Abstract
All aerobic biological systems, including N2-fixing root nodules, are subject to O2 toxicity that results from the formation of reactive intermediates such as H2O2 and free radicals of O2. H2O2 may be removed from root nodules in a series of enzymic reactions involving ascorbate peroxidase, dehydroascorbate reductase, and glutathione reductase. We confirm here the presence of these enzymes in root nodules from nine species of legumes and from Alnus rubra. Ascorbate peroxidase from soybean nodules was purified to near homogeneity. This enzyme was found to be a hemeprotein with a molecular weight of 30,000 as determined by sodium dodecyl sulfate gel electrophoresis. KCN, NaN3, CO, and C2H2 were potent inhibitors of activity. Nonphysiological reductants such as guaiacol, o-dianisidine, and pyrogallol functioned as substrates for the enzyme. No activity was detected with NAD(P)H, reduced glutathione, or urate. Ascorbate peroxidation did not follow Michaelis-Menten kinetics. The substrate concentration which resulted in a reaction rate of ½ Vmax was 70 micromolar for ascorbate and 3 micromolar for H2O2. The high affinity of ascorbate peroxidase for H2O2 indicates that this enzyme, rather than catalase, is responsible for most H2O2 removal outside of peroxisomes in root nodules.
Full text
PDF
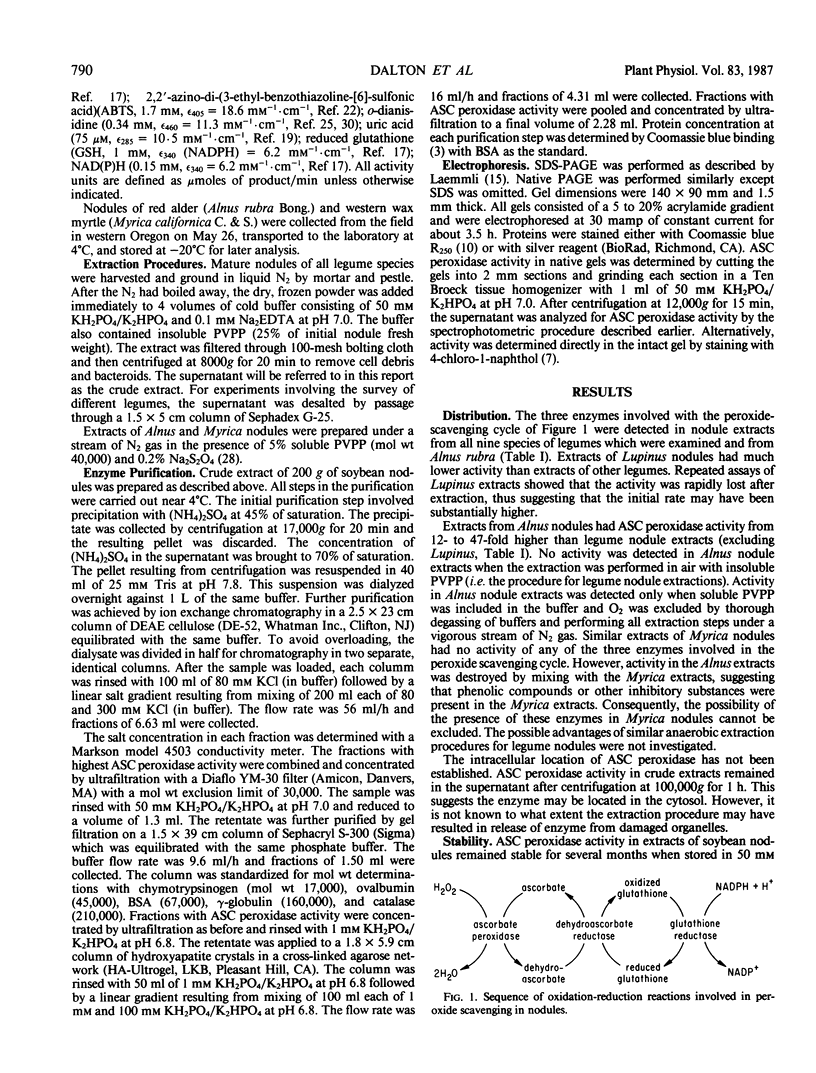
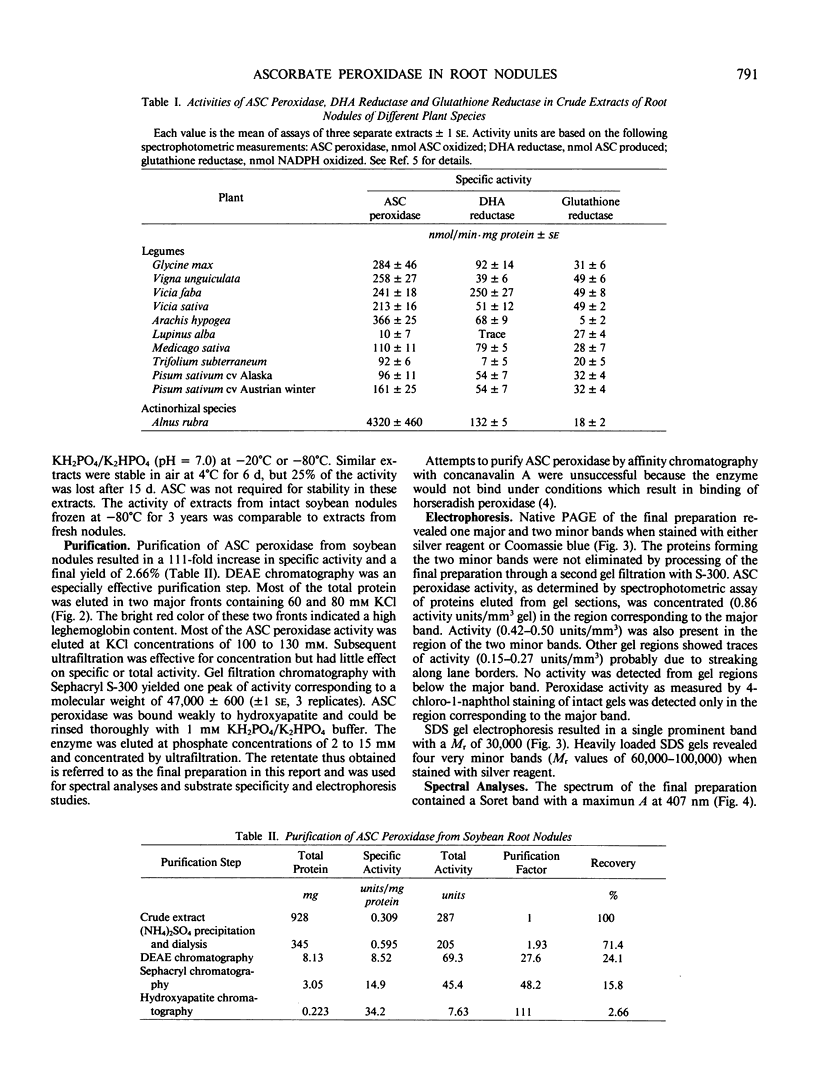
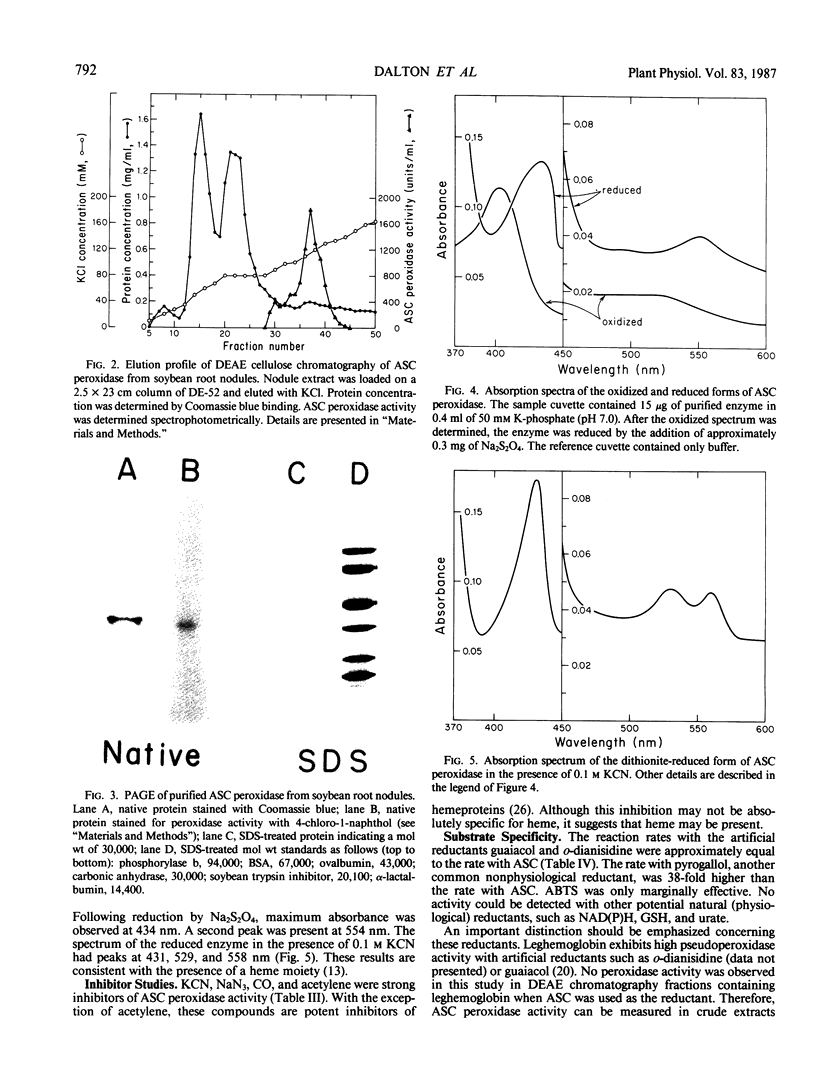


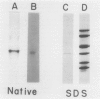
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Clarke J., Shannon L. M. The isolation and characterization of the glycopeptides from horseradish peroxidase isoenzyme C. Biochim Biophys Acta. 1976 Apr 14;427(2):428–442. doi: 10.1016/0005-2795(76)90186-0. [DOI] [PubMed] [Google Scholar]
- Dalton D. A., Russell S. A., Hanus F. J., Pascoe G. A., Evans H. J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. M. A rapid, sensitive myeloperoxidase stain using 4-chloro-1-naphthol. Am J Clin Pathol. 1980 Jun;73(6):797–799. doi: 10.1093/ajcp/73.6.797. [DOI] [PubMed] [Google Scholar]
- Groden D., Beck E. H2O2 destruction by ascorbate-dependent systems from chloroplasts. Biochim Biophys Acta. 1979 Jun 5;546(3):426–435. doi: 10.1016/0005-2728(79)90078-1. [DOI] [PubMed] [Google Scholar]
- HAYES W. J., Jr, DALE W. E., LEBRETON R. STORAGE OF INSECTICIDES IN FRENCH PEOPLE. Nature. 1963 Sep 21;199:1189–1191. doi: 10.1038/1991189b0. [DOI] [PubMed] [Google Scholar]
- Hanks J. F., Schubert K., Tolbert N. E. Isolation and characterization of infected and uninfected cells from soybean nodules : role of uninfected cells in ureide synthesis. Plant Physiol. 1983 Apr;71(4):869–873. doi: 10.1104/pp.71.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Puppo A., Rigaud J., Job D., Ricard J., Zeba B. Peroxidase content of soybean root nodules. Biochim Biophys Acta. 1980 Aug 7;614(2):303–312. doi: 10.1016/0005-2744(80)90220-x. [DOI] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]
- Shigeoka S., Nakano Y., Kitaoka S. Purification and some properties of L-ascorbic-acid-specific peroxidase in Euglena gracilis Z. Arch Biochem Biophys. 1980 Apr 15;201(1):121–127. doi: 10.1016/0003-9861(80)90495-6. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M. E., Packer L. Hydroperoxide metabolism in cyanobacteria. Arch Biochem Biophys. 1986 Apr;246(1):396–402. doi: 10.1016/0003-9861(86)90485-6. [DOI] [PubMed] [Google Scholar]