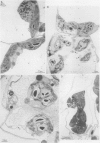
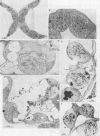
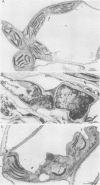
Abstract
5-[2-Chloro-4-(trifluoromethyl)phenoxy]-2-nitroacetophenone oxime-o-(acetic acid, methyl ester) (DPEI), is a potent nitrodiphenyl ether herbicide which causes rapid leaf wilting, membrane lipid peroxidation, and chlorophyll destruction in a process which is both light- and O2-dependent. These effects resemble those of other nitrodiphenyl ether herbicides. Unlike paraquat, the herbicidal effects of DPEI are only slightly reduced by pretreatment with the photosynthetic electron transport inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea. DPEI is a weak inhibitor of photosynthetic electron transport (I50 15 micromolar for water to paraquat) in vitro, with at least one site of action at the cytochrome b6f complex. Ultrastructural studies and measurements of ethane formation resulting from lipid peroxidation indicate that mutants of barley lacking photosystem I (PSI) (viridis-zb63) or photosystem II (viridis-zd69) are resistant to paraquat but susceptible to DPEI. The results indicate that electron transfer through both photosystems is not essential for the toxic effects of nitrodiphenyl ether herbicides. Furthermore, the results show that neither cyclic electron transport around PSI, nor the diversion of electrons from PSI to O2 when NADPH consumption is blocked are essential for the phytotoxicity of nitrodiphenyl ether herbicides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumelin E. E., Tappel A. L. Hydrocarbon gases produced during in vitro peroxidation of polyunsaturated fatty acids and decomposition of preformed hydroperoxides. Lipids. 1977 Nov;12(11):894–900. doi: 10.1007/BF02533308. [DOI] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Action Spectrum of the Activity of Acifluorfen-methyl, a Diphenyl Ether Herbicide, in Chlamydomonas eugametos. Plant Physiol. 1985 Feb;77(2):503–505. doi: 10.1104/pp.77.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Photosynthesis involvement in the mechanism of action of diphenyl ether herbicides. Plant Physiol. 1985 May;78(1):46–50. doi: 10.1104/pp.78.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. M. Interaction of chloroplasts with inhibitors: effects of two diphenylether herbicides, fomesafen and nitrofluorfen, on electron transport, and some comparisons with dibromothymoquinone, diuron, and paraquat. Plant Physiol. 1983 Jun;72(2):461–468. doi: 10.1104/pp.72.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettlaufer S. H., Alscher R., Strick C. Chloroplast-Diphenyl Ether Interactions II. Plant Physiol. 1985 Jun;78(2):215–220. doi: 10.1104/pp.78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M., Bendall D. S. The reduction of plastocyanin by plastoquinol-1 in the presence of chloroplasts. A dark electron transfer reaction involving components between the two photosystems. Eur J Biochem. 1976 Jan 15;61(2):337–344. doi: 10.1111/j.1432-1033.1976.tb10027.x. [DOI] [PubMed] [Google Scholar]