Abstract
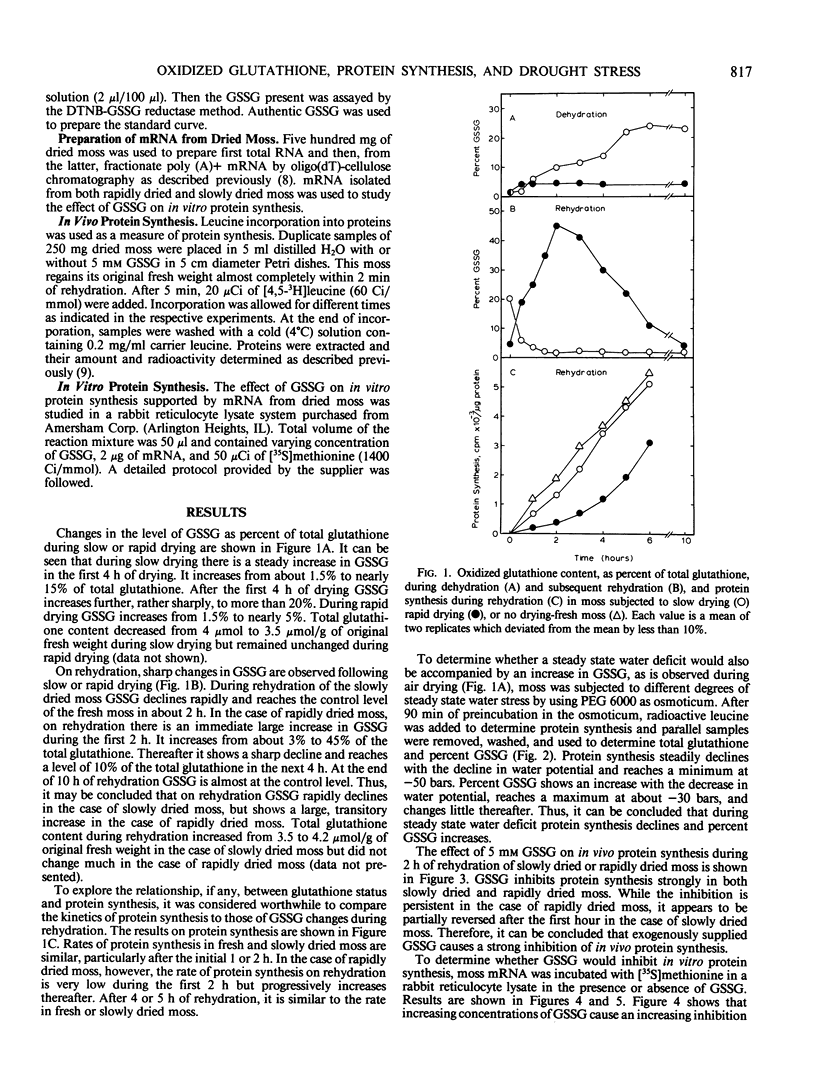
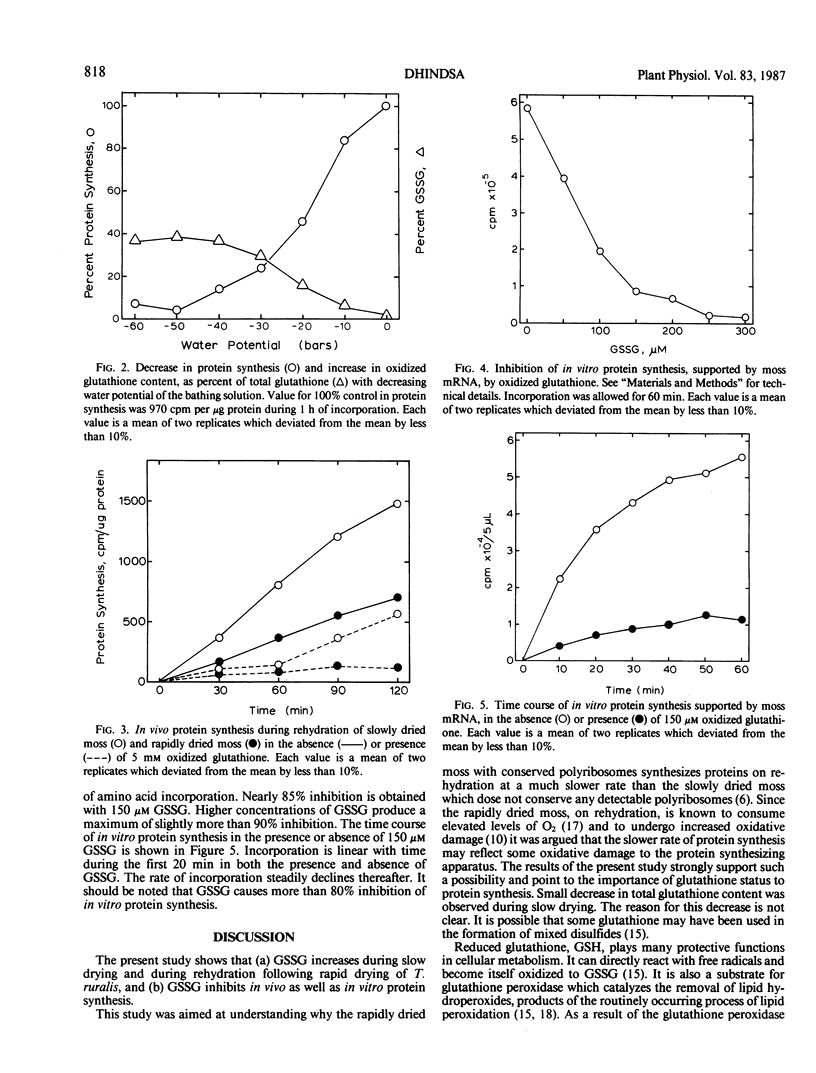
Glutathione status and its relationship to protein synthesis during water deficit and subsequent rehydration have been examined in the drought-tolerant moss, Tortula ruralis. During slow drying there is a small decrease in total glutathione but the percentage of oxidized glutathione (GSSG) increases. During rapid drying there is little change in total glutathione but a small increase in GSSG. On rehydration of slowly dried moss, GSSG rapidly declines to normal level. But when rapidly dried moss is rehydrated, there is an immediate, sharp increase in GSSG as a percentage of total glutathione. After 2 hours of rehydration GSSG starts declining and reaches a normal level in about 6 hours. When an increasing degree of steady state water deficit is imposed on the moss tissue with polyethylene glycol 6000, there is a progressive decrease in protein synthesis but an increase in oxidized glutathione. When 5 millimolar GSSG is supplied exogenously during rehydration of rapidly dried or slowly dried moss, protein synthesis is strongly inhibited. In vitro protein synthesis supported by moss mRNA is also inhibited by more than 85% by 150 micromolar GSSG. The role of glutathione status in water deficit-induced inhibition of protein synthesis is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Dhindsa R. S., Bewley J. D. Messenger RNA is conserved during drying of the drought-tolerant moss Tortula ruralis. Proc Natl Acad Sci U S A. 1978 Feb;75(2):842–846. doi: 10.1073/pnas.75.2.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa R. S., Bewley J. D. Plant desiccation: polysome loss not due to ribonuclease. Science. 1976 Jan 16;191(4223):181–182. doi: 10.1126/science.1246604. [DOI] [PubMed] [Google Scholar]
- Dhindsa R. S., Bewley J. D. Water Stress and Protein Synthesis: V. Protein Synthesis, Protein Stability, and Membrane Permeability in a Drought-sensitive and a Drought-tolerant Moss. Plant Physiol. 1977 Feb;59(2):295–300. doi: 10.1104/pp.59.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa R. S., Cleland R. E. Water stress and protein synthesis: I. Differential inhibition of protein synthesis. Plant Physiol. 1975 Apr;55(4):778–781. doi: 10.1104/pp.55.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. In situ phosphorylation of the alpha subunit of eukaryotic initiation factor 2 in reticulocyte lysates inhibited by heme deficiency, double-stranded RNA, oxidized glutathione, or the heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 May;76(5):2118–2122. doi: 10.1073/pnas.76.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Inhibition of protein synthesis initiation by oxidized glutathione: activation of a protein kinase that phosphorylates the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4110–4114. doi: 10.1073/pnas.75.9.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Campbell E. A., Herbert P., Hunt T. The preparation and properties of gel-filtered rabbit-reticulocyte lysate protein-synthesis systems. Eur J Biochem. 1983 Mar 15;131(2):289–301. doi: 10.1111/j.1432-1033.1983.tb07262.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Herbert P., Campbell E. A., Hunt T. The roles of sugar phosphates and thiol-reducing systems in the control of reticulocyte protein synthesis. Eur J Biochem. 1983 Mar 15;131(2):313–324. doi: 10.1111/j.1432-1033.1983.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Vanderhoff G. A., Benerofe B., Hunt T., Kosower E. M. Inhibition of protein synthesis by glutathione disulfide in the presence of glutathione. Biochem Biophys Res Commun. 1971 Nov 5;45(3):816–821. doi: 10.1016/0006-291x(71)90490-6. [DOI] [PubMed] [Google Scholar]
- Krochko J. E., Winner W. E., Bewley J. D. Respiration in Relation to Adenosine Triphosphate Content during Desiccation and Rehydration of a Desiccation-tolerant and a Desiccation-intolerant Moss. Plant Physiol. 1979 Jul;64(1):13–17. doi: 10.1104/pp.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Transhydrogenation in Root Tissue: Mediation by Carbon Dioxide. Science. 1965 Dec 24;150(3704):1727–1728. doi: 10.1126/science.150.3704.1727. [DOI] [PubMed] [Google Scholar]


