Abstract
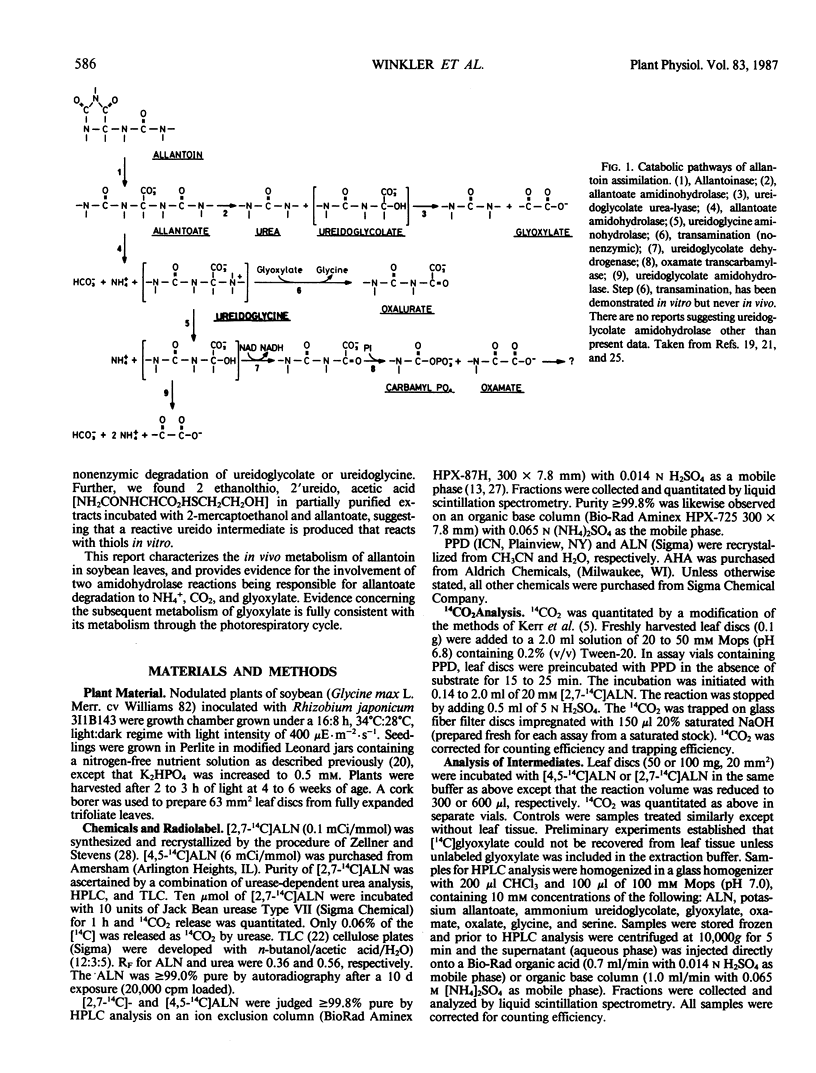
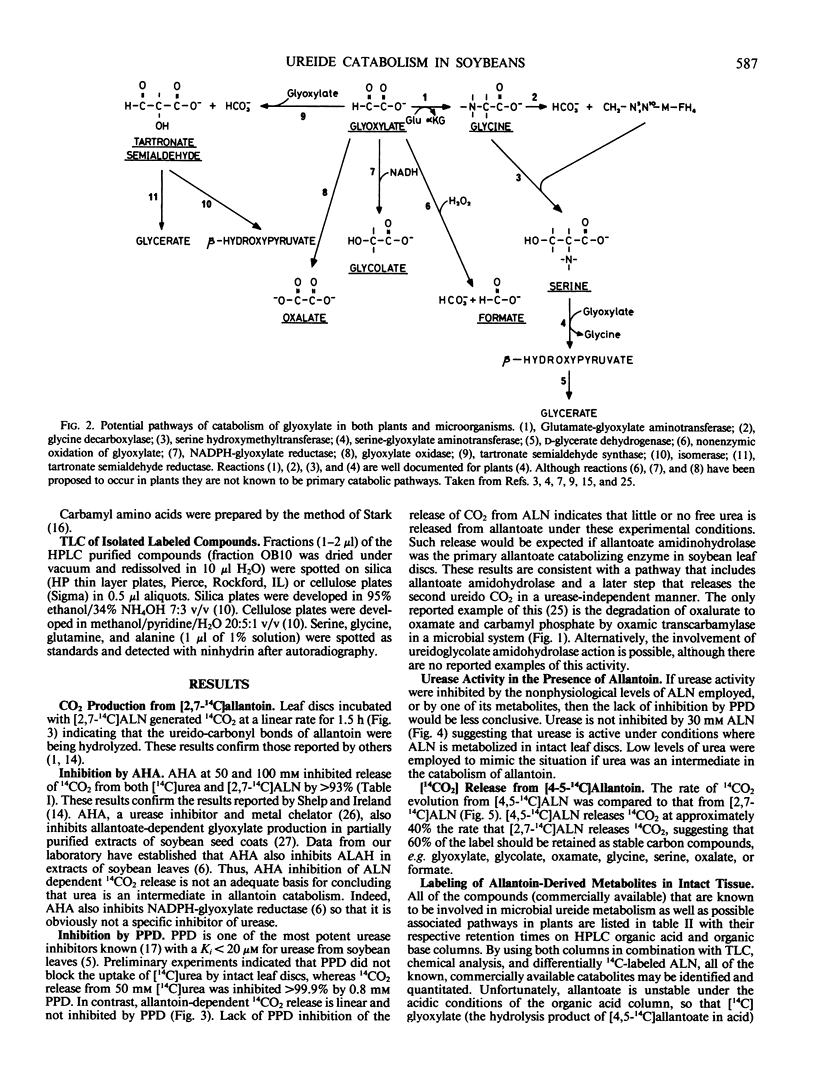
Allantoin catabolism studies have been extended to intact leaf tissue of soybean (Glycine max L. Merr.). Phenyl phosphordiamidate, one of the most potent urease inhibitors known, does not inhibit 14CO2 release from [2,7-14C]allantoin (urea labeled), but inhibits urea dependent CO2 release ≥99.9% under similar conditions. Furthermore, 14CO2 and [14C] allantoate are the only detectable products of [2,7-14C]allantoin catabolism. Neither urea nor any other product were detected by analysis on HPLC organic acid or organic base columns although urea and all commercially available metabolites that have been implicated in allantoin and glyoxylate metabolism can be resolved by a combination of these two columns. In contrast, when allantoin was labeled in the two central, nonureido carbons ([4,5-14C]allantoin), its catabolism to [14C]allantoate, 14CO2, [14C]glyoxylate, [14C]glycine, and [14C]serine in leaf discs could be detected. These data are fully consistent with the metabolism of allantoate by two amidohydrolase reactions (neither of which is urease) that occur at similar rates to release glyoxylate, which in turn is metabolized via the photorespiratory pathway. This is the first evidence that allantoate is metabolized without urease action to NH4+ and CO2 and that carbons 4 and 5 enter the photorespiratory pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Ritchie A., Peoples M. B. Metabolism and translocation of allantoin in ureide-producing grain legumes. Plant Physiol. 1982 Aug;70(2):476–482. doi: 10.1104/pp.70.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker G. T., Schaefer J. N and C NMR determination of allantoin metabolism in developing soybean cotyledons. Plant Physiol. 1985 Jan;77(1):129–135. doi: 10.1104/pp.77.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K. W., ROUSH A. H. ALLANTOINASE ASSAYS AND THEIR APPLICATION TO YEAST AND SOYBEAN ALLANTOINASES. Arch Biochem Biophys. 1964 Dec;108:460–467. doi: 10.1016/0003-9861(64)90427-8. [DOI] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Krueger R. W., Winkler R. G. Structure and possible ureide degrading function of the ubiquitous urease of soybean. Plant Physiol. 1985 Nov;79(3):794–800. doi: 10.1104/pp.79.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacco J. C., Thomas A. L., Bledsoe P. J. A soybean seed urease-null produces urease in cell culture. Plant Physiol. 1982 May;69(5):1233–1240. doi: 10.1104/pp.69.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbird R. M., Thorne J. H., Hardy R. W. Role of amides, amino acids, and ureides in the nutrition of developing soybean seeds. Plant Physiol. 1984 Feb;74(2):329–334. doi: 10.1104/pp.74.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp B. J., Ireland R. J. Ureide metabolism in leaves of nitrogen-fixing soybean plants. Plant Physiol. 1985 Mar;77(3):779–783. doi: 10.1104/pp.77.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. R., Ogren W. L. Photorespiration-deficient Mutants of Arabidopsis thaliana Lacking Mitochondrial Serine Transhydroxymethylase Activity. Plant Physiol. 1981 Apr;67(4):666–671. doi: 10.1104/pp.67.4.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. J., Schrader L. E. The Assimilation of Ureides in Shoot Tissues of Soybeans : 1. CHANGES IN ALLANTOINASE ACTIVITY AND UREIDE CONTENTS OF LEAVES AND FRUITS. Plant Physiol. 1981 May;67(5):973–976. doi: 10.1104/pp.67.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W., Blevins D. G., Randall D. D. Allantoic Acid Synthesis in Soybean Root Nodule Cytosol via Xanthine Dehydrogenase. Plant Physiol. 1980 Jun;65(6):1203–1206. doi: 10.1104/pp.65.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Glyoxylurea. Biochem Biophys Res Commun. 1961 Jul 26;5:305–308. doi: 10.1016/0006-291x(61)90168-1. [DOI] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976 Jun;40(2):403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler R. G., Polacco J. C., Blevins D. G., Randall D. D. Enzymic degradation of allantoate in developing soybeans. Plant Physiol. 1985 Nov;79(3):787–793. doi: 10.1104/pp.79.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Windt F. E., van der Drift C. Purification and some properties of hydroxypyruvate isomerase of Bacillus fastidiosus. Biochim Biophys Acta. 1980 Jun 13;613(2):556–562. doi: 10.1016/0005-2744(80)90111-4. [DOI] [PubMed] [Google Scholar]
- van der Drift C., de Windt F. E., Vogels G. D. Allantoate hydrolysis by allantoate amidohydrolase. Arch Biochem Biophys. 1970 Jan;136(1):273–279. doi: 10.1016/0003-9861(70)90351-6. [DOI] [PubMed] [Google Scholar]


