Abstract
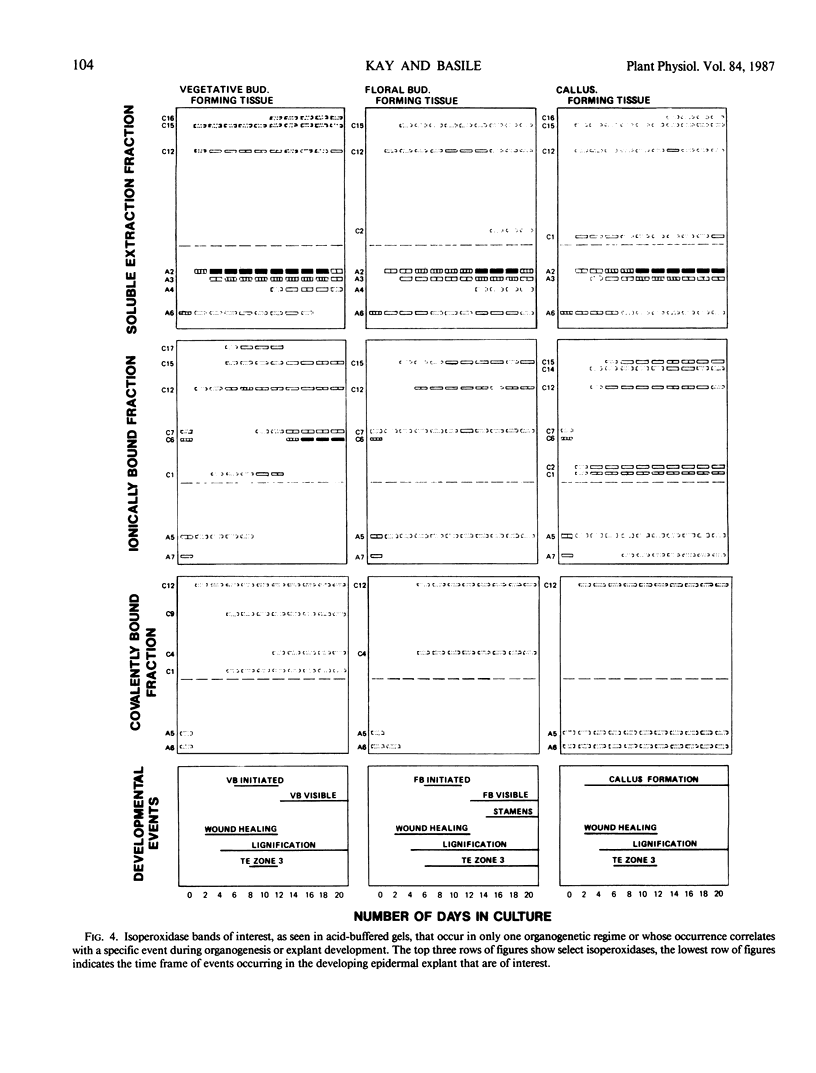
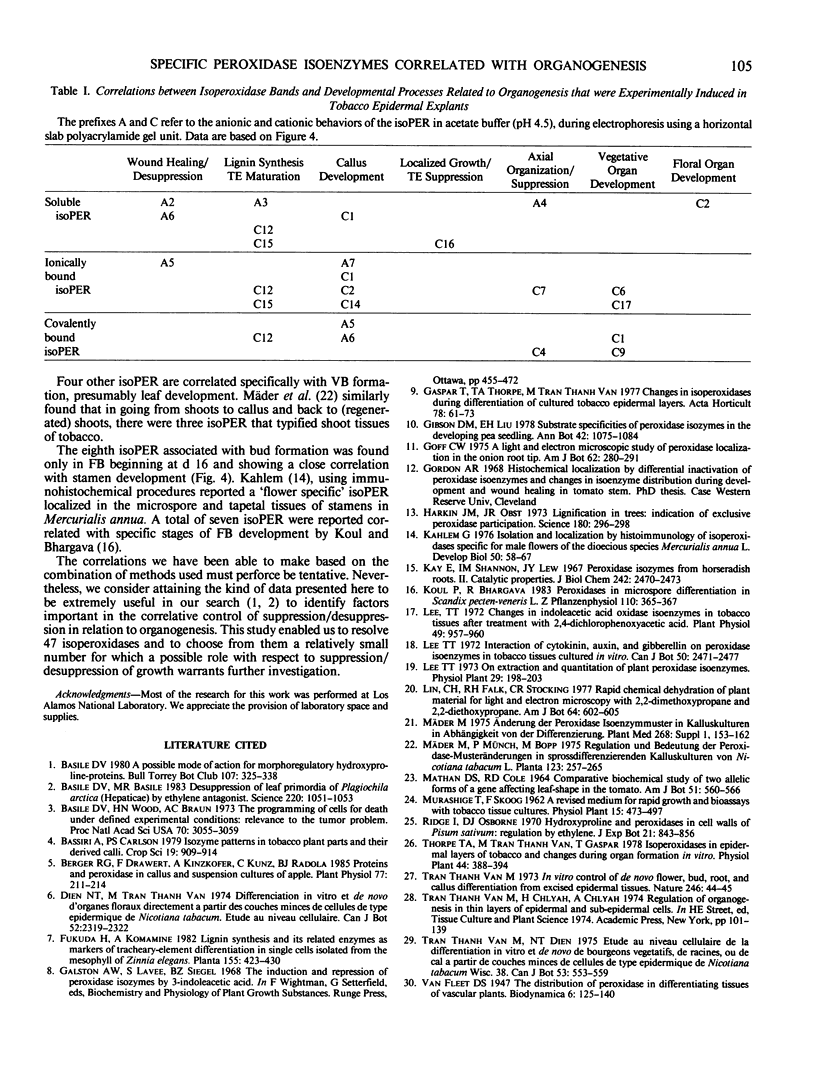
We have examined isoperoxidase patterns obtained from buffer-, salt-, and enzyme-extractable fractions and correlated them with histological changes in tobacco (Nicotiana tabacum L., cv Wisc. 38) `epidermal' explants induced to produce either callus, vegetative buds, or floral buds. By utilizing a combination of extraction and electrophoretic procedures different from any hitherto used for this kind of investigation, we were able to resolve 47 isoperoxidases distributed between the three types of fractions. The majority of these isoperoxidases were common to all explants regardless of their developmental fate. Correspondingly, a number of histological changes were observed in all explants (e.g. the initiation of cell division by day 2, lignin deposition by day 4, and the formation of clustered tracheary elements by day 8). We have made correlations between 25 isoperoxidases and specific developmental events based on the time when certain isoperoxidases were detected relative to observed histological changes: 3 were correlated with desuppressed/sustained cell division, 3 to 6 with lignification/tracheary element maturation, 7 with callus formation, 1 with localized suppression of growth, 3 with determinate axial organization, 4 with leaf development, and 1 with stamen development. These results suggest that a continued investigation using this system could lead to a better understanding of the role of specific isoperoxidases in different developmental processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basile D. V., Basile M. R. Desuppression of Leaf Primordia of Plagiochila arctica (Hepaticae) by Ethylene Antagonists. Science. 1983 Jun 3;220(4601):1051–1053. doi: 10.1126/science.220.4601.1051. [DOI] [PubMed] [Google Scholar]
- Basile D. V., Wood H. N., Braun A. C. Programming of cells for death under experimental conditions: relevance to the tumor problem. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3055–3059. doi: 10.1073/pnas.70.11.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R. G., Drawert F., Kinzkofer A., Kunz C., Radola B. J. Proteins and peroxidase in callus and suspension cultures of apple : a study using ultrathin-layer isoelectric focusing, sensitive silver staining of proteins, and peroxidase isozyme visualization. Plant Physiol. 1985 Jan;77(1):211–214. doi: 10.1104/pp.77.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin J. M., Obst J. R. Lignification in trees: indication of exclusive peroxidase participation. Science. 1973 Apr 20;180(4083):296–298. doi: 10.1126/science.180.4083.296. [DOI] [PubMed] [Google Scholar]
- Kahlem G. Isolation and localization by histoimmunology of isoperoxidases specific for male flowers of the dioecious species Mercurialis annua L. Dev Biol. 1976 May;50(1):58–67. doi: 10.1016/0012-1606(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- Lee T. T. Changes in indoleacetic Acid oxidase isoenzymes in tobacco tissues after treatment with 2,4-dichlorophenoxyacetic Acid. Plant Physiol. 1972 Jun;49(6):957–960. doi: 10.1104/pp.49.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäder M. Anderung der Peroxidase Isoenzymmuster in Kallus-Kulturen in Abhängigkeit von der Differenzierung. Planta Med. 1975;Suppl:153–162. doi: 10.1055/s-0028-1104776. [DOI] [PubMed] [Google Scholar]



