Abstract
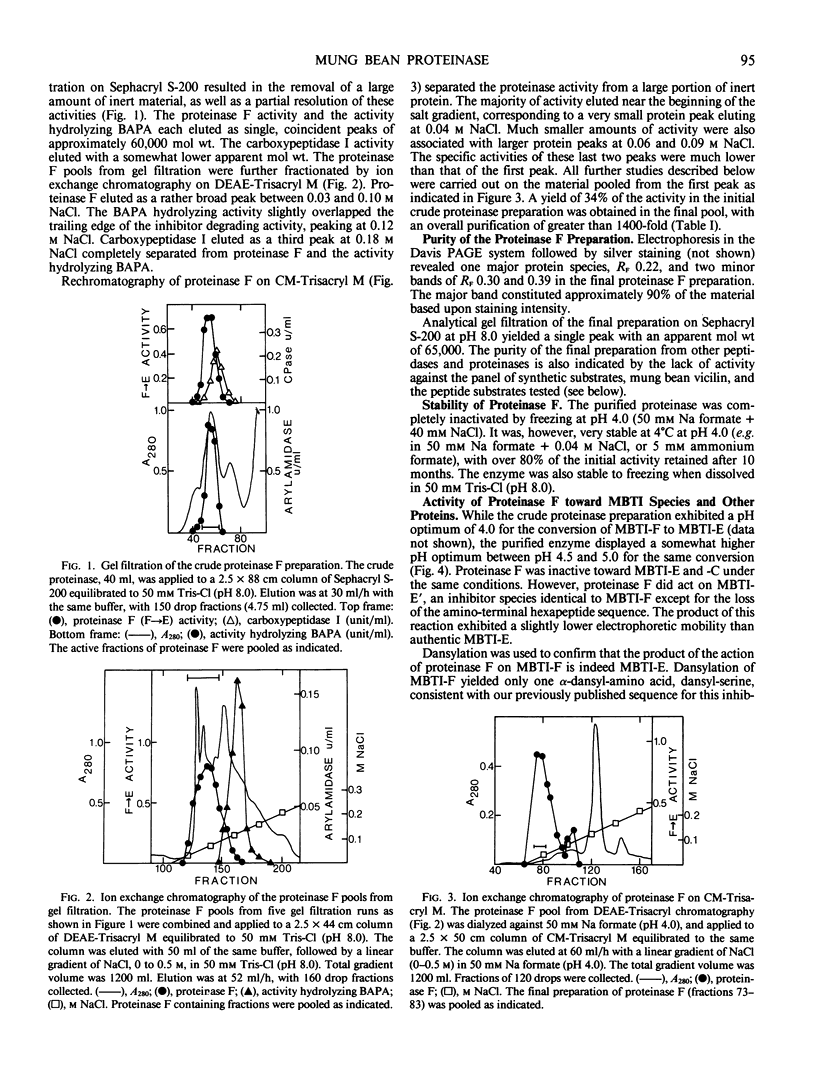
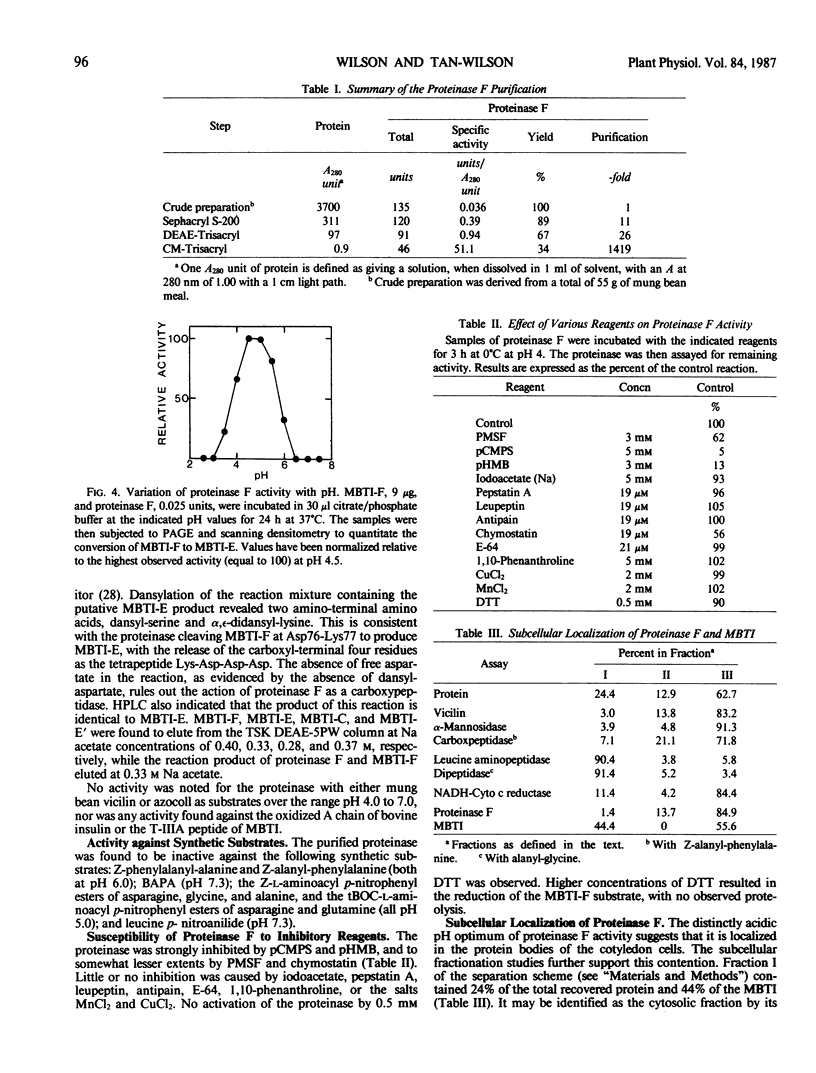
The proteinase (proteinase F) responsible for the initial proteolysis of the mung bean (Vigna radiata) trypsin inhibitor (MBTI) during germination has been purified 1400-fold from dry beans. The enzyme acts as an endopeptidase, cleaving the native inhibitor, MBTI-F, to produce the first modified inhibitor form, MBTI-E. The cleavage of the Asp76-Lys77 peptide bond of MBTI-F occurs at a pH optimum of 4.5, with the tetrapeptide Lys-Asp-Asp-Asp being released. Proteinase F exhibited no activity against the modified inhibitor forms MBTI-E and MBTI-C. Vicilin, the major storage protein of the mung bean, does not serve as a substrate for proteinase F between pH 4 and 7. Proteinase F is inhibited by phenylmethylsulfonyl fluoride, chymostatin, p-hydroxymercuribenzoate, and p-chlorophenylsulfonate, but not by iodoacetate and CuCl2. It is not activated by dithiothreitol, and is stable for extended periods of time (10 months, 4°C, pH 4.0) in the absence of reducing agents. An apparent molecular weight of 65,000 was found for proteinase F by gel filtration. Subcellular fractionation in glycerol suggests that greater than 85% of the proteinase F activity is found in the protein bodies of the ungerminated mung bean. The same studies indicate that at least 56% of the MBTI of the seed is also localized in the protein bodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Effects of leupeptin on proteinase and germination of castor beans. Plant Physiol. 1981 Oct;68(4):851–853. doi: 10.1104/pp.68.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner B., Chrispeels M. J. Purification and characterization of vicilin peptidohydrolase, the major endopeptidase in the cotyledons of mung-bean seedlings. Eur J Biochem. 1977 Jul 15;77(2):223–233. doi: 10.1111/j.1432-1033.1977.tb11661.x. [DOI] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Chappell J., Van der Wilden W., Chrispeels M. J. The biosynthesis of ribonuclease and its accumulation in protein bodies in the cotyledons of mung bean seedlings. Dev Biol. 1980 Apr;76(1):115–125. doi: 10.1016/0012-1606(80)90366-8. [DOI] [PubMed] [Google Scholar]
- Chavira R., Jr, Burnett T. J., Hageman J. H. Assaying proteinases with azocoll. Anal Biochem. 1984 Feb;136(2):446–450. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Baumgartner B. Trypsin inhibitor in mung bean cotyledons: purification, characteristics, subcellular localization, and metabolism. Plant Physiol. 1978 Apr;61(4):617–623. doi: 10.1104/pp.61.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Tacchini-Vonlanthen M. Ultrastructural localization of Bowman-Birk inhibitor on thin sections of Glycine max (soybean) cv. Maple Arrow by the gold method. Histochemistry. 1983;77(3):313–321. doi: 10.1007/BF00490894. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorensen E., Prevosto R., Wilson K. A. The Appearance of New Active Forms of Trypsin Inhibitor in Germinating Mung Bean (Vigna radiata) Seeds. Plant Physiol. 1981 Jul;68(1):88–92. doi: 10.1104/pp.68.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan-Wilson A. L., Rightmire B. R., Wilson K. A. Determination of relative antigen-antibody avidities by radial immunodiffusion. J Immunol Methods. 1983 Jun 24;61(1):99–106. doi: 10.1016/0022-1759(83)90013-3. [DOI] [PubMed] [Google Scholar]
- Van Der Wilden W., Chrispeels M. J. Characterization of the Isozymes of alpha-Mannosidase Located in the Cell Wall, Protein Bodies, and Endoplasmic Reticulum of Phaseolus vulgaris Cotyledons. Plant Physiol. 1983 Jan;71(1):82–87. doi: 10.1104/pp.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. A., Chen J. C. Amino Acid Sequence of Mung Bean Trypsin Inhibitor and Its Modified Forms Appearing during Germination. Plant Physiol. 1983 Feb;71(2):341–349. doi: 10.1104/pp.71.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. A., Tan-Wilson A. L. Proteinases involved in the degradation of trypsin inhibitor in germinating mung beans. Acta Biochim Pol. 1983;30(2):139–148. [PubMed] [Google Scholar]


