehydration, sleep disorders, too much or too little sleep, and reduced or excessive physical exercise.23,24 Pregnant women with tension-type headache or migraine should be encouraged to avoid skipping meals, to take regular exercise, to drink plenty of fluids, and to maintain a regular sleep pattern. Alcohol and smoking are potentially harmful to the fetus and should be avoided during pregnancy.
Nondrug therapies such as relaxation, biofeedback, and physical therapy are safe and may be effective in pregnancy.25,26 Acupuncture may also treat nausea and vomiting in pregnancy in addition to headache.27,28,29
Coenzyme Q10 daily is effective for migraine prophylaxis and, when taken during pregnancy, has been associated with a significant reduced risk of preeclampsia.30,31 Similarly, magnesium supplements, which can be used for migraine prophylaxis, can halve the risk of eclampsia, with no evidence of adverse effects on pregnancy.32,33 See the section on complementary medicines during pregnancy and lactation for further details about coenzyme Q10 and magnesium during pregnancy and lactation.
Drug Treatment of Headache in Pregnancy and Lactation
Drug use during pregnancy is common, and many women continue to use their usual headache medication, including triptans, throughout pregnancy.34 The most comprehensive guidelines for the drug treatment of primary headaches are those developed by the European Federation of Neurological Societies.5,32,35 Most drugs are not licensed for use in pregnancy so should only be considered if the potential benefits to the woman and fetus outweigh the potential risks. As discussed in Case 7-4, women are often very concerned about the effects that medication may have on the pregnancy. The woman should be given sufficient information about any known risks to make her own decision about drug use, with clear documentation of the discussion.
The US Food and Drug Administration (FDA) pregnancy labeling has five categories: A, B, C, D, and X (Appendix A). These categories can be misleading because categories C, D, and X are not only based on risk but consider risk versus benefit, so drugs in each of these three categories may pose similar risks. Several additional sources in the United States provide information on the safety of drugs during pregnancy and lactation, and these have been reviewed to produce the evidence for the recommendations below.
Symptomatic treatment. The symptomatic treatment of headaches during pregnancy and lactation is thesame as for the nonpregnant state, with some exceptions (Table 7-3 and Table 7-4).
Table 7-3.
Drugs Used for Acute Treatment of Headache During Pregnancy
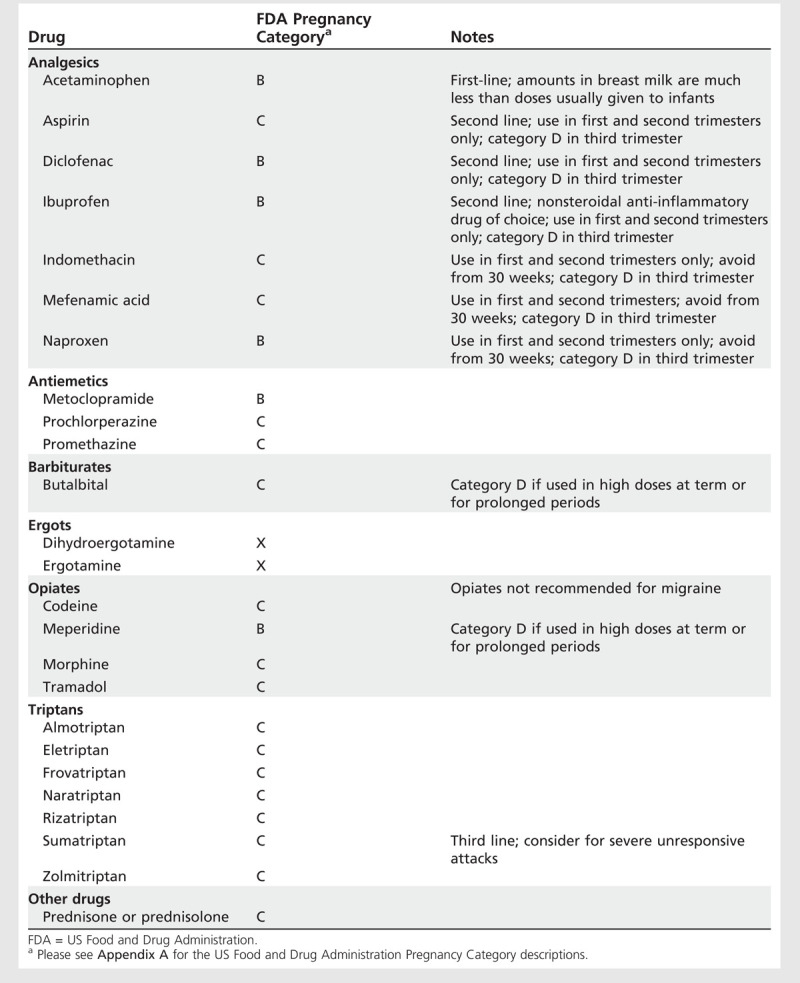
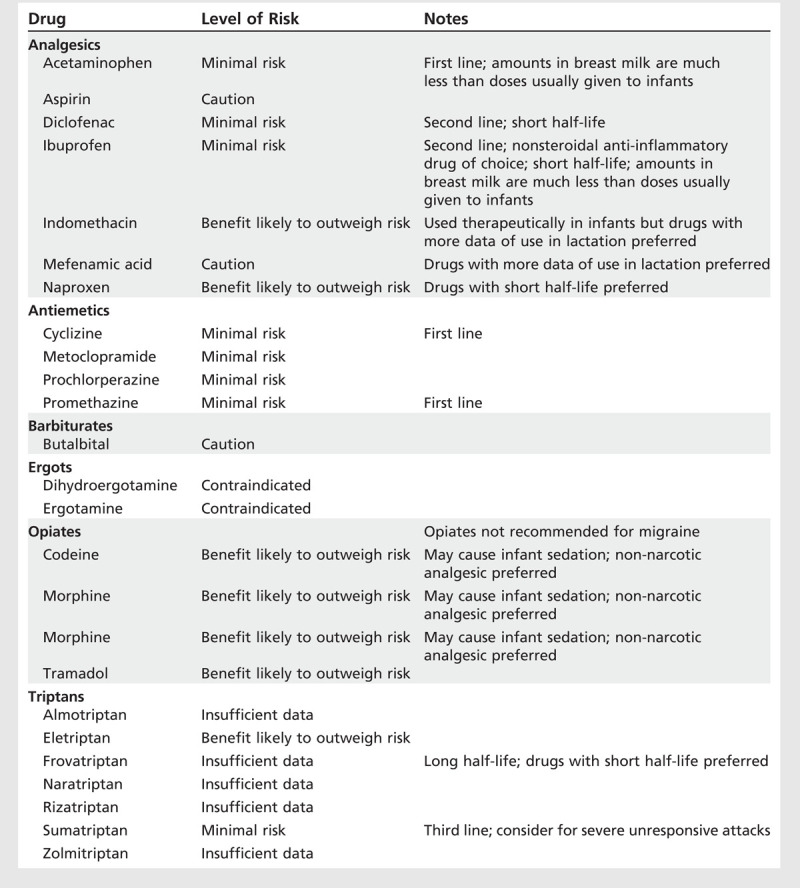
Table 7-4.
Drugs for Acute Treatment of Headache During Lactation
Analgesics. Data from large-cohort and case-control studies confirm the safety of therapeutic doses (4 g or less per day) of acetaminophen during pregnancy and lactation. It is the analgesic of choice for the short-term relief of mild to moderate pain and pyrexia.
While aspirin can be taken during the first and second trimesters of pregnancy, it is best avoided near term because of increased risk of prolonged labor, postpartum hemorrhage, and neonatal bleeding. Aspirin, in common with nonsteroidal anti-inflammatory drugs (NSAIDs), has been associated with premature closure of the fetal ductus arteriosus. Aspirin is excreted in breast milk, andalthough occasional use by the mother is unlikely to cause adverse effect, it should not be taken regularly during breast-feeding because of the theoretical risk of Reye syndrome and impaired platelet function in susceptible infants.
Nonsteroidal anti-inflammatory drugs. Ibuprofen is the NSAID of choice during the first or second trimester. NSAIDs should be avoided during the third trimester because chronic use or high doses after 30 weeks are associated with an increased risk of premature closure of the ductus arteriosus and oligohydramnios. The concentration of NSAIDs in breast milkis very low so treatment during breast-feeding is unlikely to affect the infant.
Opiates. Although safe for treatment of moderate to severe pain in pregnancy, opiates are inappropriate for migraine because they aggravate nausea and reduce gastric motility. Chronic use of opiates in later pregnancy has been associated with neonatal withdrawal symptoms. Adverse effects during lactation are unlikely. However, some women metabolize opiates slowly, which can cause higher levels of opiates to pass into their breast milk, resulting in infantile drowsiness and sedation.
Barbiturates. Only limited human data are available for butalbital during pregnancy. Phenobarbital has been associated with teratogenicity and cancause neonatal withdrawal and hemorrhagic disease of the newborn. It is excreted into breast milk and accumulates in infants’ blood, causing sedation and withdrawal symptoms.
Antiemetics. Metoclopramide, prochlorperazine, and promethazine have been used widely in pregnancy and lactation without reports of adverse effects.
Triptans. Women who have taken triptans during pregnancy can be reassured that there is evidence to support no adverse outcome. However, this is not the same as evidence to confirm safety, so continued use during pregnancy is not recommended unless no other treatment is effective.
Data from the Sumatriptan/Naratriptan/Treximet Pregnancy Registry (from 1996 to 2011) are reassuring and confirm that inadvertent exposure to sumatriptan during pregnancy has not been associated with adverse outcomes. Therefore, if clinically appropriate, sumatriptan may be considered for the acute treatment of migraine during pregnancy if no other treatments are effective. However, a small increased risk of specific birth defects cannot be excluded. The low level of excretion of sumatriptan in breast milk suggests that continued breast-feeding following its use does not pose a significant risk to the infant. Data on other triptans are insufficient.
Ergots. Ergotamine and dihydroergotamine are contraindicated, because uterine hypertonicity and vascular disruption increase the risk of miscarriage. They should not be used during breast-feeding because of reported nausea, vomiting, diarrhea, and weakness in the breast-feeding infant and suppression of prolactin secretion and lactation in the mother.
Drugs used for prophylaxis. Restriction on drugs used for prophylaxis for headache during pregnancy and lactation is greater than on drugs used for symptomatic treatment, but a number of options are available thatpose minimal risks to the unborn or breast-feeding child (Table 7-5 and Table 7-6).
Table 7-5.
Drugs Used for Prophylaxis of Headache During Pregnancy
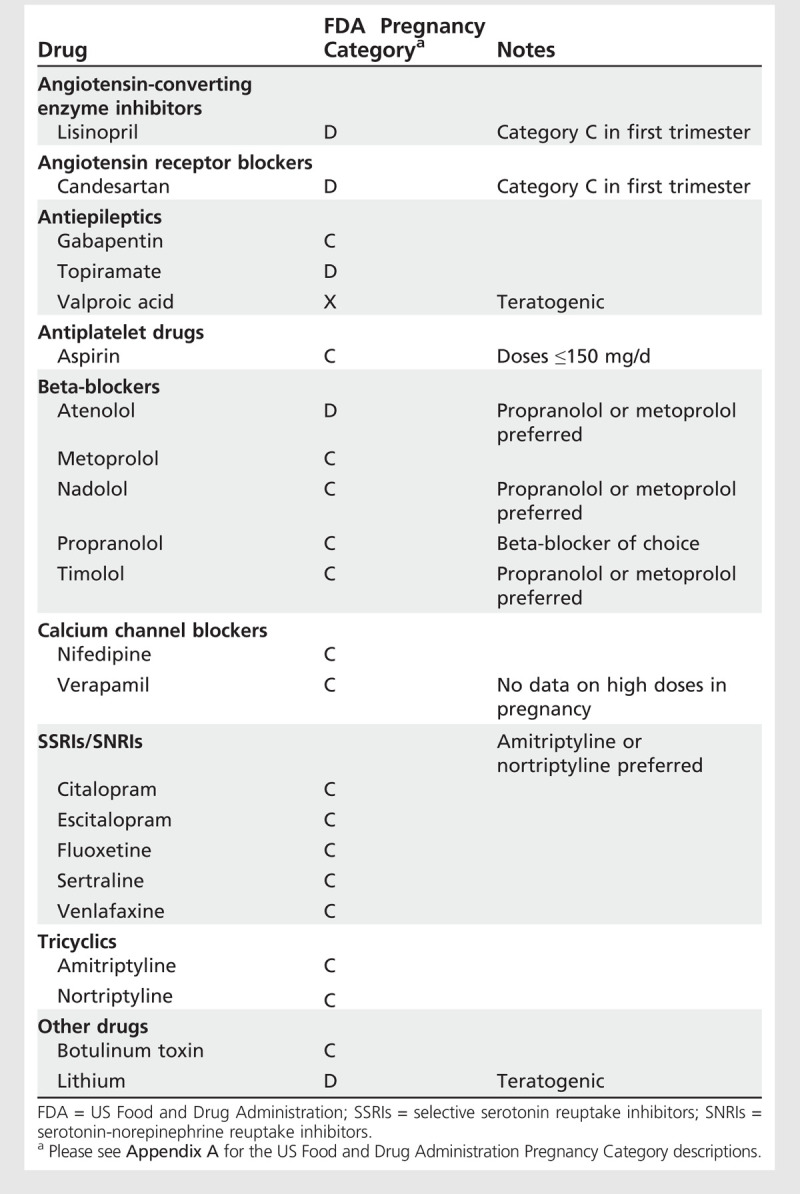
Table 7-6.
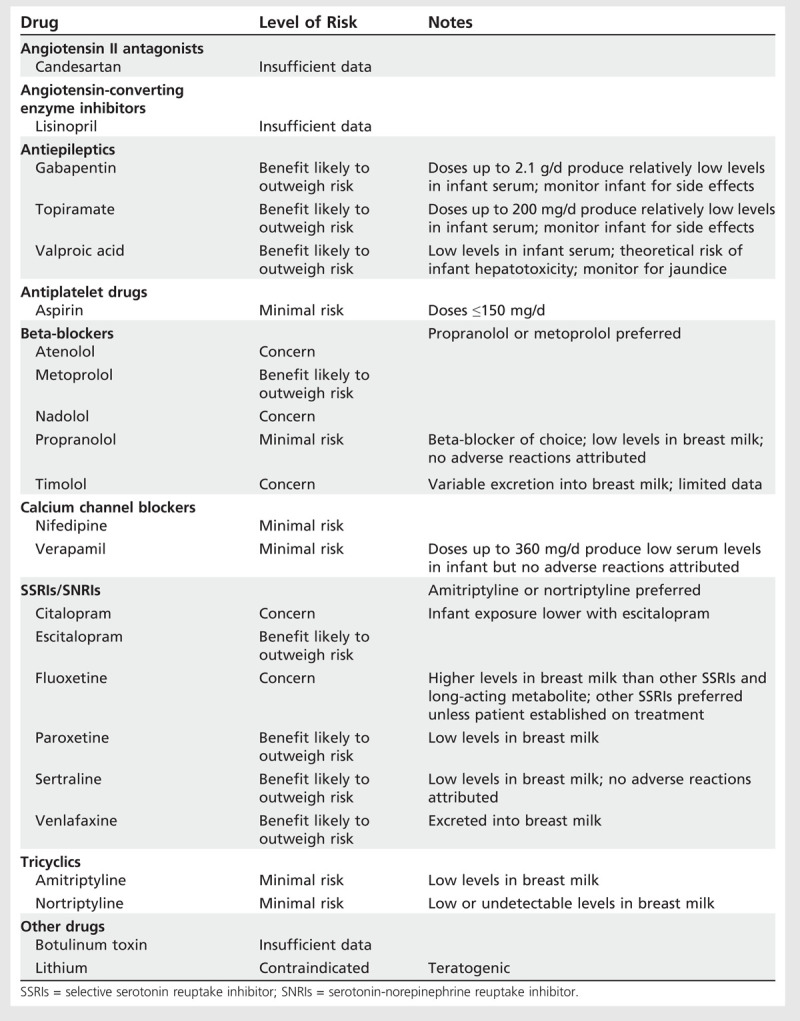
Drugs Used for Prophylaxis of Headache During Lactation
Low-dose aspirin. Low-dose aspirin (no more than 150 mg/d) has been extensively studied in preeclampsia during pregnancy, with no increase in bleeding complications and negligible effects on the ductus arteriosus.
Beta-blockers. If prophylaxis is considered necessary during pregnancy, the lowest effective doses of propranolol or metoprolol are the drugs of choice. If beta-blockers are used in the third trimester, treatment should be stopped 2 to 3 days before delivery in order to reduce the likelihood of fetal bradycardia and a reduction in uterine contraction. Infants exposed to propranolol in utero should be monitored for neonatal bradycardia, hypotension, and hypoglycemia. Propranolol or metoprolol are the beta-blockers of choice during lactation.
Antidepressants. Low-dose amitriptyline 10 mg/d to 25 mg/d is an option. While data are conflicting regarding limb deformities associated with use of high doses of amitriptyline during pregnancy, no association has been reported with low doses between 10 and 50 mg/d used for pain management. Where possible, it is recommended that the dose be tapered 3 to 4 weeks before delivery. Otherwise, the physician should monitor the baby for adverse effects such as drowsiness, jitteriness, hyperexcitability, and suckling problems. Amitriptyline or nortriptyline are barely detectable in breast milk and treatment of the mother with these drugs is unlikely to affect the infant adversely.
Data are conflicting on the risk of congenital malformations following use in early pregnancy of selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI). Although several studies have demonstrated no statistically significant increase in risk, an increased risk of cardiovascular malformations was suggested in one large study. However, the absolute risk appears to be small. Use of any SSRI in late pregnancy may result in a mild, transient neonatal withdrawal syndrome, which includes continuous crying, irritability, jitteriness, or restlessness; shivering; fever; tremors; hypertonia or rigidity; tachypnea or respiratory distress; feeding difficulty; sleep disturbance; hypoglycemia; and seizures. Exposure to SSRIs beyond 20 weeks of gestation has been associated with persistent pulmonary hypertension of the newborn, although the estimated absolute risk is less than 0.5%.
Antiepileptics. Risk of oral clefts is increased after exposure to topiramate in pregnancy. Sodium valproate is associated with a high risk of fetal abnormalities and is contraindicated during pregnancy in the absence of epilepsy; it can be used during lactation. Currently available data do not suggest that gabapentin has any adverse effect on pregnancy, but it is considered at best to be a third-line migraine prophylactic agent. Refer to the article “Pregnancy and Epilepsy” by Dr Cynthia Harden in this issue of CONTINUUM for a detailed discussion of antiepileptic agents and pregnancy.
Calcium channel blockers. Data for verapamil are limited. It has a tocolytic effect on the uterus, so should be avoided in late pregnancy. No association with congenital anomalies has been reported. Verapamil is excreted in human milk with potential for adverse reactions in infants, which is of particular concern with high doses necessary for cluster headache, although no data are available.
Angiotensin receptor blockers. Case reports have described congenital malformations, fetal renal damage, oligohydramnios, skull ossification defects, and death in infants exposed to angiotensin receptor blockers in utero.
Angiotensin-converting enzyme inhibitors. Lisinopril is used for migraine prophylaxis. In common with other angiotensin-converting enzyme (ACE) inhibitors, it poses no significant fetal risk during the first trimester. During the second and third trimesters, it is toxic and teratogenic, causing prematurity, intrauterine growth retardation, renal tubular dysplasia, severe oligohydramnios leading to fetal distress, lung hypoplasia, skull hypoplasia, limb contractures, neonatal hypotension, and anuria.
Botulinum toxin. No human data have been published regarding teratogenicity of botulinum toxin, licensed for chronic migraine, in pregnancy and lactation.
Lithium. Exposure to therapeutic doses of lithium in pregnancy are associated with teratogenic and other toxic effects in the fetus and neonate. Opinions are conflicting as to whether lithium should be stopped during the first trimester of pregnancy and restarted in the second trimester. If treatment with lithium is indicated, combined specialist supervision by a neurologist and obstetrician is essential. Lithium is excreted in breast milk and has been associated with adverse effects in the infant.
Case 7-4
A 35-year-old woman presented when she was 13 weeks pregnant with her second child. She had migraine without aura as a teenager but was free of attacks during her twenties. Her first pregnancy, 7 years earlier, had been uneventful; 4 months before this presentation, she had a miscarriage at 9 weeks. Since the birth of her first child, she had only occasional attacks, but over the previous year, she had migraine twice a month that did not always respond to sumatriptan. She had started amitriptyline 50 mg/d, which reduced the frequency to one attack every 4 to 6 weeks, which she could then control with sumatriptan. She continued on this combination during the second pregnancy and was concerned that this was the reason for her miscarriage. She stopped sumatriptan and amitriptyline as soon as she realized that she was pregnant again. During the past 12 weeks, she had had three migraine attacks, treated with acetaminophen and codeine. This treatment was not effective, and she had missed several days from work. She was concerned that any medication may increase her chances of another miscarriage but was equally worried that she would not be able to continue work. Other than mild pregnancy-related nausea in the mornings, she was fit and well and took only pregnancy supplements. It was explained to her that migraine did not have any adverse effect on the outcome of pregnancy and neither did most migraine medications, including amitriptyline. The importance of regular snacks and hydration to help migraine and the morning nausea was discussed. A combination of a nonsteroidal anti-inflammatory drug and an antiemetic was considered for symptomatic treatment, understanding that she could use this until the 30th week of pregnancy, with the option of taking sumatriptan if necessary. Given that migraine without aura often improves after the first trimester of pregnancy, preventive treatment was deferred. At her review appointment 6 weeks later, she reported only one further attack at 11 weeks of pregnancy and none since.
Comment. Women are often concerned that they are responsible for a miscarriage, so it is important to remind them that at least 10% of pregnancies result in miscarriage, most occurring during the first trimester as a result of a genetic abnormality. Balancing risk and benefit of medication for migraine is an important consideration particularly since migraine itself has no adverse effect on the pregnancy. However, an untreated migraine can result in significant morbidity.
Approach to Drug Treatment of Tension-Type Headache During Pregnancy and Lactation
Symptomatic treatment with nonopioid analgesics is appropriate for episodic attacks occurring on fewer than 2 days per week.5 Prophylactic medications are seldom necessary and are only indicated for chronic tension-type headache or when headaches regularly occur more than 2 to 3 days a week. Amitriptyline is the drug of first choice for prophylaxis of tension-type headache during pregnancy and lactation.
Approach to Drug Treatment of Migraine During Pregnancy and Lactation
Symptomatic treatment of attacks includes nonopioid analgesics and prokinetic antiemetics, which should be limited to a maximum of 2 to 3 treatment days per week (12 days per month). Sumatriptan may be indicated for severe attacks during pregnancy that do not respond to first-line drugs and can be used during lactation without disruption to breast-feeding. Prophylaxis should be considered when attacks are frequent or fail to respond to symptomatic management.
Analgesics can be used for symptomatic treatment. Most antiemetics can be continued throughout pregnancy and lactation. If prophylaxis is indicated during pregnancy or lactation, the lowest effective dose of propranolol is the first-line recommendation. Amitriptyline is also an option.
Approach to Drug Treatment of Cluster Headache During Pregnancy and Lactation
Prophylaxis is the mainstay of management of cluster headache. Preferred treatments during pregnancy and lactation are verapamil or prednisone/prednisolone.20 Cardiac conduction problems affect about 20% of patients with cluster headache taking verapamil and are dependent on neither duration nor dose. ECGs to assess PR interval prolongation should be undertaken at baseline, before each dose increment, and every 6 months during long-term treatment. Gabapentin is a second-line prophylactic option during pregnancy and lactation. Lithium is an additional option for prophylaxis during lactation but should not be used during pregnancy.
Acute treatment includes oxygen (100% at 7 L/min for 10 to 15 minutes at onset of attack) or subcutaneous or intranasal sumatriptan.20 No safety concerns exist regarding use of oxygen during pregnancy.21
Complementary Medicines During Pregnancy and Lactation
Many pregnant women use dietary supplements during pregnancy, incorrectly assuming that because they are not “drugs” they must be safe (Table 7-7). Although some evidence suggests that supplementing deficient vitamins and minerals can be beneficial, megadose regimens should be avoided.
Table 7-7.
Complementary Supplements and Pregnancy and Lactationa

The daily recommended dietary allowances of riboflavin (Vitamin B2) for pregnant women is 1.4 mg, and 1.6 mg for breast-feeding women.
Pregnant women are at risk of vitamin D deficiency due to increased fetal and neonatal demands during intrauterine growth and development. Vitamin D deficiency has also been associated with increased risk of migraine.Vitamin D supplements of 10 μg/d (or equivalent) are currently recommended for all pregnant and breast-feeding women. Very high doses of vitamin D can increase the serum calcium concentration, which could result in maternal, fetal, or neonatal hypercalcemia.
Scientific evidence is insufficient to support the safe use of coenzyme Q10 during early pregnancy or breast-feeding. Supplementation with 100 mg coenzyme Q10 twice daily from week 20 of pregnancy has been shown to reduce the risk of preeclampsia in women at risk for the condition.
Magnesium sulfate is considered to be compatible with pregnancy and breast-feeding, but the FDA recommends an upper limit of 350 mg magnesium sulfate daily.
EMERGENCY TREATMENT OF HEADACHE
During pregnancy and lactation, prochlorperazine 10 mg or chlorpromazine 25 mg to 50 mg by IM injection are effective for emergency headache relief even without additional analgesia and, together with IV fluids, are usually sufficient to abort an attack. IV magnesium sulfate 1 g given intravenously over 15 minutes was well tolerated and effective in a randomized, single-blind, placebo-controlled trial of 30 patients with migraine.36 A combination of IV prochlorperazine 10 mg every 8 hours and IV magnesium sulfate 1 g every 12 hours was used successfully to abort two cases ofprolonged migraine aura during pregnancy.37 Continuous administration ofmagnesium sulfate injection beyond 5to 7 days in pregnancy should be avoided because it can cause low calcium levels and bone changes in the baby.
Corticosteroids successfully treat intractable nausea and vomiting in hyperemesis gravidarum. A 6-day reducing course of prednisolone (60 mg/d for 2 days, 40 mg/d for 2 days, and 20 mg/d for 2 days) can be considered to treat long-duration headache attacks, particularly if evidence of medication overuse is present.38 Chronic exposure to high doses of steroids in pregnancy should be avoided as it may cause fetal/neonatal adrenal suppression, and increased incidence of congenital anomalies, neonatal cataracts, and stillbirth have been reported when the drug is used throughout pregnancy. Prednisone/prednisolone is compatible with breast-feeding.
CONCLUSIONS
The majority of headaches occurring during pregnancy and lactation are benign and likely to improve with minimal intervention. However, pregnancy can be a risk factor for certain potentially life-threatening headaches that require urgent evaluation. The assessment of the pregnant patient with headache relies on a directed history to elicit warning symptoms that require immediate investigation and management. Investigation of headache is the same as for nonpregnant women, although the threshold to consider certain disorders must be lower, and routine investigations should be deferred until postpartum.
It is important to minimize drug exposure in women who are planning pregnancy or who are at high risk ofunplanned pregnancy. Many drugs and other teratogens exert their greatest effects on the fetus during the second and third months of gestation. If a drug is taken at any stage ofpregnancy, it is essential that the woman be provided with sufficient information about any known risks in order for her to make her own decision about its use.
Nonpharmacologic management and lifestyle changes can be effectivewithout the need for additional drugintervention and have the advantage of continued benefit beyond pregnancy.
USEFUL WEBSITES
Sumatriptan/Naratriptan/Treximet Pregnancy Registry, Kendle International
pregnancyregistry.gsk.com/sumatriptan.html
Rizatriptan Pregnancy Registry, Merck & Co, Inc
www.merckpregnancyregistries.com/maxalt.html
Summary of Proposed Rule on Pregnancy and Lactation Labelling
www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093310.htm
National Library of Medicine Drugs and Lactation Database (LactMed)
toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT
Organization of Teratology Information Specialists (OTIS)
Teratogen Information Service (TERIS)
depts.washington.edu/terisweb/teris/
The International Classification of Headache Disorders, 3rd edition (beta version)
KEY POINTS
Of the primary headaches, tension-type headache and migraine generally improve during pregnancy.
A common cause of daily headache in a patient with a history of primary headache is medication overuse headache.
Because secondary headaches can occur in a patient with a long-standing history of primary headache, it is important to elicit new symptoms.
Each primary headache has a specific pattern of symptoms in the absence of clinical signs.
Headaches that lack associated symptoms, in an otherwise well person who is not overusing medication, are likely to be tension-type headaches.
Recurrent episodic headaches that last between 4 and 72 hours and are associated with photophobia, nausea, and disability in an otherwise well person are typical features of migraine.
Cluster headache is frequently misdiagnosed as migraine despite stereotypical symptoms of strictly unilateral headache and autonomic symptoms lasting up to 2 hours in clusters typically lasting 6 to 8 weeks.
Pregnant women with tension-type headache or migraine should be encouraged to avoid skipping meals, to take regular exercise, to drink plenty of fluids, and to maintain a regular sleep pattern.
Nondrug therapies such as relaxation, biofeedback, and physical therapy are safe and may be effective in pregnancy.
During pregnancy and lactation, prochlorperazine 10 mg or chlorpromazine 25 mg to 50 mg by IM injection are effective for emergency headache relief even without additional analgesia.
Footnotes
Relationship Disclosure: Professor MacGregor has acted as a paid consultant to and/or her department has received research funding from Addex Therapeutics; Allergan, Inc; AstraZeneca; Berlin-Chemie AG; BTG International Ltd; Endo Pharmaceuticals Inc; GlaxoSmithKline; the Menarini Group; Merck & Co, Inc; POZEN Inc; and UniPath.
Unlabeled Use of Products/Investigational Use Disclosure: Professor MacGregor discusses the use of several drugs for the treatment of headaches, none of which are labeled by the US Food and Drug Administration for use in pregnancy.
REFERENCES
- 1.Bonati M,, Bortolus R,, Marchetti F, et al. Drug use in pregnancy: an overview of epidemiological (drug utilization) studies. Eur J Clin Pharmacol 1990; 38 (4): 325–328. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33 (9): 629–808. [DOI] [PubMed] [Google Scholar]
- 3.Cripe SM,, Frederick IO,, Qiu C,, Williams MA. Risk of preterm delivery and hypertensive disorders of pregnancy in relation to maternal co-morbid mood and migraine disorders during pregnancy. Paediatr Perinat Epidemiol 2011; 25 (2): 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aromaa M,, Rautava P,, Helenius H,, Sillanpää ML. Prepregnancy headache and the well-being of mother and newborn. Headache 1996; 36 (7): 409–415. [DOI] [PubMed] [Google Scholar]
- 5.Bendtsen L,, Evers S,, Linde M, et al. EFNS guideline on the treatment of tension-type headache—report of an EFNS task force. Eur J Neurol 2010; 17 (11): 1318–1325. [DOI] [PubMed] [Google Scholar]
- 6.Granella F,, Sances G,, Pucci E, et al. Migraine with aura and reproductive life events: a case control study. Cephalalgia 2000; 20 (8): 701–707. [DOI] [PubMed] [Google Scholar]
- 7.Cupini LM,, Matteis M,, Troisi E, et al. Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia 1995; 15 (2): 140–144. [DOI] [PubMed] [Google Scholar]
- 8.Wainscott G,, Sullivan M,, Volans G,, Wilkinson M. The outcome of pregnancy in women suffering from migraine. Postgrad Med J 1978; 54 (628): 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotton WN,, Sachtleben MR,, Friedman EA. Migraine and eclampsia. Obstet Gynecol 1959; 14: 322–330. [PubMed] [Google Scholar]
- 10.Moore MP,, Redman CW. Case-control study of severe pre-eclampsia of early onset. Br Med J (Clin Res Ed) 1983; 287 (6392): 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcoux S,, Berube S,, Brisson J,, Fabia J. History of migraine and risk of pregnancy-induced hypertension. Epidemiology 1992; 3 (1): 53–56. [DOI] [PubMed] [Google Scholar]
- 12.Facchinetti F,, Allais G,, D’Amico R, et al. The relationship between headache and preeclampsia: a case-control study. Eur J Obstet Gynecol Reprod Biol 2005; 121 (2): 143–148. [DOI] [PubMed] [Google Scholar]
- 13.Scher AI,, Terwindt GM,, Picavet HS, et al. Cardiovascular risk factors and migraine: the GEM population-based study. Neurology 2005; 64 (4): 614–620. [DOI] [PubMed] [Google Scholar]
- 14.Banhidy F,, Acs N,, Horvath-Puho E,, Czeizel AE. Pregnancy complications and delivery outcomes in pregnant women with severe migraine. Eur J Obstet Gynecol Reprod Biol 2007; 134 (2): 157–163. [DOI] [PubMed] [Google Scholar]
- 15.Adeney KL,, Williams MA,, Miller RS, et al. Risk of preeclampsia in relation to maternal history of migraine headaches. J Matern Fetal Neonatal Med 2005; 18 (3): 167–172. [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti F,, Allais G,, Nappi R, et al. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia 2009; 29 (3): 286–292. [DOI] [PubMed] [Google Scholar]
- 17.Adeney KL,, Williams MA. Migraine headaches and preeclampsia: an epidemiologic review. Headache 2006; 46 (5): 794–803. [DOI] [PubMed] [Google Scholar]
- 18.Ekbom K,, Waldenlind E. Cluster headache in women: evidence of hypofertility(?) headaches in relation to menstruation and pregnancy. Cephalalgia 1981; 1 (3): 167–174. [DOI] [PubMed] [Google Scholar]
- 19.van Vliet JA,, Favier I,, Helmerhorst FM, et al. Cluster headache in women: relation with menstruation, use of oral contraceptives, pregnancy, and menopause. J Neurol Neurosurg Psychiatry 2006; 77 (5): 690–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen BK. Migraine and tension-type headache in a general population: psychosocial factors. Int J Epidemiol 1992; 21 (6): 1138–1143. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain 1993; 53 (1): 65–72. [DOI] [PubMed] [Google Scholar]
- 22.Marcus DA,, Scharff L,, Turk DC. Nonpharmacological management of headaches during pregnancy. Psychosom Med 1995; 57 (6): 527–535. [DOI] [PubMed] [Google Scholar]
- 23.Nestoriuc Y,, Rief W,, Martin A. Meta-analysis of biofeedback for tension-type headache: efficacy, specificity, and treatment moderators. J Consult Clin Psychol 2008; 76 (3): 379–396. [DOI] [PubMed] [Google Scholar]
- 24.Linde K,, Allais G,, Brinkhaus B, et al. Acupuncture for tension-type headache. Cochrane Database Syst Rev 2009; (1): CD007587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linde K,, Allais G,, Brinkhaus B, et al. Acupuncture for migraine prophylaxis. Cochrane Database Syst Rev 2009; (1): CD001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neri I,, Allais G,, Schiapparelli P, et al. Acupuncture versus pharmacological approach to reduce hyperemesis gravidarum discomfort. Minerva Ginecol 2005; 57 (4): 471–475. [PubMed] [Google Scholar]
- 27.Sandor PS,, Di Clemente L,, Coppola G, et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology 2005; 64 (4): 713–715. [DOI] [PubMed] [Google Scholar]
- 28.Teran E,, Hernandez I,, Nieto B, et al. Coenzyme Q10 supplementation during pregnancy reduces the risk of pre-eclampsia. Int J Gynaecol Obstet 2009; 105 (1): 43–45. [DOI] [PubMed] [Google Scholar]
- 29.Evers S,, Áfra J,, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol 2009; 16 (9): 968–981. [DOI] [PubMed] [Google Scholar]
- 30.Altman D,, Carroli G,, Duley L, et al. Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002; 359 (9321): 1877–1890. [DOI] [PubMed] [Google Scholar]
- 31.Nezvalová-Henriksen K,, Spigset O,, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache 2010; 50 (4): 563–575. [DOI] [PubMed] [Google Scholar]
- 32.May A,, Leone M,, Afra J, et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol 2006; 13 (10): 1066–1077. [DOI] [PubMed] [Google Scholar]
- 33.Jüergens TP,, Schaefer C,, May A. Treatment of cluster headache in pregnancy and lactation. Cephalalgia 2009; 29 (4): 391–400. [DOI] [PubMed] [Google Scholar]
- 34.The National Teratology Information Service. Hyperbaric oxygen exposure in pregnancy. 2006. www.toxbase.org/upload/Pregnancy%20pdfs/Carbon%20Monoxide%202012.pdf. Accessed November 23, 2013.
- 35.Demirkaya S,, Vural O,, Dora B,, Topcuoglu MA. Efficacy of intravenous magnesium sulfate in the treatment of acute migraine attacks. Headache 2001; 41 (2): 171–177. [DOI] [PubMed] [Google Scholar]
- 36.Rozen TD. Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. Headache 2003; 43 (8): 901–903. [PubMed] [Google Scholar]
- 37.Krymchantowski AV,, Barbosa JS. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia 2000; 20 (2): 107–113. [DOI] [PubMed] [Google Scholar]
- 38.MacGregor A. Migraine. In: MacGregor EA,, Frith A, eds. ABC of headache. Hoboken, NJ: BMJ/Wiley Blackwell, 2009. [Google Scholar]


