Abstract
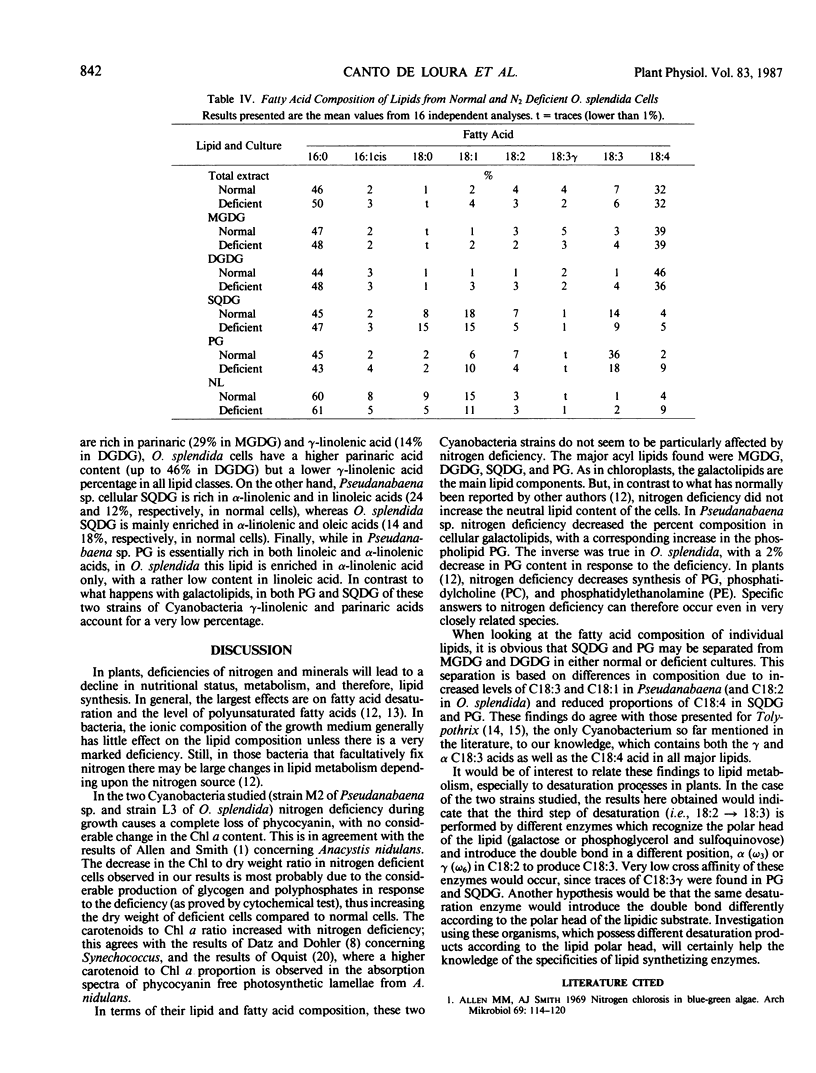
In contrast to what happens in higher plants and eukaryotic algae, a nitrogen deficiency during growth causes a change in pigment composition but no significant changes in whole cell lipid and fatty acid composition of the two Cyanobacteria, Pseudanabaena sp. (strain M2) and Oscillatoria splendida (strain L3). Nitrogen deficiency does not affect the cellular content in chlorophyll a, but it causes a selective loss in phycobiliproteins; carotenoid content increases with phycocyanin depletion. The major cellular lipids in both Cyanobacteria studied are monogalactosyl diacylglycerol, digalactosyl diacylglycerol, sulfoquinovosyl diacylglycerol, and phosphatidylglycerol. The fatty acid composition is particularly interesting as both these filamentous Oscillatoriaceae show important contents in α- and γ-linolenic (18:3) and parinaric (18:4) acids. This seems to be very unusual in Cyanobacteria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennoun P., Jupin H. Spectral properties of system I-deficient mutants of Chlamydomonas reinhardi. Possible occurrence of uphill energy transfer. Biochim Biophys Acta. 1976 Jul 9;440(1):122–130. doi: 10.1016/0005-2728(76)90118-3. [DOI] [PubMed] [Google Scholar]
- Cruden D. L., Stanier R. Y. The characterization of chlorobium vesicles and membranes isolated from green bacteria. Arch Mikrobiol. 1970;72(2):115–134. doi: 10.1007/BF00409518. [DOI] [PubMed] [Google Scholar]
- Fork D. C. Effect of Growth Temperature on the Lipid and Fatty Acid Composition, and the Dependence on Temperature of Light-induced Redox Reactions of Cytochrome f and of Light Energy Redistribution in the Thermophilic Blue-Green Alga Synechococcus lividus. Plant Physiol. 1979 Mar;63(3):524–530. doi: 10.1104/pp.63.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol. 1972 Feb;109(2):827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N., Rippka R., Stanier R. Y. Fatty acid composition and physiological properties of some filamentous blue-green algae. Arch Mikrobiol. 1972;83(3):216–236. doi: 10.1007/BF00645123. [DOI] [PubMed] [Google Scholar]
- Lang N. J. The fine structure of blue-green algae. Annu Rev Microbiol. 1968;22:15–46. doi: 10.1146/annurev.mi.22.100168.000311. [DOI] [PubMed] [Google Scholar]
- Nichols B. W., Wood B. J. The occurrence and biosynthesis of gamma-linolenic acid in a blue-green alga,Spirulina platensis. Lipids. 1968 Jan;3(1):46–50. doi: 10.1007/BF02530968. [DOI] [PubMed] [Google Scholar]
- Sato N., Murata N., Miura Y., Ueta N. Effect of growth temperature on lipid and fatty acid compositions in the blue-green algae, Anabaena variabilis and Anacystis nidulans. Biochim Biophys Acta. 1979 Jan 29;572(1):19–28. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- ZEHNDER A., GORHAM P. R. Factors influencing the growth of Microcystis aeruginosa Kutz, emend, Elenkin. Can J Microbiol. 1960 Dec;6:645–660. doi: 10.1139/m60-077. [DOI] [PubMed] [Google Scholar]