Abstract
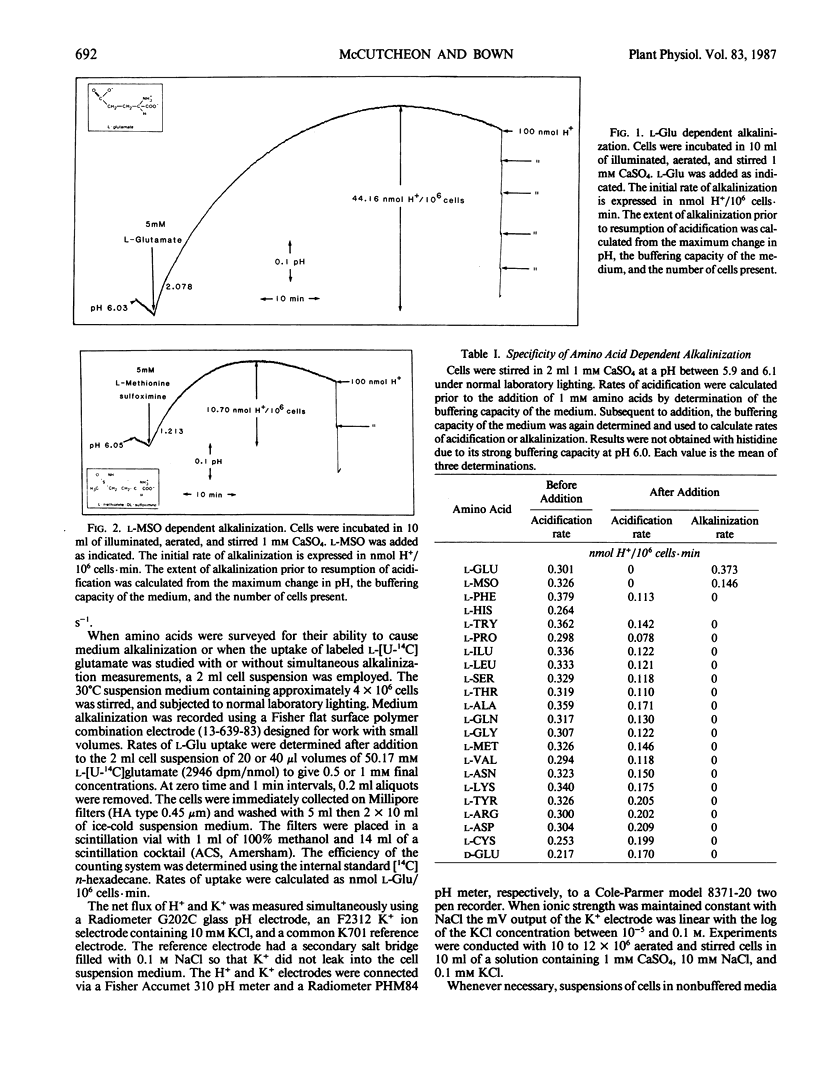
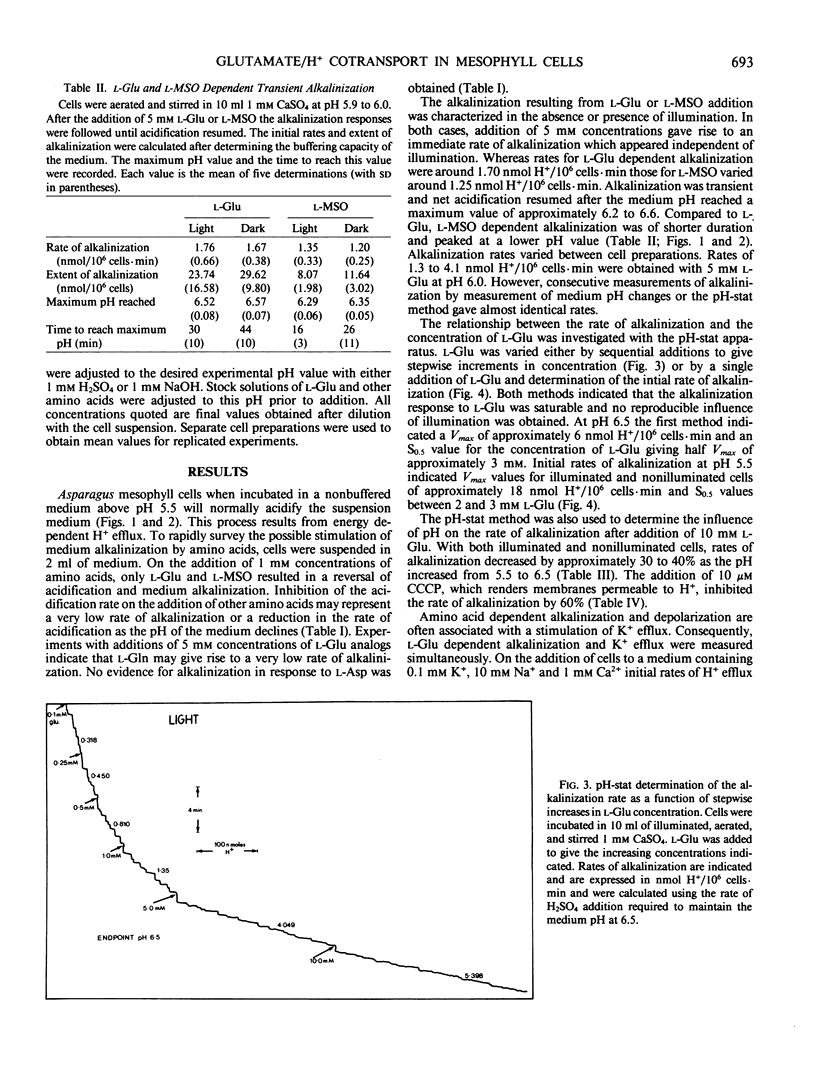
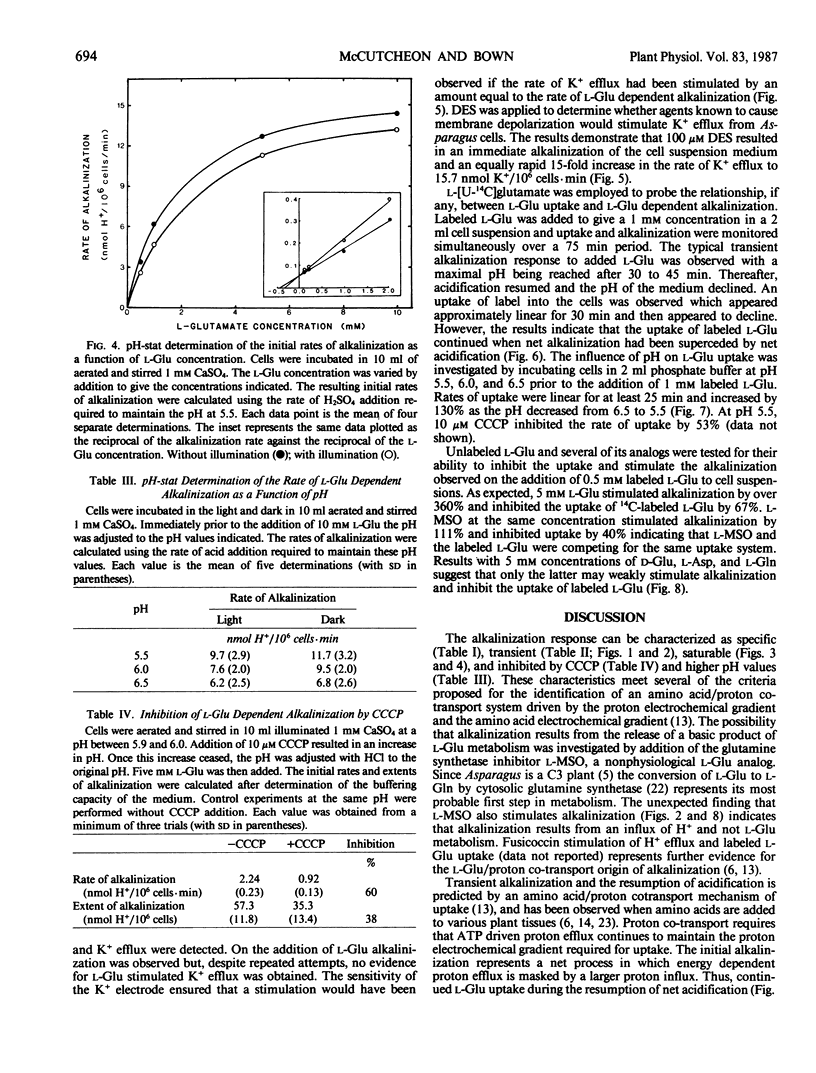
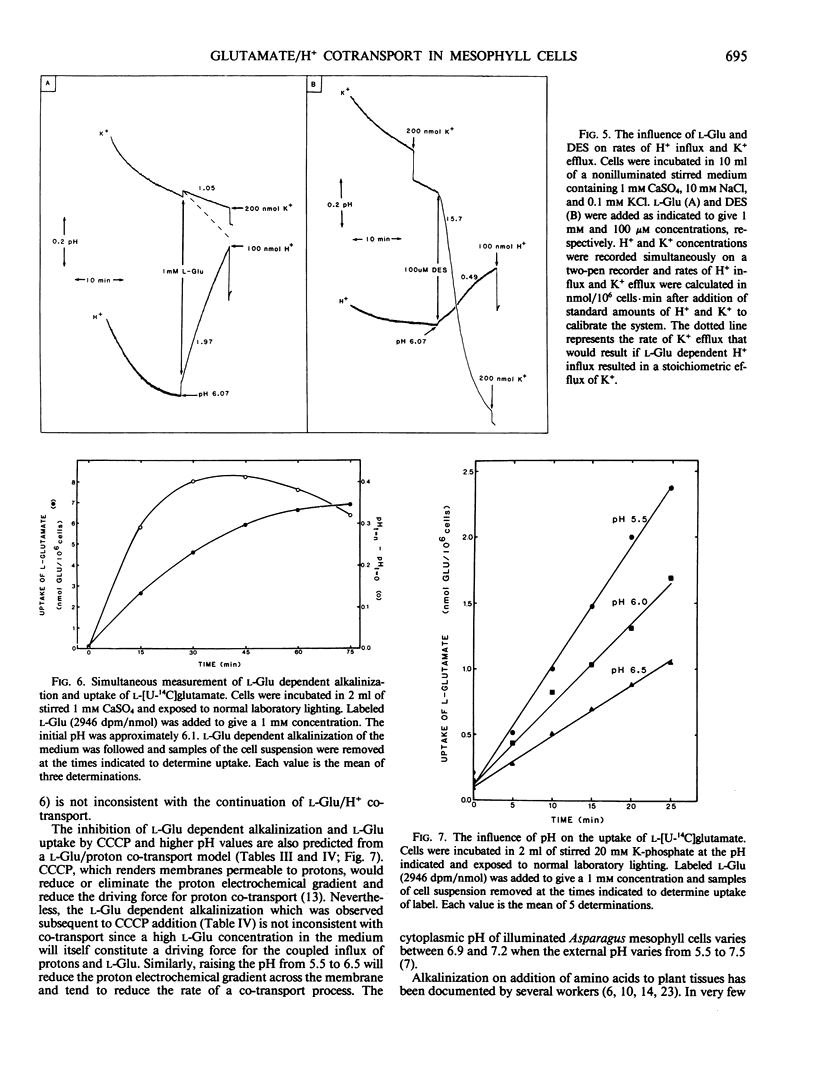
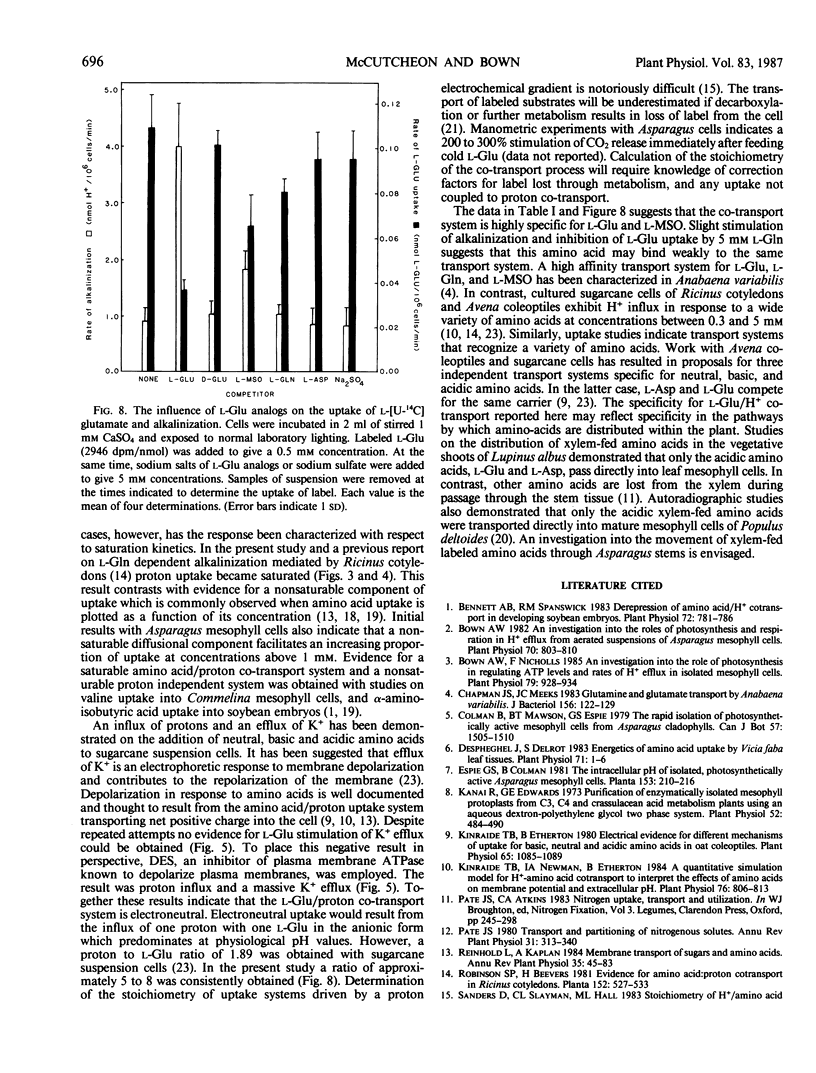
Mechanically isolated Asparagus sprengeri Regel mesophyll cells were suspended in 1 millimolar CaSO4. Immediate alkalinization of the medium occured on the addition of 1 millimolar concentrations of l-glutamate (Glu) and its analog l-methionine-d,l-sulfoximine (l-MSO). d-Glu and the l isomers of the protein amino acids did not elicit alkalinization. l-Glu dependent alkalinization was transient and acidification resumed after approximately 30 to 45 minutes. At pH 6.0, 5 millimolar l-Glu stimulated initial rates of alkalinization that varied between 1.3 to 4.1 nmol H+/106 cells·minute. l-Glu dependent alkalinization was saturable, increased with decreasing pH, was inhibited by carbonyl cyanide-p-trichloromethoxyphenyl hydrazone (CCCP), and was not stimulated by light. Uptake of l-[U-14C]glutamate increased as the pH decreased from 6.5 to 5.5, and was inhibited by l-MSO. l-Glu had no influence on K+ efflux. Although evidence for multiple amino acid/proton cotransport systems has been found in other tissues, the present report indicates that a highly specific l-Glu/proton uptake process is present in Asparagus mesophyll cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., Spanswick R. M. Derepression of amino Acid-h cotransport in developing soybean embryos. Plant Physiol. 1983 Jul;72(3):781–786. doi: 10.1104/pp.72.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W. An investigation into the roles of photosynthesis and respiration in h efflux from aerated suspensions of asparagus mesophyll cells. Plant Physiol. 1982 Sep;70(3):803–810. doi: 10.1104/pp.70.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown A. W., Nicholls F. An investigation into the role of photosynthesis in regulating ATP levels and rates of h efflux in isolated meosphyll cells. Plant Physiol. 1985 Dec;79(4):928–934. doi: 10.1104/pp.79.4.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Meeks J. C. Glutamine and glutamate transport by Anabaena variabilis. J Bacteriol. 1983 Oct;156(1):122–129. doi: 10.1128/jb.156.1.122-129.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeghel J. P., Delrot S. Energetics of Amino Acid Uptake by Vicia faba Leaf Tissues. Plant Physiol. 1983 Jan;71(1):1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Purification of enzymatically isolated mesophyll protoplasts from c(3), c(4), and crassulacean Acid metabolism plants using an aqueous dextran-polyethylene glycol two-phase system. Plant Physiol. 1973 Nov;52(5):484–490. doi: 10.1104/pp.52.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Newman I. A., Etherton B. A Quantitative Simulation Model for H-Amino Acid Cotransport To Interpret the Effects of Amino Acids on Membrane Potential and Extracellular pH. Plant Physiol. 1984 Nov;76(3):806–813. doi: 10.1104/pp.76.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann T. C., Dickson R. E., Larson P. R. Comparative Distribution and Metabolism of Xylem-Borne Amino Compounds and Sucrose in Shoots of Populus deltoides. Plant Physiol. 1985 Feb;77(2):418–428. doi: 10.1104/pp.77.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Komor E. Mechanism of amino Acid uptake by sugarcane suspension cells. Plant Physiol. 1984 Dec;76(4):865–870. doi: 10.1104/pp.76.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]


