Abstract
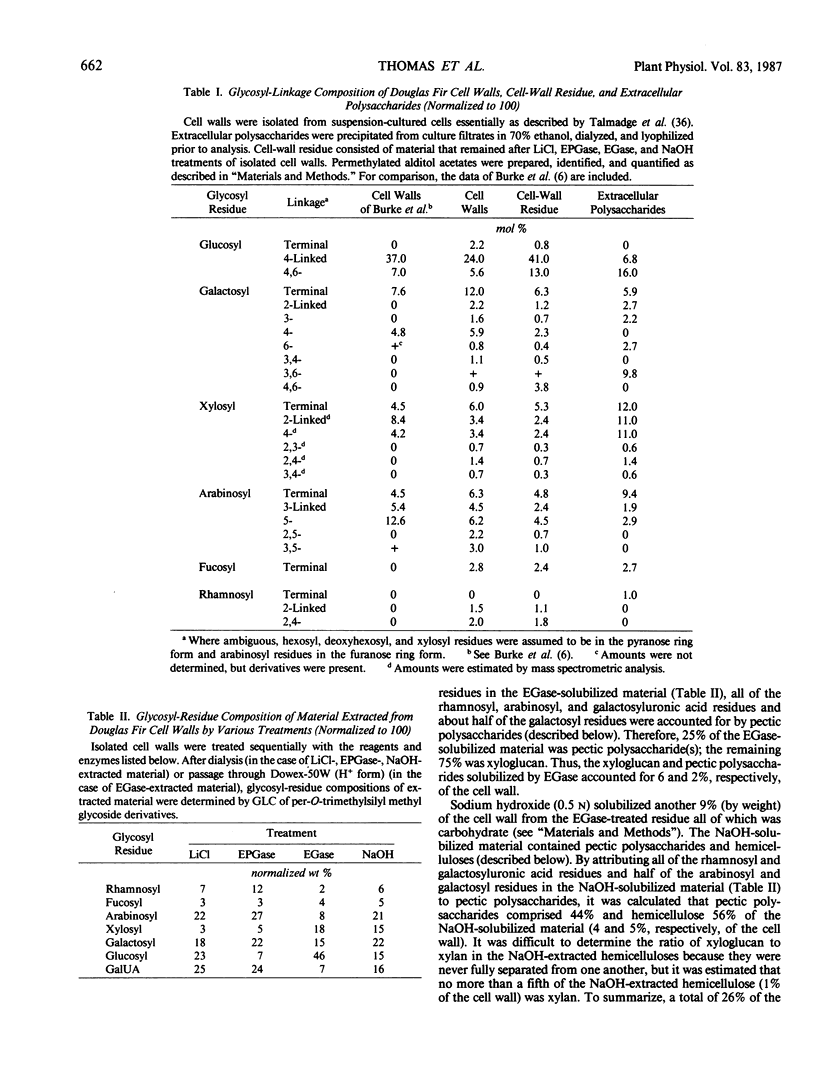
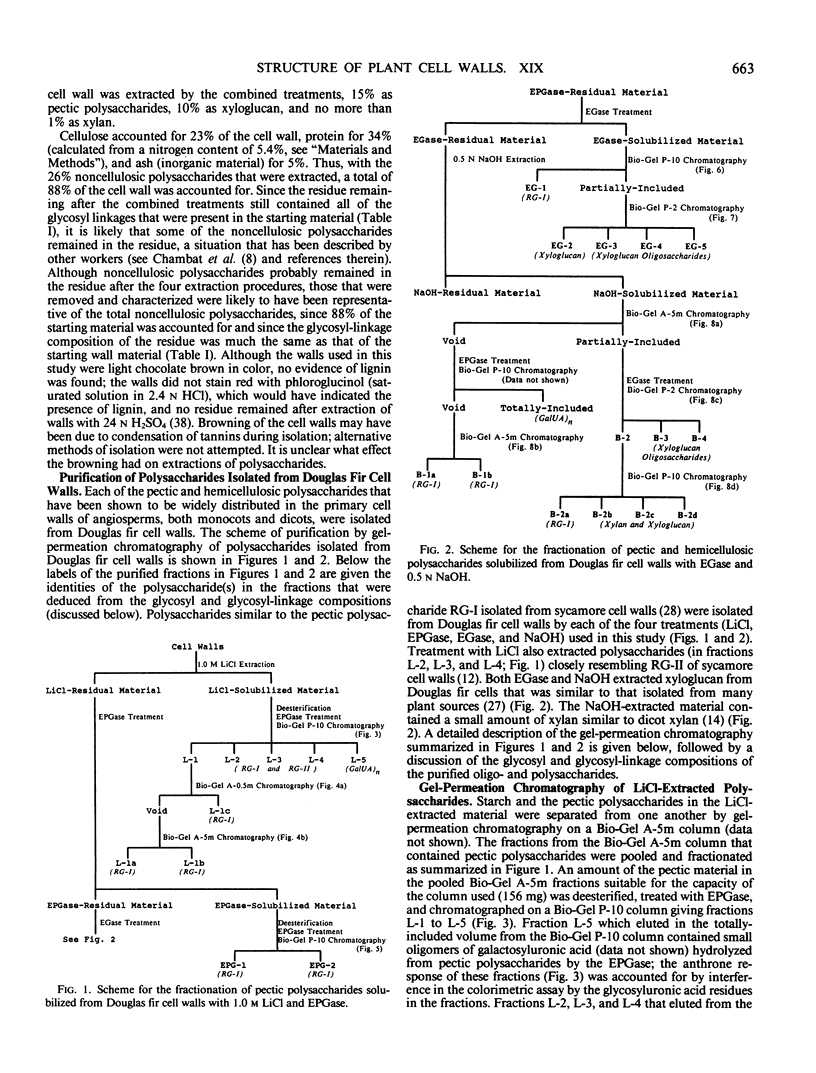
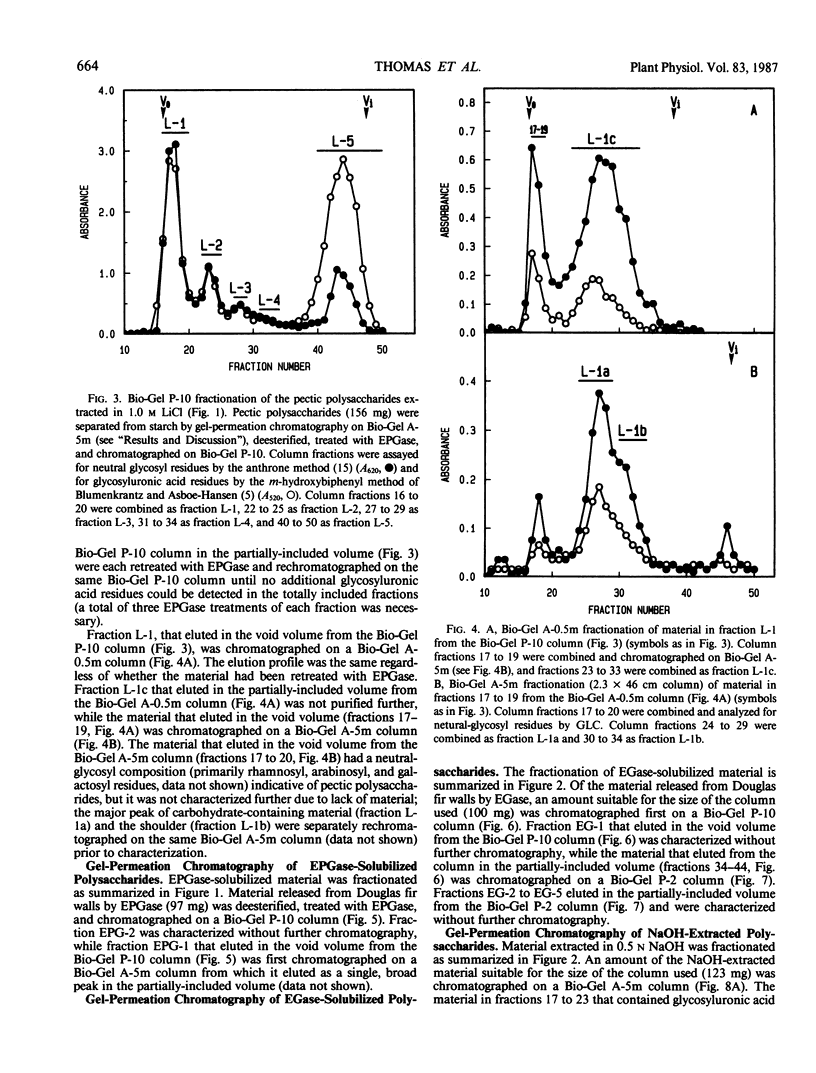
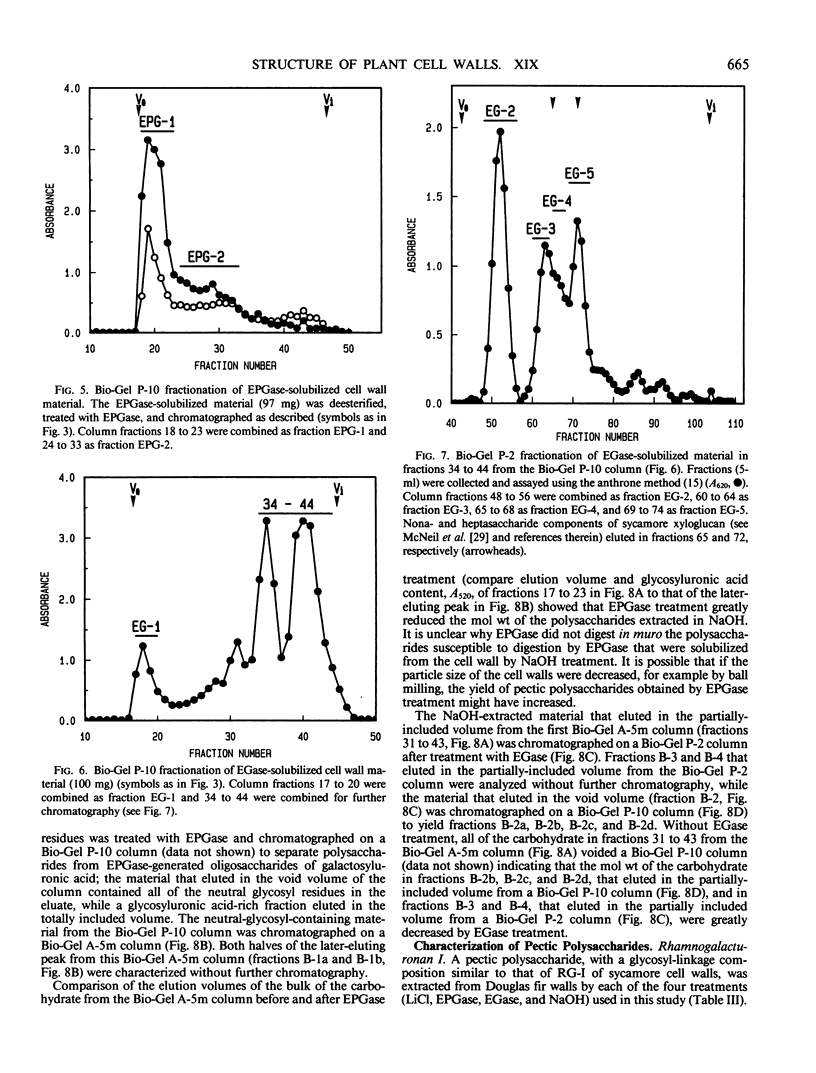
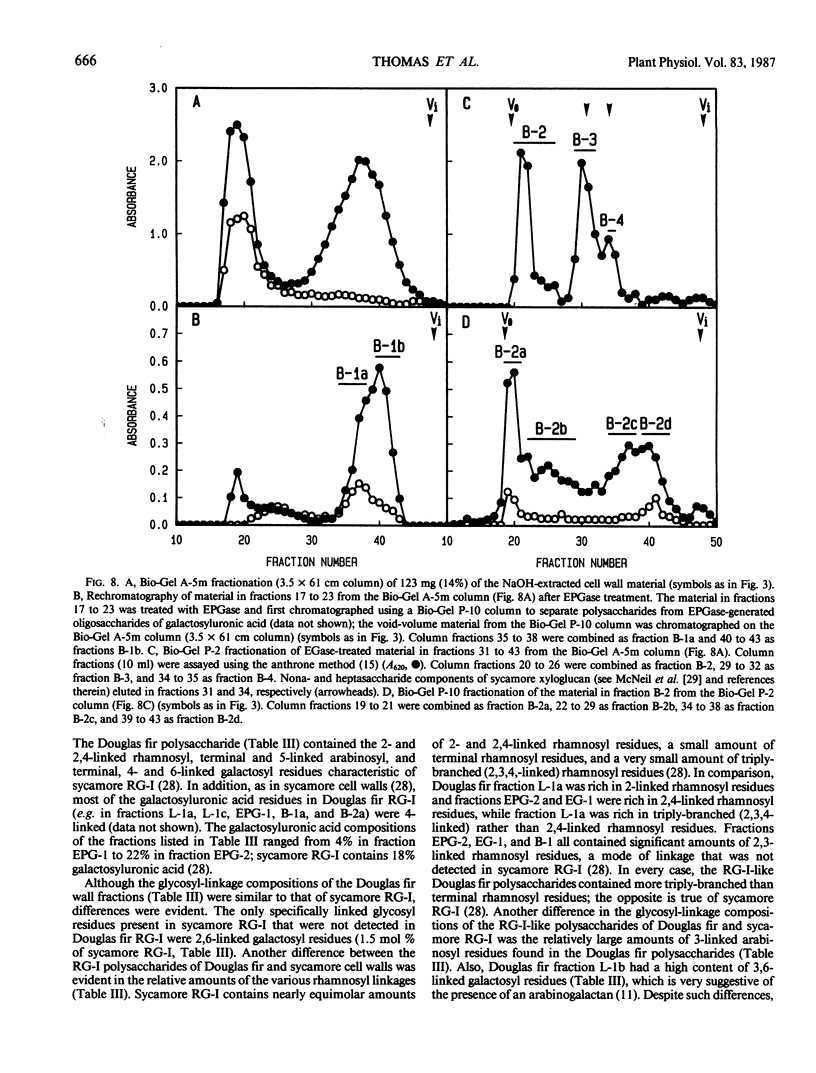
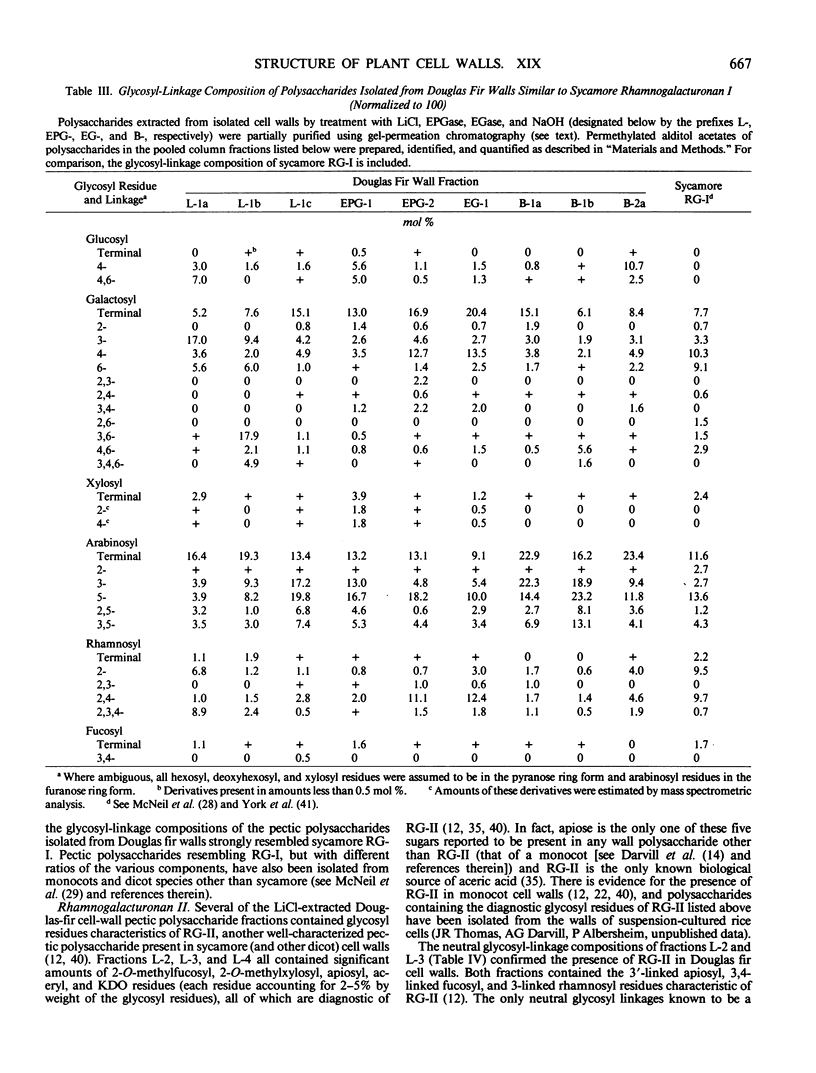
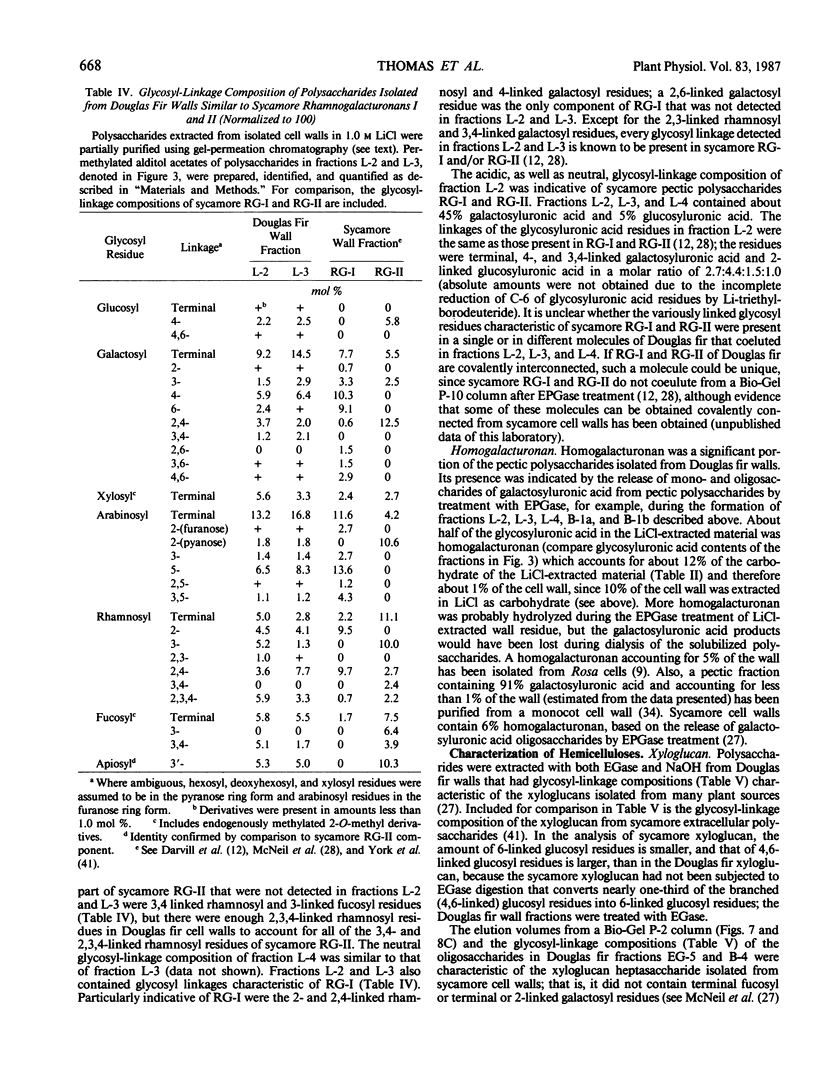
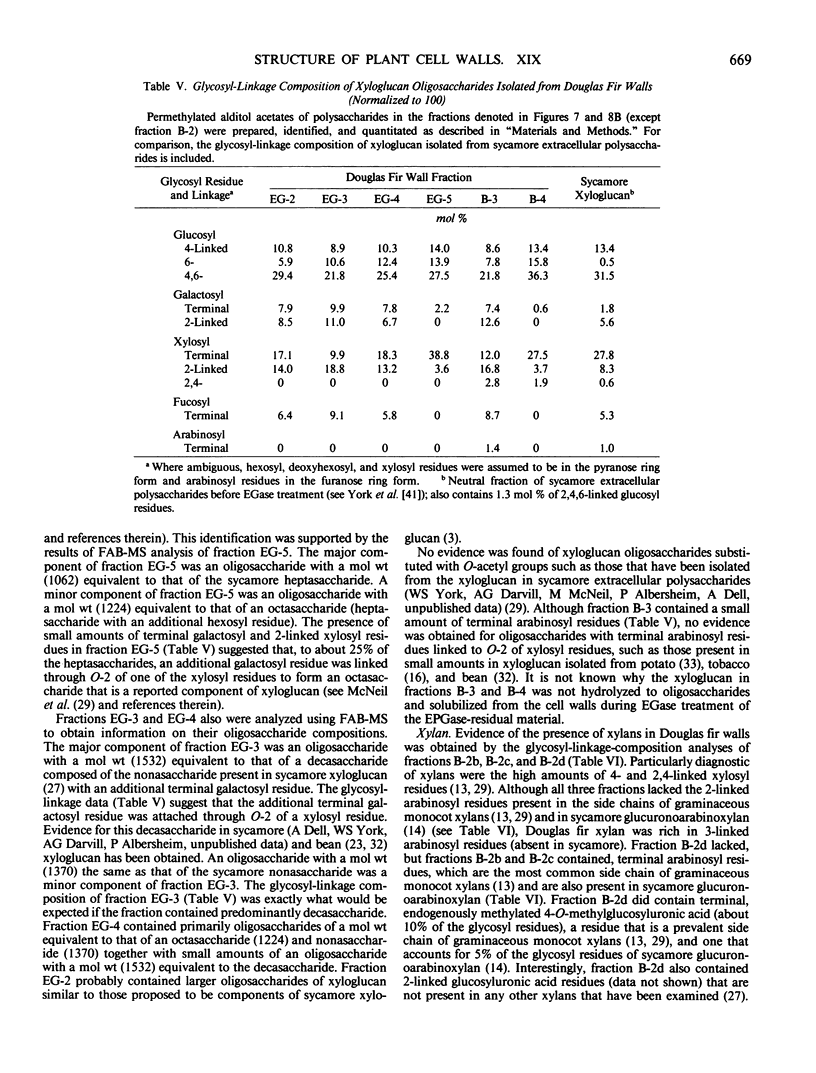
The partial purification and characterization of cell wall polysaccharides isolated from suspension-cultured Douglas fir (Pseudotsuga menziesii) cells are described. Extraction of isolated cell walls with 1.0 m LiCl solubilized pectic polysaccharides with glycosyl-linkage compositions similar to those of rhamnogalacturonans I and II, pectic polysaccharides isolated from walls of suspension-cultured sycamore cells. Treatment of LiCl-extracted Douglas fir walls with an endo-α-1,4-polygalacturonase released only small, additional amounts of pectic polysaccharide, which had a glycosyl-linkage composition similar to that of rhamnogalacturonan I. Xyloglucan oligosaccharides were released from the endo-α-1,4-polygalacturonase-treated walls by treatment with an endo-β-1,4-glucanase. These oligosaccharides included hepta- and nonasaccharides similar or identical to those released from sycamore cell walls by the same enzyme, and structurally related octa- and decasaccharides similar to those isolated from various angiosperms. Finally, additional xyloglucan and small amounts of xylan were extracted from the endo-β-1,4-glucanase-treated walls by 0.5 n NaOH. The xylan resembled that extracted by NaOH from dicot cell walls in that it contained 2,4- but not 3,4-linked xylosyl residues. In this study, a total of 15% of the cell wall was isolated as pectic material, 10% as xyloglucan, and less than 1% as xylan. The noncellulosic polysaccharides accounted for 26% of the cell walls, cellulose for 23%, protein for 34%, and ash for 5%, for a total of 88% of the cell wall. The cell walls of Douglas fir were more similar to dicot (sycamore) cell walls than to those of graminaceous monocots, because they had a predominance of xyloglucan over xylan as the principle hemicellulose and because they possessed relatively large amounts of rhamnogalacturonan-like pectic polysaccharides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Hemicellulosic polymers of cell walls of zea coleoptiles. Plant Physiol. 1983 Jun;72(2):515–521. doi: 10.1104/pp.72.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambat G., Barnoud F., Joseleau J. P. Structure of the Primary Cell Walls of Suspension-Cultured Rosa glauca Cells: I. Polysaccharides Associated with Cellulose. Plant Physiol. 1984 Mar;74(3):687–693. doi: 10.1104/pp.74.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill J. E., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: XI. GLUCURONOARABINOXYLAN, A SECOND HEMICELLULOSE IN THE PRIMARY CELL WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P. D., Maglothin A., Keegstra K., Albersheim P. A Cell Wall-degrading Endopolygalacturonase Secreted by Colletotrichum lindemuthianum. Plant Physiol. 1972 Mar;49(3):293–298. doi: 10.1104/pp.49.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. Preparation and Properties of a beta-d-Glucanase for the Specific Hydrolysis of beta-d-Glucans. Plant Physiol. 1977 Aug;60(2):300–304. doi: 10.1104/pp.60.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson U., Fägerstam L., Pettersson G., Andersson L. Purification and characterization of a low molecular weight 1,4-beta-glucan glucanohydrolase from the cellulolytic fungus Trichoderma viride QM 9414. Biochim Biophys Acta. 1978 Jun 9;524(2):385–392. doi: 10.1016/0005-2744(78)90175-4. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: X. RHAMNOGALACTURONAN I, A STRUCTURALLY COMPLEX PECTIC POLYSACCHARIDE IN THE WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1128–1134. doi: 10.1104/pp.66.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- THORNBER J. P., NORTHCOTE D. H. Changes in the chemical composition of a cambial cell during its differentiation into xylem and phloem tissue in trees. 3. Xylan, glucomannan and alpha-cellulose fractions. Biochem J. 1962 Feb;82:340–346. doi: 10.1042/bj0820340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]


