Abstract
Chlorate-resistant Nicotiana plumbaginifolia (cv Viviani) mutants were found to be deficient in the nitrate reductase apoprotein (NR−nia). Because they could not grow with nitrate as sole nitrogen source, they were cultivated as graftings on wild-type Nicotiana tabacum plants. The grafts of mutant plants were chlorotic compared to the grafts of wild type. Mutant leaves did not accumulate nitrogen and nitrate but contained less malate and more glutamine than wild leaves. They exhibited a slight increase of the proportion of the light-harvesting chlorophyll a/b protein complexes and a lowering of the efficiency of energy transfer between these complexes and the active centers. After a 3 second 14CO2 pulse, the total 14C incorporation of the mutant leaves was approximately 20% of that of the control. The 14C was essentially recovered in ribulose bisphosphate in these plants. It was consistent with a decline of ribulose bisphosphate carboxylase activity observed in the mutant. After a 3 second 14CO2 pulse followed by a 60 second chase with normal CO2, 14C was mainly accumulated in starch which was labeled more in the mutant than in the wild type. These results confirm the observation that in the nitrate reductase deficient leaves, chloroplasts were loaded with large starch inclusions preceding disorganization of the photosynthetic apparatus.
Full text
PDF


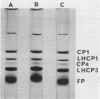
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Pernollet J. C., Huet J. C., Moutot F., Morot-Gaudry J. F. Relationship between Photosynthesis and Protein Synthesis in Maize: II. Interconversions of the Photoassimilated Carbon in the Ear Leaf and in the Intermediary Organs to Synthesize the Seed Storage Proteins and Starch. Plant Physiol. 1986 Jan;80(1):216–222. doi: 10.1104/pp.80.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]