Abstract
Background:
Cerebral perfusion is directly affected by systemic blood pressure, which has been shown to be negatively correlated with cerebral blood flow (CBF). The impact of aging on these effects is not fully understood.
Purpose:
To determine whether the relationship between mean arterial pressure (MAP) and cerebral hemodynamics persists throughout the lifespan.
Study Type:
Retrospective, cross-sectional study.
Population:
669 participants from the Human Connectome Project-Aging ranging between 36-100+ years and without a major neurological disorder.
Field Strength/Sequence:
Imaging data was acquired at 3.0 Tesla using a 32-channel head coil. CBF and arterial transit time (ATT) were measured by multi-delay pseudo-continuous arterial spin labeling (ASL).
Assessment:
The relationships between cerebral hemodynamic parameters and MAP were evaluated globally in gray and white matter and regionally using surface-based analysis in the whole group, separately within different age groups (young: <60 years; younger-old: 60-79 years; oldest-old: ≥80 years).
Statistical Tests:
Chi-squared, Kruskal-Wallis, ANOVA, Spearman rank correlation and linear regression models. The general linear model setup in FreeSurfer was used for surface-based analyses. p<0.05 was considered significant.
Results:
Globally, there was a significant negative correlation between MAP and CBF in both gray (ρ=−0.275) and white matter (ρ=−0.117). This association was most prominent in the younger-old [gray matter CBF (β=−0.271); white matter CBF (β=−0.241)]. In surface-based analyses, CBF exhibited a widespread significant negative association with MAP throughout the brain, whereas a limited number of regions showed significant prolongation in ATT with higher MAP. The associations between regional CBF and MAP in the younger-old showed a different topographic pattern in comparison to young subjects.
Data Conclusion:
These observations further emphasize the importance of cardiovascular health in mid-to-late adulthood for healthy brain aging. The differences in the topographic pattern with aging indicate a spatially heterogeneous relationship between high blood pressure and CBF.
Keywords: arterial spin labeling, aging, cerebral blood flow, arterial transit time, mean arterial pressure
INTRODUCTION
Cerebral metabolic demand is supported by the delivery of oxygen-rich blood, which is often measured as cerebral blood flow (CBF)(1). CBF is regulated by various systemic factors, including blood pressure, neurovascular coupling, cerebrovascular reactivity, cardiac output, autonomic neural activity, and endothelium-dependent regulation(2). These overlapping regulatory mechanisms mediate proper vascular responses to changes in metabolic demand(1). Steady upstream cardiovascular physiology, including cardiac output, systemic blood pressure, balanced cerebrovascular resistance, and metabolic factors, is therefore critical not only for the regulation of CBF but also for maintaining cerebrovascular and neural integrity(3).
Structural and functional integrity of the brain declines with aging, and this effect varies as a function of cardiovascular health(4). Vascular risk factors, including hypertension, diabetes, obesity, hyperlipidemia, and smoking, contribute to changes in the brain independently of age(5). In particular, hypertension is one of the twelve modifiable risk factors that has been emphasized by the Lancet Commission for Dementia Prevention, Intervention and Care(6). Chronic hypertension may contribute to disruption of vasoregulatory functions throughout the brain, resulting in small vessel disease, hypoperfusion and disrupted white matter integrity(7, 8). Pathology studies have also revealed the impact of elevated blood pressure on brain tissue, including elevated amyloid-beta deposition, neurofibrillary tangles, neuritic plaque formation, and parenchymal volume loss, all of which are linked to neurodegeneration(9). However, hypertension represents a categorical threshold for a certain level of risk and does not capture the dynamic nature of blood pressure physiology. Indeed, previous studies have demonstrated that blood pressure (as a continuous measure) is associated with brain health and cognition, even in individuals in the pre-hypertensive range(10, 11).
Mean arterial pressure (MAP), which represents the average arterial pressure throughout one cardiac cycle, is a critical hemodynamic factor in maintaining sufficient cerebral perfusion. In addition, alterations in cardiac output and systemic vascular resistance are reflected in MAP(1). Furthermore, age-related physiological changes lead to increases in MAP that contribute to cardiac ventricular remodeling, vascular injury, and end-organ damage(12). Together, these observations suggest that MAP can serve as one of the key systemic vascular indicators governing cerebral hemodynamics. Indeed, elevated MAP has been shown to be inversely related to CBF in cross-sectional and prospective cohorts(13, 14). However, the extent of the impact of blood pressure on brain vascular health may also vary depending on age(15-17). Although the association between midlife hypertension and decreased CBF has been consistently demonstrated, discrepant results have been reported in the elderly (older than 65 years) and more specifically in the oldest-old (older than 80 years) populations(17-19).
This study aimed to extend prior observations regarding MAP and cerebral hemodynamics using arterial spin labeling (ASL) data from the Human Connectome Project-Aging (HCP-A) cohort. Specifically, the aim was to investigate the associations between MAP and cerebral perfusion metrics, globally and regionally, across the adult lifespan.
MATERIALS AND METHODS
Study Participants
This retrospective study was approved by the local Institutional Review Board. Cross-sectional analysis of publicly-available data acquired in the HCP-A study was performed, which enrolled typically aging adults without any major disorders, including dementia, clinical stroke, or other identified causes of cognitive decline(20-22). Details on screening, additional exclusion criteria, and cognitive assessments in the HCP-A cohort have been described previously(20). A total of 669 participants (age range: 36 to >100 years; [median (IQR) age: 59 (47-73) years, 56% female]) in whom both ASL and blood pressure data were available were included. Study participants were divided into three age groups and referred to as “young” [36 to 59 years; n=353(52.8%), 204 female], “younger-old” [60 to 79 years; n=221(33%); 119 female], and “oldest-old” [≥80 years; n=95(14.2%); 52 female].
Clinical and Imaging Data Acquisition
The following demographic and clinical information was extracted for all study participants from the study database: age, sex, race, ethnicity, blood pressure (systolic and diastolic), and use of anti-hypertensive medications. Blood pressure measurements were obtained once while the subjects were in a seated position.
Mean arterial pressure (i.e., MAP) was calculated as follows:
MRI data were acquired at 3.0 Tesla (Prisma; Siemens Healthcare, Erlangen Germany) using a 32-channel head coil at four different sites using the same acquisition parameters. T1-weighted imaging was performed with a multi-echo magnetization-prepared rapid gradient echo (MPRAGE) sequence using the following parameters: repetition time (TR)=2500 ms; inversion time (TI)=1000 ms; echo times (TE)=1.8/3.6/5.4/7.2 ms; spatial resolution=0.8x0.8x0.8 mm3; number of echoes=4. Pseudo-continuous ASL was acquired without background or vascular suppression using a labeling duration=1500 ms with the standard body transmit coil, five post-labeling delays (PLD)=200 ms, 700 ms, 1200 ms, 1700 ms, and 2200 ms, and with a multi-band 2D gradient echo echo-planar imaging (EPI) readout: TR=3580 ms; TE=19 ms; in-plane spatial resolution=2.5x2.5 mm2; slice thickness=2.5 mm; field-of-view=215 mm x 215 mm x 182 mm; scan time=5:29 min; and slice distance factor=10%. Equilibrium magnetization (M0) images were acquired at the end of the scan for normalization of the ASL difference images, and a pair of spin echo echo-planar imaging scans with opposite phase encoding directions was separately acquired for correction of field inhomogeneity-related distortions.
Image Processing
T1-weighted images were automatically processed to reconstruct cortical surfaces and to segment region-of-interest (ROI) volumes using the FreeSurfer recon-all procedure(23, 24). FreeSurfer’s ‘gtmseg’ tool was then used to generate a high-resolution segmentation for each participant for use in partial volume correction.
ASL data were corrected for susceptibility distortion, motion, and signal loss incurred due to spin history effects from the multi-band readout as previously described(25). Control and label ASL data were pair-wise subtracted, averaged for each PLD, and normalized to the second M0 image. Arterial transit time (ATT) values were calculated on a voxel-wise basis through a two-stage normalized cross-correlation approach using a cross-correlation approach similar to that which has been previously described(25). CBF values were then calculated on a voxel-wise basis by fitting a two-compartment model using a least-squares approach and the independently derived ATT information. The model parameters used have been described previously(25). FreeSurfer's PetSurfer partial volume correction (PVC) stream was used to account for the tissue fraction effect in CBF and ATT volumes. For subcortical ROIs, the symmetric geometric transfer matrix (SGTM) method was used to directly estimate ROI means. In addition, the Muller-Gartner method was applied to obtain voxel-wise values. These corrected results were registered to each participant’s structural space(26, 27).
Mean ATT and CBF values were calculated inside each ROI in the Desikan-Killiany atlas(28). For subcortical regions, the ROI-based calculations output by the SGTM algorithm were used directly(26, 27). For cortical regions, the CBF and ATT volumes output by the Muller-Gartner algorithm were mapped to the cortical surfaces of each participant, and surface-based smoothing was applied with a FWHM (full-width/half-maximum) of 15 mm. Finally, the vertex-wise mean of each cortical Desikan-Killiany ROI was calculated for regionally specified analyses.
Statistical Analysis
Categorical variables are expressed as n (percentage), and continuous variables are described as either mean ± standard deviation (SD) or median (interquartile range, IQR) depending on the normality of distribution as evaluated by Kolmogorov-Smirnov test. Group-wise comparisons were performed by chi-square test for categorical variables and Kruskal-Wallis or analysis of variance (ANOVA) for continuous variables. Associations between continuous variables were assessed using Spearman rank correlation. Steiger’s z tests were used to compare two dependent correlation coefficients with one variable in common(29). Multivariate analyses were performed using linear regression models. The dependent variable was log normalized to meet the assumption of normality in regression models. All statistical tests were performed using R Project for Statistical Computing (software version 3.5.1), and p<0.05 was considered statistically significant.
First, we examined the patterns of associations between MAP and global white and gray matter cerebral perfusion parameters in all subjects. Next, we performed multivariate analyses to determine independent factors related to CBF and ATT. Along with MAP, anti-hypertensive medication use, age and sex were introduced in these models as covariates. Subsequently, we repeated these analyses within each age group (‘young’, ‘younger-old’, and ‘oldest-old’) separately. Finally, we constructed regression models in the whole group by including interaction terms (age group * MAP) in order to elucidate how age impacts the association between MAP and cerebral hemodynamics. In addition to studying the impact of blood pressure measures on mean CBF and ATT values globally, age-adjusted regional variations of these associations were examined in the whole group and separate age groups by performing surface-based analyses using general linear model setup in FreeSurfer(23). Correlations between MAP and CBF vs. MAP and ATT were compared using Steiger’s z test among five regions with the highest correlations in surface-based analyses of CBF and ATT.
RESULTS
Table 1 summarizes the demographic variables, use of anti-hypertensive medication, and blood pressure levels together with CBF and ATT values of the study population. There were no significant sex differences or differences (p= 0.626) in diastolic blood pressure (p= 0.226) between age groups, but other blood pressure parameters showed significant differences. There was a weak yet significant correlation between MAP and age (Spearman's ρ= 0.171). The median (IQR) values of global gray and white matter CBF observed in the entire cohort were 56.9 (49.8 – 65.9) ml/100 g/min, and 32.5 (29.3 – 36.2) ml/100 g/min, respectively, and were negatively correlated with age in the gray (Spearman's ρ= −0.448) and white matter (Spearman's ρ= − 0.082). A significant and moderately positive correlation between ATT and age was observed in both tissue types (gray matter: Spearman's ρ= 0.552, white matter: Spearman's ρ= 0.409).
Table 1:
Summary of demographic variables, blood pressure information and cerebral perfusion metrics
| All (n=669) | Young group (n= 353) | Younger-old group (n= 221) |
Oldest-old group (n= 95) |
p | |
|---|---|---|---|---|---|
| Female | 375 (56.1%) | 204 (57.8%) | 119 (53.8%) | 52 (54.7%) | 0.626 |
| Race | <0.001 | ||||
| American Indian/Alaska native | 2 (0.3%) | 1 (0.3%) | 0 (0.0%) | 1 (1.1%) | |
| Asian | 49 (7.3%) | 32 (9.1%) | 17 (7.7%) | 0 (0.0%) | |
| Black/African American | 91 (13.6%) | 66 (18.7%) | 21 (9.5%) | 4 (4.2%) | |
| Caucasian | 486 (72.6%) | 217 (61.5%) | 180 (81.4%) | 89 (93.7%) | |
| More than one race | 30 (4.5%) | 26 (7.4%) | 3 (1.4%) | 1 (1.1%) | |
| Unknown/not reported | 11 (1.6%) | 11 (3.1%) | 0 (0.0%) | 0 (0.0%) | |
| Ethnicity | <0.001 | ||||
| Hispanic/Latino | 70 (10.5%) | 56 (15.9%) | 14 (6.3%) | 0 (0.0%) | |
| Not Hispanic/Latino | 597 (89.2%) | 297 (84.1%) | 206 (93.2%) | 94 (98.9%) | |
| Unknown/not reported | 2 (0.3%) | 0 (0.0%) | 1 (0.5%) | 1 (1.1%) | |
| Anti-hypertensive medication | 167 (25.0%) | 40 (11.3%) a | 76 (34.4%) b | 51 (53.7%) c | <0.001 |
| Systolic blood pressure (mmHg) | 129 (118–140) | 124 (114–134) a | 133 (122–143) b | 142 (127–152) c | <0.001 |
| Diastolic blood pressure (mmHg) | 79±10 | 80±11 | 79±10 | 78±10 | 0.226 |
| Mean arterial pressure (mmHg) | 96±11 | 95±11 a | 97±11 b | 98±11 b | 0.004 |
| Gray matter CBF (ml/100 g/min) | 56.9 (49.8–65.9) | 61.7 (54.8–69.6) a | 53.5 (47.1–60.2) b | 48.8 (39.3–55.3) c | <0.001 |
| White matter CBF (ml/100 g/min) | 32.5 (29.3–36.2) | 32.7 (29.9–36.1) a | 32.7 (29.1–36.6) a | 30.6 (26.5–34.4) b | <0.001 |
| Gray matter ATT (s) | 1.33 (1.18–1.50) | 1.23 (1.11–1.34) a | 1.42 (1.28–1.56) b | 1.52 (1.41–1.66) c | <0.001 |
| White matter ATT (s) | 1.64±0.15 | 1.59±0.14 a | 1.68±0.15 b | 1.75±0.13 c | <0.001 |
Values represent n (%), mean±SD or median (IQR). p-values denote statistical significance of group-wise comparisons conducted by chi-square test for categorical variables and by analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables. Different superscript letters indicate significant difference among age groups based on post-hoc pairwise comparison.
Associations Between MAP and Global Cerebral Perfusion Parameters
Higher MAP was associated with lower CBF in both gray (Spearman's ρ= −0.275) and white matter (Spearman's ρ= −0.117) in the overall population. In addition, a significant positive correlation was observed between MAP and ATT in both gray (Spearman’s ρ= 0.171) and white matter (Spearman’s ρ= 0.137) (Figure 1). The relationship between higher MAP and CBF in gray (β= −0.140) and white matter (β= −0.103) remained significant after adjusting for age, sex and anti-hypertensive medication use in multivariate analyses. However, there was no significant association between MAP and ATT in multivariate models after adjusting for age, sex, and antihypertensive use (for gray matter, p= 0.757; for white matter, p= 0.686).
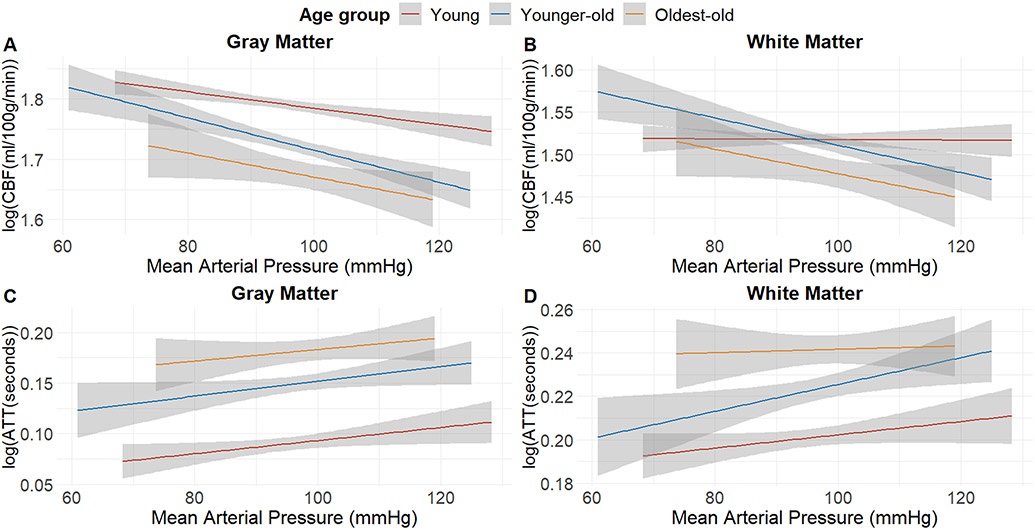
Figure 1: The relationship between MAP and cerebral hemodynamics in the whole sample.
Elevated MAP is inversely correlated with CBF and positively correlated with ATT in all age groups. The relationship with CBF is more prominent in the gray matter (A) compared to that in the white matter (B). GM CBF decreases 0.3 ml/100 g/min for each 1 mmHg increase in MAP, whereas this decrease is 0.06 ml/100 g/min per 1 mmHg MAP increase in white matter. A similar yet minor difference is seen between gray matter ATT (0.003 seconds per mmHg increase) (C) and white matter ATT (0.002 seconds per mmHg increase) (D). The shaded areas represent the 95% CI of the regression line.
Across different age groups, the most robust association between MAP and CBF was observed in individuals aged 60 to 79 years [for gray matter CBF (β= −0.271); for white matter CBF (β= −0.241)] (Table 2). The oldest-old group displayed a relationship in the same direction, but these results did not meet significance criteria [for gray matter CBF (β= −0.176, p= 0.079); for white matter CBF (β= −0.177, p= 0.089)]. The young group had a significant yet weaker association between MAP and gray matter CBF (β= −0.101). No significant association was observed between MAP and white matter CBF in this age group (β= −0.012; p= 0.834).
Table 2:
Summary of the correlation and regression (adjusted for age, sex and antihypertensive medication) analyses evaluating the effect of MAP on cerebral hemodynamics across age groups.
| Spearman’s correlation |
Linear regression | |||||
|---|---|---|---|---|---|---|
| Age groups |
Dependent variable |
ρ | p value | Beta | B (SE) | p value |
| Young group | Gray matter CBF | −0.180 | 0.001 | −0.101 | −0.001 (0.000) | 0.040 |
| White matter CBF | 0.011 | 0.841 | −0.012 | −0.000 (0.000) | 0.834 | |
| Gray matter ATT | 0.083 | 0.120 | −0.016 | 0.000 (0.000) | 0.741 | |
| White matter ATT | 0.044 | 0.411 | −0.018 | 0.000 (0.000) | 0.713 | |
| Younger-old group | Gray matter CBF | −0.295 | <0.001 | −0.271 | −0.002 (0.001) | <0.001 |
| White matter CBF | −0.204 | 0.002 | −0.241 | −0.002 (0.000) | <0.001 | |
| Gray matter ATT | 0.130 | 0.053 | 0.032 | 0.000 (0.000) | 0.598 | |
| White matter ATT | 0.125 | 0.064 | 0.085 | 0.000 (0.000) | 0.185 | |
| Oldest-old group | Gray matter CBF | −0.284 | 0.005 | −0.176 | −0.002 (0.001) | 0.079 |
| White matter CBF | −0.232 | 0.024 | −0.177 | −0.001 (0.001) | 0.089 | |
| Gray matter ATT | 0.119 | 0.249 | 0.095 | 0.000 (0.000) | 0.334 | |
| White matter ATT | 0.022 | 0.834 | −0.002 | 0.000 (0.000) | 0.981 | |
The dependent variables were log transformed in linear regression models, which include age, sex, anti-hypertensive use and MAP as independent variables. ρ= Spearman’s rho correlation coefficient. Beta and B represent standardized and unstandardized coefficients, respectively. SE=Standard error.
The analyses in the overall population after inclusion of the MAP * age group (reference category: younger old) interaction term, revealed no significant difference between the younger-old and the oldest-old groups regarding the associations of MAP with gray matter (β= 0.001; p= 0.686) or white matter CBF (β= 0.001; p= 0.893). However, a significant interaction was observed in between the young group and the younger-old for the association of MAP with gray matter (βMAP*age= 0.003) and white matter CBF (βMAP*age= 0.003) (Supplemental table 1).
Surface-based Analyses
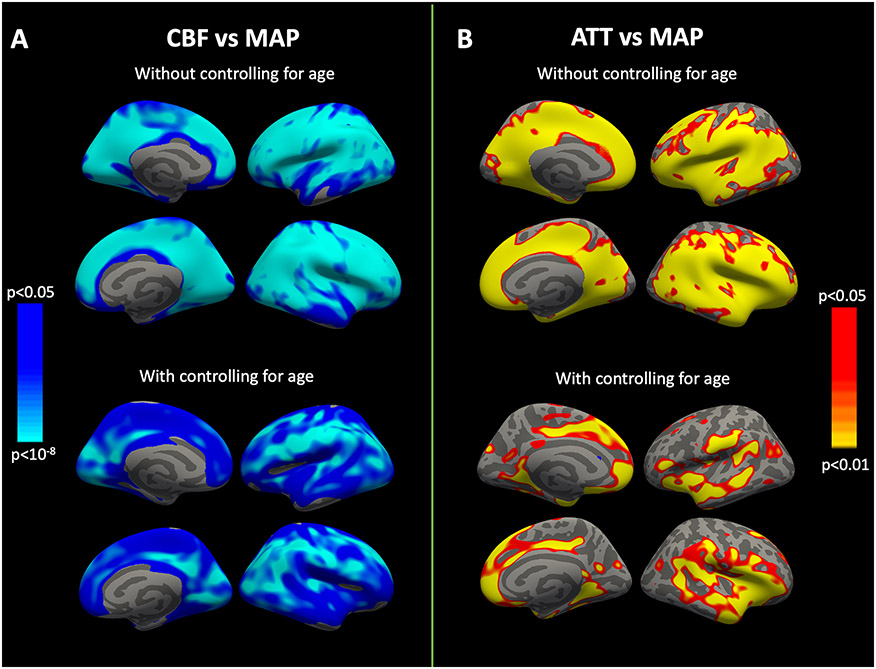
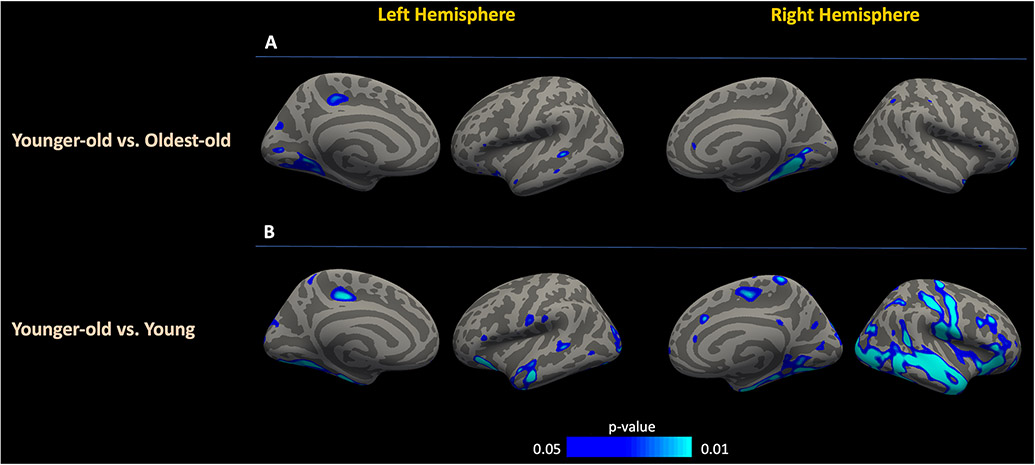
Both unadjusted and age-adjusted surface-based image analyses in the overall population showed that MAP was strongly associated with CBF in multiple cortical areas, with the highest correlations observed in the cuneus, inferior parietal, transverse temporal, pericalcarine and supramarginal regions. (Figure 2A). Meanwhile, the relationship between MAP and ATT was significant only in limited number of cortical areas, including temporal pole, medial and lateral orbitofrontal cortices, pars triangularis and insula (Figure 2B). In regions showing the highest correlation between MAP and CBF, the coefficients were significantly higher when compared to the correlations between MAP and ATT (Supplemental table 2). Meanwhile, there was no significant difference between the correlation coefficients of MAP vs. CBF and MAP vs. ATT in the top-tier of regions highlighted in surface-based analyses of ATT (Supplemental table 3). Pairwise comparisons of the age groups showed no significant topographical difference in the association of MAP with CBF between the oldest-old and the younger-old groups (Figure 3A). However, in the pairwise comparison of younger-old and young groups, there was a significant group-wise difference of CBF vs. MAP slopes in a number of regions (i.e. right medial, inferior, occipital and lateral parietal areas) with more negative slopes observed in the former group (Figure 3B). Regions showing the highest partial correlations between MAP and CBF across age groups are summarized in Supplemental table 4, highlighting a consistent association between CBF and MAP in all age groups in the left inferior frontal and right middle temporal lobes.
Figure 2: Vertex-wise analyses between MAP, CBF and ATT with and without adjustment for age.
Age-unadjusted associations between MAP and hemodynamic parameters display a widespread relationship (top row). After controlling for age effects (bottom row), this pattern mostly endured for CBF compared to ATT. Color bars indicate p-values and the direction of correlation; blue represents a negative correlation whereas red/yellow represents a positive correlation. CBF, Cerebral blood flow; ATT, Arterial transit time; MAP, Mean arterial pressure.
Figure 3: Vertex-wise comparative group analyses between MAP and CBF.
The relationship between CBF and MAP displayed a significant difference only in a limited number of regions among the oldest-old and younger-old groups (A). Surface-based analyses among the younger-old and young groups highlighted various anatomic regions with more negative slopes in the younger-old with respect to the relationship between MAP and CBF (B). Color bars indicate p values and the direction of the association, with blue representing a more negative slope. CBF, Cerebral blood flow; ATT, Arterial transit time; MAP, Mean arterial pressure.
DISCUSSION
In this study, we aimed to investigate associations between systemic blood pressure and cerebral hemodynamics across the adult lifespan. Our results showed that higher MAP values were associated with lower global CBF in both gray and white matter. This association was more prominent in younger-old participants in comparison to the young group. Furthermore, the comparative surface-based analyses showed significant group-wise differences between the younger-old and young groups in certain anatomic areas with steeper slopes in the MAP vs. gray matter CBF relationship of the younger-old subjects.
Substantial work examining the associations between cardiovascular risk factors and cerebral hemodynamics, has highlighted the adverse effects of hypertension on CBF in middle-aged and older individuals(19, 30, 31). The change in vascular structure (arteriolar thickening and luminal narrowing) and elasticity are considered to underlie the decreased CBF observed in subjects with chronic hypertension(32, 33). However, there are some discrepant observations questioning whether the negative influence of high blood pressure on cerebral perfusion still persists at later periods of life and especially in the oldest-old individuals. In a longitudinal study, Suri et al. found that the significant relationship between cardiovascular risk and lower CBF observed in midlife attenuated during the periods of later life(34). Similarly, van Dalen et al. found no associations between blood pressure and CBF cross-sectionally or longitudinally over a three-year period in a cohort of older hypertensive individuals(35). In another study, Foster-Dingley et al. found no effect of antihypertensive drug cessation on CBF in a cohort of patients with mild cognitive impairment and mean age of 81 years(18). Moreover, numerous longitudinal and observational studies have highlighted an association between low instead of high blood pressure with negative health outcomes such as brain atrophy (36), dementia (37) and even mortality (38) in later life. On the other hand, Deverdun et al. have shown an association between increased MAP over 12 years and lower CBF across the whole gray matter in healthy older adults(13). In the current study, we found a negative correlation between MAP and global CBF in the gray matter throughout the lifespan. The strength of this relationship was greater in gray matter and most prominent in individuals aged 60-79 years. A similar positive correlation was observed in the oldest-old group, but this association did not reach the level of statistical significance. However, both the regression analyses testing for the interaction of the age and MAP, and the comparative surface-based analyses suggested that the oldest-old individuals do not differ significantly from the younger-old subjects from the perspective of MAP-CBF interplay. Therefore, we believe that the non-significant results in the oldest-old population stem from statistical power issues associated with the lower number of participants in this age group. Meanwhile, our findings highlight that the association between MAP and global CBF may be more subtle in younger individuals and show topographic differences in comparison to older subjects. Although this might reflect the duration of hypertension and thereby the associated vascular effects, which probably are milder in the younger cases, it is not possible to rule out spatial heterogeneity between different age groups regarding the interplay between MAP and cerebral perfusion. Longitudinal studies evaluating cerebral hemodynamics and their spatial patterns from younger age to midlife extending to the oldest-old period are needed to further understand these relationships.
In addition to CBF, we evaluated ATT measures from the gray and white matter using a new approach that has recently been presented(25). The analyses on white matter ATT may be more novel, as many prior studies focusing on the cerebral hemodynamic effects of age or vascular risk factors were primarily based on CBF measurements in the gray matter(13, 18, 34). Consistent with our previous work, ATT was found to be higher in the white matter in comparison to the gray matter in the current larger cohort(25). In addition, global gray matter ATT showed a positive correlation with increasing age, replicating the observations in previous studies(39, 40). There was also a similar prolongation of ATT with age in the white matter. From the perspective of MAP, there was a positive correlation with gray and white matter ATT in bivariate analyses, which became non-significant after adjusting for age, sex and anti-hypertensive use. Despite the neutral relationship observed in global analyses, significant associations between MAP and ATT were found in surface-based image analyses, particularly in insula, medial and lateral orbitofrontal cortices, pars triangularis of inferior frontal gyrus and temporal pole. Although the negative effects of systemic vascular risk factors on cerebral perfusion are generally known, there are fewer studies that directly examine the relationship with ATT. In one of these studies, similar to our results, a significant relationship was found between blood pressure metrics and ATT in the insular region of men aged 55-80 years, suggesting that vascular risk factors may have region-specific differential effects on cerebral hemodynamics(31). Overall, these observations suggest that MAP levels have more widespread adverse effects on CBF in comparison to ATT. This finding could be related to the fact that CBF is more directly influenced by blood pressure, whereas ATT may be more related to macrovascular mechanisms, including vessel tortuosity and slower blood velocity(25).
Limitations
First, the study cohort was limited in terms of ethnic and racial diversity, with the vast majority of participants being of Caucasian descent. More data in diverse populations are needed to allow for generalization of our observations to populations that are known to have a higher prevalence of elevated blood pressure. Second, our findings rely on a single cross-sectional retrospective evaluation of blood pressure data and perfusion imaging data acquired at one field strength (i.e., 3 Tesla). Longitudinal follow-up of individuals with measurements at multiple time points may be more informative about the role played by aging and blood pressure levels in cerebral hemodynamic changes. Third, additional vascular risk factors such as diabetes mellitus, hyperlipidemia, obesity or smoking that might adversely affect cerebral perfusion were not accounted for in our analyses. Fourth, we have defined our study groups as those <60 years, between 60 and 79 years, and ≥80 years; however, a more ideal analysis that was not possible in our cohort due to the sample size would be to stratify the study population to multiple age categories (e.g., to within a decade) to more finely delineate the effect of aging on blood pressure and cerebral hemodynamic relationships. Fifth, although the study did not include subjects with a history of stroke, we did not have angiographic information to rule out asymptomatic, yet significant stenosis that might confound the assessment of cerebral hemodynamics. Finally, although younger participants of the current study are considered to be typically aging, it is unclear whether they will remain free of major neurological disorders in their later years. In this regard, there is an inevitable selection bias towards healthier participants in the oldest-old group.
Conclusion
In the current study, we demonstrated a strong relationship of MAP, an important systemic physiological parameter, with CBF in both the gray and white matter and detailed the spatial distribution differences of this relationship by surface-based analysis in different age groups using ASL MRI data from the HCP-A population. We found compelling evidence that the most prominent effects were observed in individuals within the mid-to-late-life which is consistent with the mounting evidence in the literature highlighting the role of vascular health during this period of life on cognitive health in following years.
Supplementary Material
Acknowledgments
E. Yetim, M.R. Juttukonda, and D.H. Salat were involved in study conception and design; analysis and interpretation of data; E. Yetim drafted the manuscript. J. Jacoby, R. Almaktoum and N.L. Damestani performed analyses and interpreted the results. A.E. Lovely was involved in data acquisition. All authors contributed to manuscript revision. The final version of the manuscript has been read and approved by all named authors.
Grant Support
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (U01AG052564, K01AG070318) and the American Heart Association (19CDA34790002). Dr. Yetim was funded by TUBITAK (The Scientific and Technological Research Council of Turkey) as part of the international postdoctoral research fellowship program.
Footnotes
Disclosures
M.R. Juttukonda receives research-related support from Siemens Healthcare. All other authors declare that they have no conflicts of interests.
REFERENCES
- 1.Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144(5):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne SJ. Cerebral blood flow and metabolism: a quantitative approach. 1st ed. Hackensack, NJ: World Scientific; 2018. 468 p. [Google Scholar]
- 4.Dhamoon MS, Dong C, Elkind MS, Sacco RL. Ideal cardiovascular health predicts functional status independently of vascular events: the Northern Manhattan Study. J Am Heart Assoc. 2015;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palta P, Albert MS, Gottesman RF. Heart health meets cognitive health: evidence on the role of blood pressure. Lancet Neurol. 2021;20(10):854–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassenaar TM, Yaffe K, van der Werf YD, Sexton CE. Associations between modifiable risk factors and white matter of the aging brain: insights from diffusion tensor imaging studies. Neurobiol Aging. 2019;80:56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox SR, Lyall DM, Ritchie SJ, Bastin ME, Harris MA, Buchanan CR, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging. 2000;21(1):57–62. [DOI] [PubMed] [Google Scholar]
- 10.Salat DH, Williams VJ, Leritz EC, Schnyer DM, Rudolph JL, Lipsitz LA, et al. Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage. 2012;59(1):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leritz EC, Salat DH, Williams VJ, Schnyer DM, Rudolph JL, Lipsitz L, et al. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage. 2011;54(4):2659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol. 2012;590(9):2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deverdun J, Akbaraly TN, Charroud C, Abdennour M, Brickman AM, Chemouny S, et al. Mean arterial pressure change associated with cerebral blood flow in healthy older adults. Neurobiol Aging. 2016;46:49–57. [DOI] [PubMed] [Google Scholar]
- 14.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308–15. [DOI] [PubMed] [Google Scholar]
- 15.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4(8):487–99. [DOI] [PubMed] [Google Scholar]
- 16.Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia: a double edged sword. Ageing Res Rev. 2009;8(2):61–70. [DOI] [PubMed] [Google Scholar]
- 17.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement. 2017;13(2):103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster-Dingley JC, Moonen JE, de Craen AJ, de Ruijter W, van der Mast RC, van der Grond J. Blood Pressure Is Not Associated With Cerebral Blood Flow in Older Persons. Hypertension. 2015;66(5):954–60. [DOI] [PubMed] [Google Scholar]
- 19.Tryambake D, He J, Firbank MJ, O'Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61(6):1309–15. [DOI] [PubMed] [Google Scholar]
- 20.Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, et al. The Lifespan Human Connectome Project in Aging: An overview. NeuroImage. 2019;185:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, et al. The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5-21 year olds. Neuroimage. 2018;183:456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. Neuroimage. 2018;183:972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. NeuroImage. 1999;9(2):179–94. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 25.Juttukonda MR, Li B, Almaktoum R, Stephens KA, Yochim KM, Yacoub E, et al. Characterizing cerebral hemodynamics across the adult lifespan with arterial spin labeling MRI data from the Human Connectome Project-Aging. Neuroimage. 2021;230:117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, et al. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, et al. Different partial volume correction methods lead to different conclusions: An (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. [DOI] [PubMed] [Google Scholar]
- 29.Hoerger M ZH: An updated version of Steiger's Z and web-based calculator for testing the statistical significance of the difference between dependent correlations. 2013. [Google Scholar]
- 30.Bangen KJ, Nation DA, Clark LR, Harmell AL, Wierenga CE, Dev SI, et al. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Frontiers in Aging Neuroscience. 2014;6(159). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntosh BJ, Swardfager W, Robertson AD, Tchistiakova E, Saleem M, Oh PI, et al. Regional cerebral arterial transit time hemodynamics correlate with vascular risk factors and cognitive function in men with coronary artery disease. AJNR Am J Neuroradiol. 2015;36(2):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veglio F, Paglieri C, Rabbia F, Bisbocci D, Bergui M, Cerrato P. Hypertension and cerebrovascular damage. Atherosclerosis. 2009;205(2):331–41. [DOI] [PubMed] [Google Scholar]
- 33.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28(6):1071–85. [DOI] [PubMed] [Google Scholar]
- 34.Suri S, Topiwala A, Chappell MA, Okell TW, Zsoldos E, Singh-Manoux A, et al. Association of Midlife Cardiovascular Risk Profiles With Cerebral Perfusion at Older Ages. JAMA Network Open. 2019;2(6):e195776–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Dalen JW, Mutsaerts HJ, Petr J, Caan MW, van Charante EPM, MacIntosh BJ, et al. Longitudinal relation between blood pressure, antihypertensive use and cerebral blood flow, using arterial spin labelling MRI. J Cereb Blood Flow Metab. 2021;41(7):1756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jochemsen HM, Muller M, Visseren FL, Scheltens P, Vincken KL, Mali WP, et al. Blood pressure and progression of brain atrophy: the SMART-MR Study. JAMA Neurol. 2013;70(8):1046–53. [DOI] [PubMed] [Google Scholar]
- 37.Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B, et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. 2001;12(1):33–9. [DOI] [PubMed] [Google Scholar]
- 38.Lv YB, Gao X, Yin ZX, Chen HS, Luo JS, Brasher MS, et al. Revisiting the association of blood pressure with mortality in oldest old people in China: community based, longitudinal prospective study. BMJ. 2018;361:k2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutsaerts HJ, Petr J, Vaclavu L, van Dalen JW, Robertson AD, Caan MW, et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J Cereb Blood Flow Metab. 2017;37(9):3184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai W, Fong T, Jones RN, Marcantonio E, Schmitt E, Inouye SK, et al. Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. J Magn Reson Imaging. 2017;45(2):472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.