Abstract
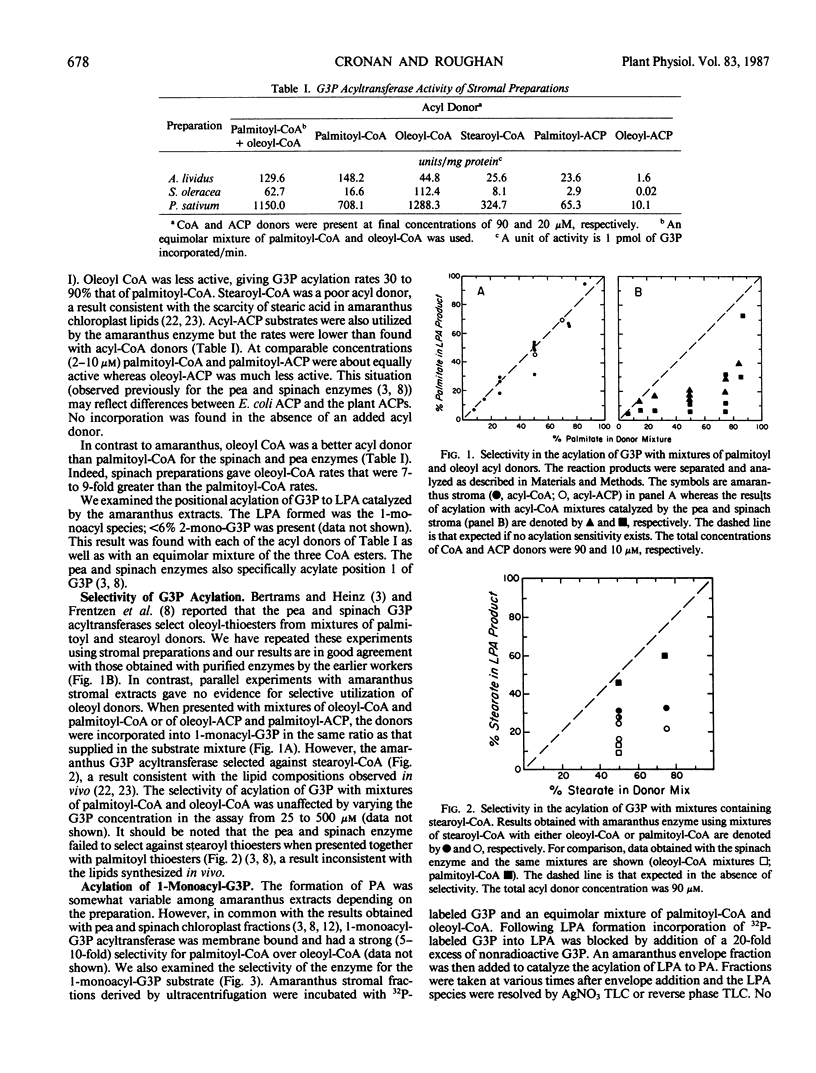
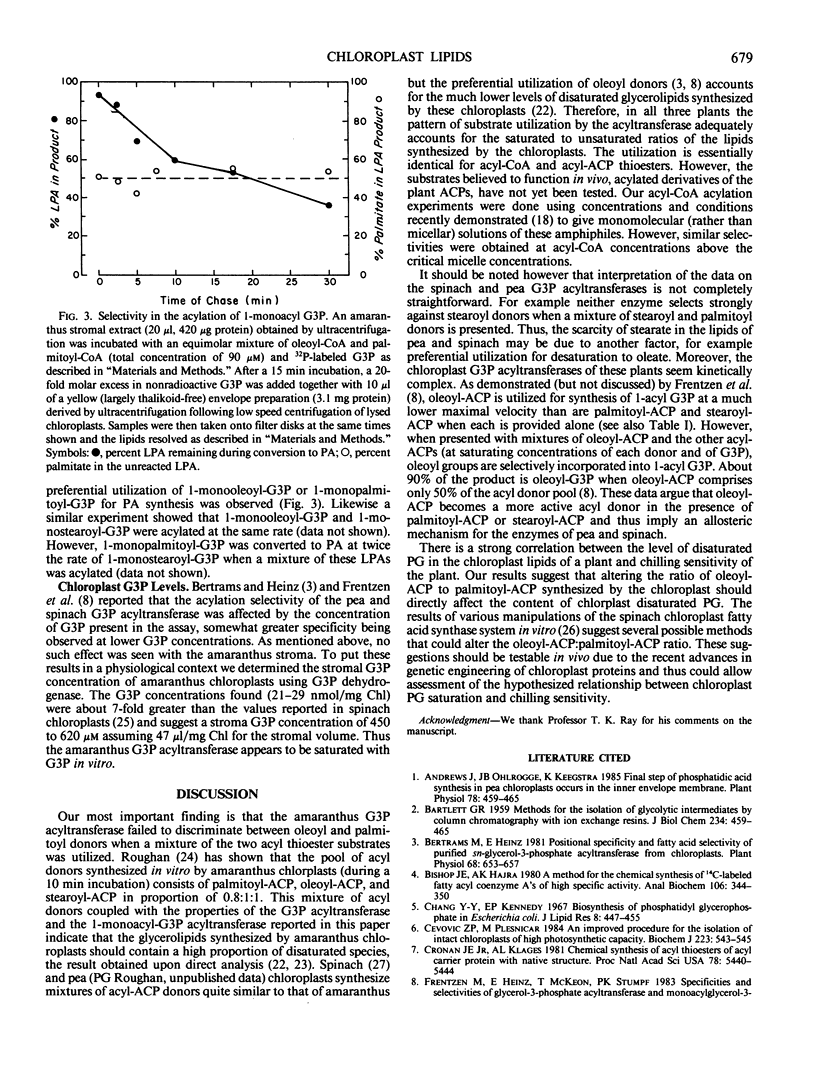
Chilling sensitivity of plants is strongly correlated with the presence of high levels of a species of chloroplast phosphatidylglycerol that contains two saturated fatty acids. The most straightforward synthetic pathway for this lipid would require the primary acylation of sn-glycerol 3-phosphate (G3P) with a saturated fatty acid (palmitic acid) rather than with oleic acid, an unsaturated acid. This selective incorporation would differ markedly from the reported properties of the chloroplast G3P acyltransferases of pea and spinach, two chilling resistant plants and thus we have studied the chloroplast G3P acyltransferase of Amaranthus lividus, a chilling sensitive plant. In contrast to our results and those of others (M. Frentzen et al. 1983 Eur J Biochem 129: 629-636 and previous work) with the pea and spinach enzymes, the amaranthus chloroplast G3P acyltranferase did not select oleic acid donors from a mixture of oleic and palmitic acid donors (either coenzyme A or acyl carrier protein thioesters). Instead the fatty acid composition of the synthesized 1-acyl G3P faithfully reflected the composition of the acyl donor mixture. However, the amaranthus enzyme did strongly select against incorporation of stearic acid. The properties of the amaranthus G3P acyltransferase are consistent with this enzyme having the major role in synthesis of the disaturated phosphatidylglycerol species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gardiner S. E., Heinz E., Roughan P. G. Rates and products of long-chain Fatty Acid synthesis from [1-C]acetate in chloroplasts isolated from leaves of 16:3 and 18:3 plants. Plant Physiol. 1984 Apr;74(4):890–896. doi: 10.1104/pp.74.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A., Yoshimura T., Okuda S. A new method for the preparation of acyl-CoA thioesters. J Biochem. 1981 Feb;89(2):337–339. doi: 10.1093/oxfordjournals.jbchem.a133207. [DOI] [PubMed] [Google Scholar]
- Murata N., Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984 Apr;74(4):1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell G. L., Grothusen J. R., Zimmerman J. K., Evans C. A., Fish W. W. A re-examination of some properties of fatty acyl-CoA micelles. J Biol Chem. 1981 Dec 25;256(24):12740–12747. [PubMed] [Google Scholar]
- Renkonen O. Mono- and dimethyl phosphatidates from different subtypes of choline and ethanolamine glycerophosphatides. Biochim Biophys Acta. 1968 Jan 10;152(1):114–135. doi: 10.1016/0005-2760(68)90014-3. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Acyl carrier protein from Escherichia coli. Methods Enzymol. 1981;71(Pt 100):341–351. doi: 10.1016/0076-6879(81)71043-7. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Garwin J. L., Cronan J. E., Jr Preparative enzymatic synthesis of acyl-acyl carrier protein. Methods Enzymol. 1981;72:397–403. doi: 10.1016/s0076-6879(81)72029-9. [DOI] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 1985 Mar;77(3):740–746. doi: 10.1104/pp.77.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The purification and function of acetyl coenzyme A:acyl carrier protein transacylase. J Biol Chem. 1983 Mar 25;258(6):3592–3598. [PubMed] [Google Scholar]
- Spencer A. K., Greenspan A. D., Cronan J. E., Jr Thioesterases I and II of Escherichia coli. Hydrolysis of native acyl-acyl carrier protein thioesters. J Biol Chem. 1978 Sep 10;253(17):5922–5926. [PubMed] [Google Scholar]


