Abstract
Background:
The appearance of the scalp and hair is very important aesthetically regardless of age or sex. Although there are many drugs and treatment methods for scalp problems and hair loss, the treatment response is still insufficient.
Aims and Objectives:
To evaluate the efficacy of low-level light therapy in a helmet-like device.
Materials and Methods:
This study was designed as a 24-week trial with 50 participants. All participants used a helmet-shaped device emitting 630–690, 820–880, and 910–970 nm light wavelengths, for 20 minutes, daily for 24 weeks. A phototrichogram for hair density and thickness, Global Aesthetic Improvement Scale score, erythema index, and sebum secretions of the scalp were evaluated at baseline and at 12 and 24 weeks.
Results:
After 24 weeks of treatment, hair density and hair thickness were found to have significantly increased (P <.01 and P =0.013, respectively) and sebum secretion of vertex area had decreased significantly (P <.01). Of 49 participants, 73.47% of the participants showed improvement in the overall appearance of the scalp (n = 36).
Conclusion:
A helmet-like low-level light therapy device can improve the appearance of the hair, with thickening and increase in the density of the hair, and can improve scalp condition by decreasing sebum secretion.
Key Words: Hairloss, low-level light therapy, scalp
Introduction
Androgenetic alopecia is the most common type of hair loss in both males and females and can occur at any age after puberty.[1,2] The severity of androgenetic alopecia progresses gradually with age; thus, the therapeutic goal is to prevent rapid progression or stop it entirely.[3] Since hair loss is an important aesthetic factor, it can seriously damage quality of life regardless of sex, age, and severity. While many drugs have been developed for hair loss, most patients are highly concerned about the systemic side effects of these drugs,[4] and it is difficult for them to continue taking these drugs for a long time due to poor response or adverse effects. Additionally, there are many off-label topical agents and medications for hair loss, whose efficacy and safety have not been proven clinically.
Low-level light therapy (LLLT) has been used for many years in the field of dermatology to treat a variety of clinical conditions and symptoms, including wounds, pain, inflammation, and hair loss.[5,6] Depending on the wavelength of light used in LLLT, the effect may vary.[7]
LLLT has been used in various fields of dermatology, including stimulation of wound healing, reduction of inflammation, pain reduction, and skin rejuvenation.[8] The basic biological mechanism of LLLT is prolongation of the anagen phase through activation of the b-catenin/Wnt and extracellular-signal-regulated kinase signaling pathways.[9,10] A recent study has shown that LLLT also promotes protein synthesis, increases cell migration and proliferation, and modulates the growth factors and cytokine levels by changing the expression of genes such as activator protein 1, nuclear factor kappa B, and hypoxia-inducible factor 1.[11,12]
LLLT devices have been proved to promote hair growth, but the optimum wavelength has not yet been clearly identified by research yet.[13] We aimed to conduct our study with red light of 630–690 nm and infrared wavelengths of 820–880 nm and 910–970 nm. This single-arm study evaluated the efficacy and safety of a helmet-like LLLT device in the treatment of the androgenetic alopecia. We also examined sebum secretion and erythema index as secondary outcomes because previous studies have reported that LLLT inhibits sebaceous gland activity and has anti-inflammatory effect.[14,15]
Materials and Methods
Study design
A 24-week, prospective, single-arm clinical trial was conducted at Seoul, Korea. The study protocol was approved by our Institutional Review Board, and informed consent was obtained from all participants.
Healthy adult males and females interested in improving the appearance of their scalp and hair were recruited as participants. They were screened to verify that they met the inclusion criteria for the study. This study included men and women aged 19 to 65 years with androgenetic alopecia based on the Norwood-Hamilton classification for men and Ludwig classification for women. The patients were required to maintain the same hair color and style and to avoid using hair products that could affect the scalp or hair growth during clinical trial period. We excluded individuals who had used topical or systemic medications affecting hair growth, such as steroids, finasteride, cyclosporine, or minoxidil, within the previous 6 months or who had hair disorders other than androgenetic alopecia that might have affected hair growth.
The device used was a helmet-shaped machine emitting 1.3 mW/cm2 of power at wavelengths of 630–690, 820–880, and 910–970 nm, and the number of diodes for each wavelength was 240, which made up a total of 720 diodes [Figure 1]. The participants used this device at home, and the device turned off automatically 20 minutes after treatment.
Figure 1.

A helmet-shaped LLLT device containing 720 diodes
Efficacy assessments
The primary end points were changes from baseline in hair density and thickness on the vertex of the scalp at 12 and 24 weeks. Using a phototrichogram (Folliscope, LeadM Corporation, Seoul, Korea), a virtual circle with an area of 1 cm2 was drawn on the vertex of the scalp. The number and thickness of hair strands whose follicle was within this area were measured. The global assessment of hair and scalp were scored with Global Aesthetic Improvement Scale by the participants and the investigators, and the data on subjective satisfaction experienced by the participants were also collected [Table 1].
Table 1.
Questionnaire about global assessment of hair and scalp, scored with global aesthetic improvement scale by the participants and the investigators
| Assessments | Global aesthetic improvement scale* (-3~3) |
|---|---|
| By investigators | |
| Global photographic assessment | |
| By participants | |
| Overall appearance of the hair and scalp | |
| Changes in the richness of the hair | |
| Changes in the number of dropout hair strands | |
| Changes in hair thickness | |
| Changes in hair growth rate |
*Global aesthetic assessment scale points; -3: severe deterioration, -2: moderate deterioration, -1: mild deterioration, 0: no change, +1: mild improvement, +2: moderate improvement, +3: marked improvement
Biophysical measurements were done before treatment (baseline) and at 12 and 24 weeks of treatment. A combination of corneometer/mexameter/reviscometer/sebumeter device, MPA5 (Courage and Khazaka, Cologne, Germany), was used to measure skin melanin indices, erythema indices, and sebum secretion. Each parameter was measured three times, and the mean value was used in the analysis.
Safety assessments
Safety assessments including vital signs and adverse events were observed by the investigators and reported by the participants at all three visits (baseline, week 12, and week 24).
Statistical methods
The nonparametric paired Wilcoxon test and Friedman test were used to compare the changes in hair density, thickness, erythema index, sebum secretion, and global assessment of hair appearance score. The analysis was performed using the SPSS version 17.0 software for Windows (SPSS, Chicago, IL, USA). The cut-off for statistical significance was set at a P value <.05.
Results
Participants
In total, 50 participants were enrolled. One participant was excluded from the study due to the contraction of coronavirus disease-19. Overall, 49 participants completed the study, of which 51.02% (n = 25) were males and 48.98% (n = 24) were females. The mean age was 44.5 ± 11.9 years. Hair density, thickness, sebum secretion, melanin index, and erythema index before the treatment are shown in Table 2.
Table 2.
Baseline characteristics of participants
| Baseline characteristics | Overall population (n=49) | |
|---|---|---|
| Patients, n | 49 | |
| male, n (%) | 25 (51.02%) | |
| Nordwood-Hamilton classification, n | ||
| I | 5 | |
| II | 5 | |
| III` | 10 | |
| IV | 1 | |
| V | 2 | |
| VI | 2 | |
| female, n (%) | 24 (48.98%) | |
| Ludwig classification | ||
| I | 23 | |
| II | 0 | |
| III | 1 | |
| Age | 44.5±11.9 | |
| Hair density/cm², mean ± SD | 117.67±28.21 | |
| Hair thickness, µm, mean ± SD | 48.96±9.56 | |
| Frontal | ||
| Sebum secretion, mean ± SD | 46.51±55.29 | |
| Melanin index, mean ± SD | 281.04±77.00 | |
| Erythema index, mean ± SD | 177.18±77.31 | |
| Vertex | ||
| Sebum secretion, mean ± SD | 43.61±54.85 | |
| Melanin index, mean ± SD | 297.04±76.09 | |
| Erythema index, mean ± SD | 164.73±73.50 |
The correlations between the participant's age at enrollment, the thickness of the hair, the number of hair strands, erythema index, and sebum secretion were assessed. Older participants were found to have significantly fewer number of hair strands (correlation coefficient -0.433, P =0.002) and significantly thinner hair (correlation coefficient -0.438, P =0.002). They were also found to have higher erythema indices in both the frontal and vertex areas (correlation coefficient 0.419, P =.003; correlation coefficient 0.415, P =.003, respectively). There was no relationship between age and amount of sebum secretion and also none between the amount of sebum secretion and erythema.
Compliance evaluation
At each visit, participants submitted a questionnaire that mentioned the date on which the machine was actually used for 20 minutes. The following formula was used to calculate compliance, and the results are shown in Table 3.
Table 3.
Treatment compliance
| Compliance (%) | Results | |
|---|---|---|
| Total | N | 49 |
| Mean±SD | 94.52±3.21 | |
| Min-Max | 83.83-97.14 |
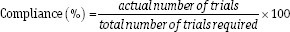
The total number of trials required = the number of days between last visit date (the machine return date) and initial visit date (the machine received date).
Safety
None of the participants experienced serious adverse effects including severe pain, allergic reaction, and hair loss during or after the study.
Improvement score
The participants were surveyed for overall satisfaction with the device through a questionnaire at the 24-week visit. There were five survey items, including the overall appearance of the hair and scalp, the changes in the richness of the hair, the number of dropout hair strands, hair thickness, and hair growth rate, which were evaluated subjectively in seven-graded scale. Of 49 participants, 73.47% of the participants showed improvement in the overall appearance of the scalp (n = 36), 61.22% showed an increase in hair richness (n = 30), 65.31% showed a decrease in the number of dropout hairs (n = 32), 59.18% showed an increase in hair thickness (n = 29), and 67.35% showed an increase in hair growth rate. The representative photographs taken of three participants are shown in Figure 2.
Figure 2.
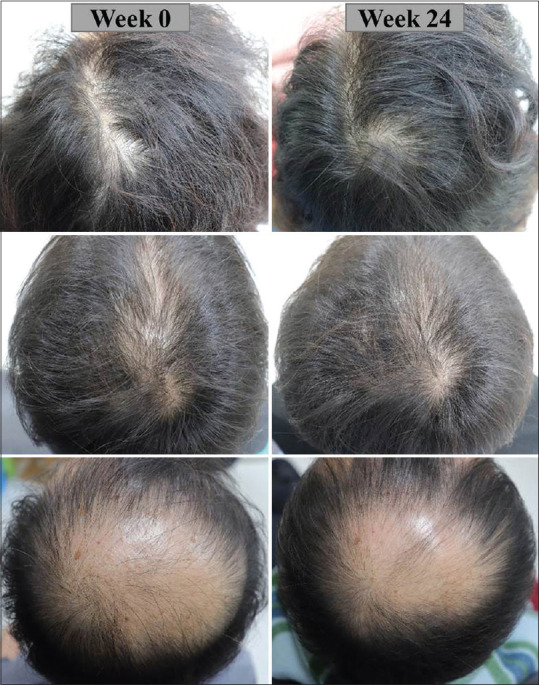
Baseline and 24-week global photographs of 3 participants are shown
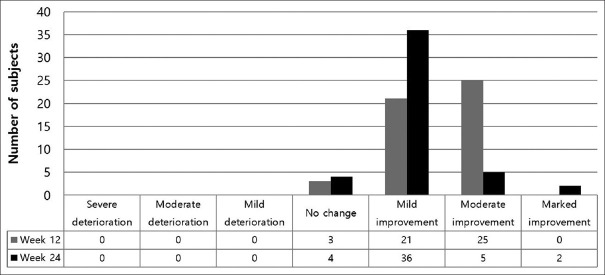
At the 12-week visit, the investigator's evaluation showed that 46 participants (93.55%) showed an overall improvement in hair condition compared to the baseline, and at the 24-week visit, 45 participants (91.84%) showed overall improvement compared to the previous visit. There was no decrease in participant number at each visit, and the number of cases with no changes in hair were three (6.12%) at the 12-week visit and four (8.16%) at the 24-week visit. Details of the investigator's evaluation are shown in Figure 3.
Figure 3.
Assessment of global aesthetic improvement by investigators at week 12 and 24 is shown
Biophysical assessments
Hair density in the vertex area increased significantly between baseline and at week 12 and between week 12 and 24 (P <.01 and P <.01, respectively) [Figure 4]. Hair thickness measured in the vertex area increased significantly during these periods as well (P <.01 and P =0.013, respectively) [Figure 5]. The sebum index decreased significantly in vertex area of the scalp throughout the 24-week study period (P <.01). However, the melanin index and erythema index did not differ significantly during the study period.
Figure 4.
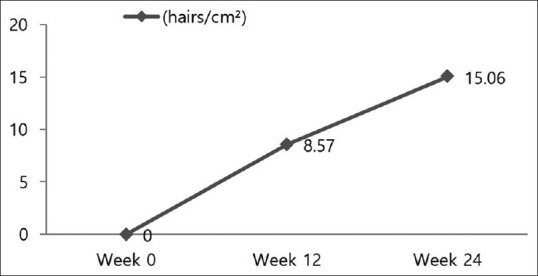
Mean change from baseline in hair density (P <.01 and P <.01, respectively) is shown at the two time points
Figure 5.
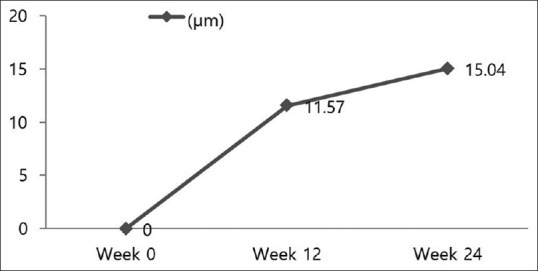
Mean change from baseline in hair diameter (P <.01 and P =0.013, respectively) is shown at the two time points
Discussion
We used a helmet-shaped device emitting 630–690, 820–880, and 910–970 nm wavelengths. Many studies have shown that the 600–700 nm wavelength range has an effect on hair growth effect; however, another study showed that LLLT at 830 nm showed greater efficacy than other wavelengths (632, 670, and 785 nm).[13,16,17] Among the current medical devices, LLLT is conducted with 600–700 nm wavelengths, but there are relatively fewer machines that emit wavelengths more than 800 nm. Based on this, we tried to maximize the effect of hair growth by including these three different and broad ranges of wavelengths. We selected device emitting 630–690 and 820–880 nm wavelengths based on previous studies, and we believe that deeper penetration of longer-wavelength light played an important role in facilitating the proliferation of hair follicle cells, we added 910-970 nm wavelengths. However, it was not possible to compare the effects of each wavelength. Therefore, further in depth research is warranted to understand the optimal combination of various wavelengths.
Patients can easily use this helmet-shaped machine at home. Since the machine is automatically turned off after 20 minutes of use, the risk of over-use is prevented, and since it is helmet-shaped, there is little concern about ocular damage owing to light exposure. There were some complaints of mild headache (8.2%, n = 4) and itching (4.1%, n = 2) due to the use of the helmet, but there were no complaints of severe discomfort that could interfere with its use.
When we compared the response of males and females to LLLT, the results revealed no statistically significant differences with respect to changes in hair density and diameter and rating scores for hair regrowth.
Analyzing the age of participants, the older ones had thinner hair, fewer number of hair strands, and a higher erythema index. There was no correlation between erythema index and sebum secretion and none between age and sebum secretion. A previous study showed not only that the number and depth of sebaceous units were decreased in the elderly but also that factors such as sex, diet, race, and environment affected the amount of sebum secretion.[18]
The strength of our study is that it is the first to demonstrate how LLLT has other effects on the physiological changes of the scalp, such as sebum secretion, melanin, and erythema index. We found no change in erythema index, but sebum secretion was significantly decreased compared to that before using the LLLT device. One of the important mechanisms of LLLT is the Wnt signaling pathway, which promotes differentiation of follicular cell leading hair growth. In addition, Wnt signaling inhibits differentiation of preadipocytes, which leads to decreased activity of sebaceous gland. This study found that LLLT can help prevent excessive sebum secretion. This study was targeted at people without scalp disease, and it is thought that if the test is performed on a group of participants with seborrheic dermatitis, the effect on the sebum secretion or skin erythema treatment will be better known.
There are some limitations to our study. First, it was a single-arm study, and there was no control group using the same device. There is an inability to distinguish between the effect of the treatment and the natural course of hair change. And it is also difficult to interpret the response without a treatment of reference for comparison. However, since the results were comparatively analyzed including objective numerical methods, we consider the above shortcomings were partially compensated. Second, we have confirmed that the treatment is effective for 24 weeks, but it would be necessary to elaborate on the stability and effectiveness evaluation through a longer study. Third, the hair color needs to be considered because the amount of light reflected and absorbed may vary depending on the color of the participant's hair, and we did not properly consider the effect of hair color.
Additionally, to understand the effects of LLLT on seborrheic dermatitis, scalp erythema, and sebum secretion, patients with conditions such as seborrheic dermatitis should be studied in the future. Moreover, the change in sebum secretion can differ depending on the time the participants wash their hair before the sebum examination, and the seasonal effect also should be considered. Finally, although participants did not have long-term follow-up, after discontinuing use of the device, analysis of how long the effect of using the device lasted and whether hair loss began again after discontinuation is difficult.
In conclusion, this novel helmet-shaped LLLT device is an effective and well-tolerated treatment option for androgenetic alopecia and prevents excessive sebum secretion of the scalp regardless of age and sex. LLLT can be used as an alternative for patients who respond poorly to pharmacological treatments or refuse to receive pharmacological treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by Cell-return, Seoul, Republic of Korea.
References
- 1.Yeo IK, Jang WS, Min PK, Cho HR, Cho SW, Hong NS, et al. An epidemiological study of androgenic alopecia in 3114 Korean patients. Clin Exp Dermatol. 2014;39:25–9. doi: 10.1111/ced.12229. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues M, Antunes I, Magalhaes S, Pereira N. Androgenic alopecia: An entity to consider in adolescence. BMJ Case Rep. 2017;2017:bcr2017220679. doi: 10.1136/bcr-2017-220679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElwee KJ, Shapiro JS. Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 4.Khera M, Than JK, Anaissie J, Antar A, Song W, Losso B, et al. Penile vascular abnormalities in young men with persistent side effects after finasteride use for the treatment of androgenic alopecia. Transl Androl Urol. 2020;9:1201–9. doi: 10.21037/tau.2020.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashmi JT, Huang Y-Y, Sharma SK, Kurup DB, Taboada LD, Carroll JD, et al. Effect of pulsing in low-level light therapy. Lasers Surg Med. 2010;42:450–66. doi: 10.1002/lsm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Dai T, Hamblin MR. Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci. 2014;29:257–65. doi: 10.1007/s10103-013-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figurova M, Ledecky V, Karasova M, Hluchy M, Trbolova A, Capik I, et al. Histological assessment of a combined low-level laser/light-emitting diode therapy (685 nm/470 nm) for sutured skin incisions in a porcine model: A short report. Photomed Laser Surg. 2016;34:53–5. doi: 10.1089/pho.2015.4013. [DOI] [PubMed] [Google Scholar]
- 8.Moskvin SV. Low-level laser therapy and light energy. Photobiomodul Photomed Laser Surg. 2019;37:267–8. doi: 10.1089/photob.2019.4622. [DOI] [PubMed] [Google Scholar]
- 9.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JE, Woo YJ, Sohn KM, Jeong KH, Kang H. Wnt/beta-catenin and ERK pathway activation: A possible mechanism of photobiomodulation therapy with light-emitting diodes that regulate the proliferation of human outer root sheath cells. Lasers Surg Med. 2017;49:940–7. doi: 10.1002/lsm.22736. [DOI] [PubMed] [Google Scholar]
- 11.Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MVP, Bagnato VS. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem Photobiol. 2015;91:411–6. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer AP. Revisiting the photon/cell interaction mechanism in low-level light therapy. Photobiomodul Photomed Laser Surg. 2019;37:336–41. doi: 10.1089/photob.2018.4606. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Qu Q, Chen J, Miao Y, Hu Z. Proposed mechanisms of low-level light therapy in the treatment of androgenetic alopecia. Lasers Med Sci. 2021;36:703–13. doi: 10.1007/s10103-020-03159-z. [DOI] [PubMed] [Google Scholar]
- 14.Kwon HH, Lee JB, Yoon JY, Park SY, Ryu HH, Park BM, et al. The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: A double-blind, randomized controlled trial. Br J Dermatol. 2013;168:1088–94. doi: 10.1111/bjd.12186. [DOI] [PubMed] [Google Scholar]
- 15.Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–33. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mai-Yi Fan S, Cheng YP, Lee MY, Lin SJ, Chiu HY. Efficacy and safety of a low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, self-comparison, sham device-controlled trial. Dermatol Surg. 2018;44:1411–20. doi: 10.1097/DSS.0000000000001577. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Choi JW, Kim JY, Shin JW, Lee S-J, Huh C-H. Low-level light therapy for androgenetic alopecia: A 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177–83. doi: 10.1111/dsu.12200. [DOI] [PubMed] [Google Scholar]
- 18.Pappas A, Fantasia J, Chen T. Age and ethnic variations in sebaceous lipids. Dermatoendocrinol. 2013;5:319–24. doi: 10.4161/derm.25366. [DOI] [PMC free article] [PubMed] [Google Scholar]