Abstract
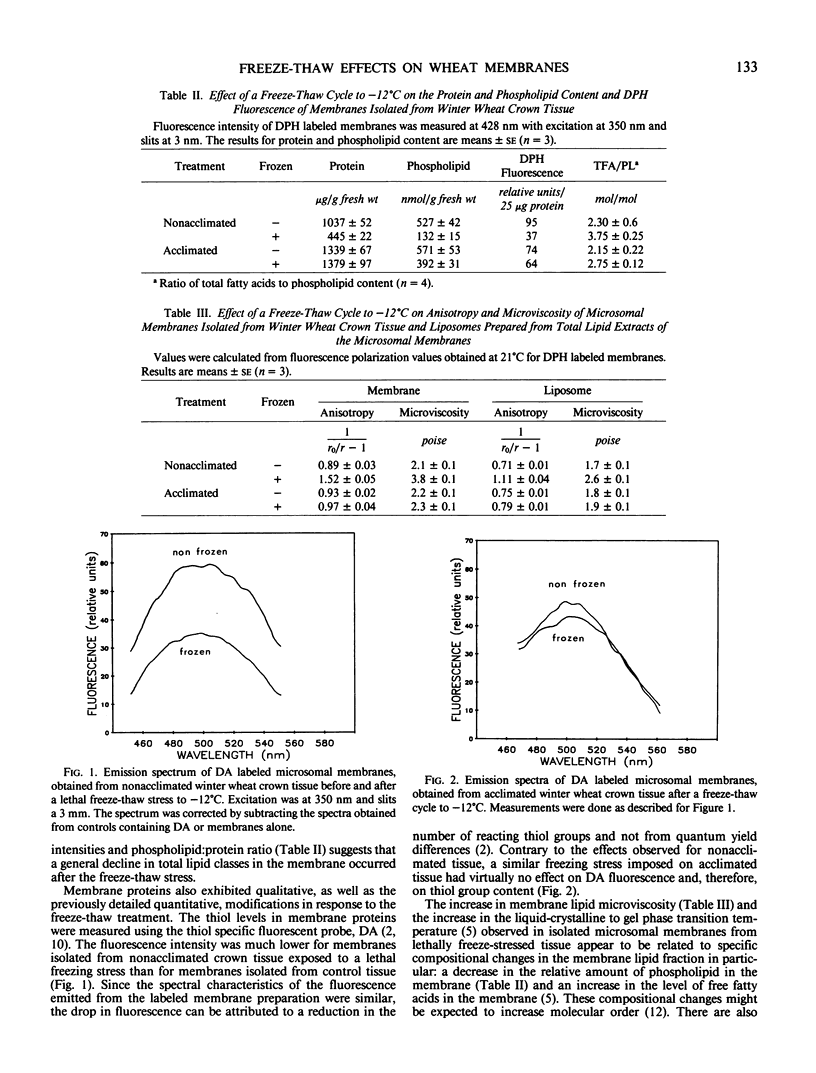
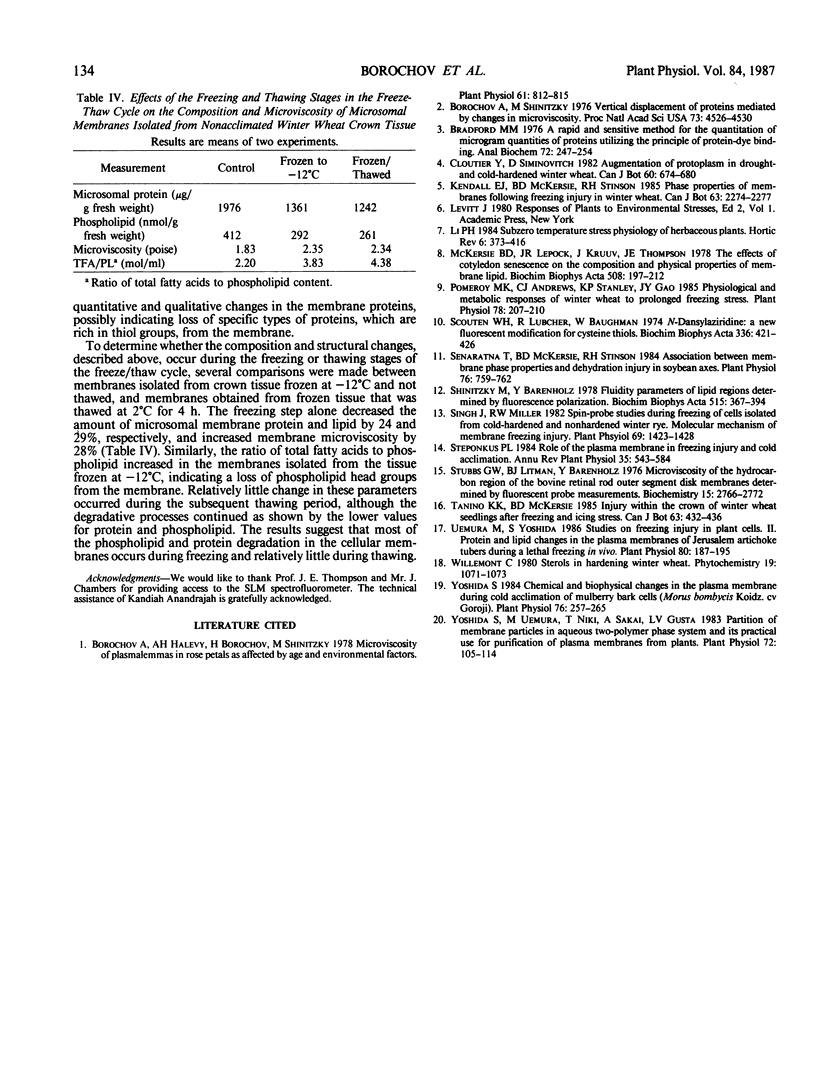
A freeze-thaw cycle to −12°C induced several physical and compositional changes in the microsomal membranes isolated from crown tissue of winter wheat (Triticum aestivum L. cv Frederick). Exposing 7-day-old, nonacclimated seedlings to a single freeze-thaw cycle prevented regrowth of the crown and resulted in increased membrane semipermeability. The phospholipid and protein content of microsomal membranes isolated from the crowns decreased by 70 and 50%, respectively. Microsomal membranes isolated after the lethal freeze-thaw stress, and liposomes prepared from total membrane lipids, exhibited greater microviscosity, measured by fluorescence polarization of 1,6-diphenyl-1,3,5-hexatriene. The number of free thiol groups per milligram membrane protein, measured using the specific fluorescent probe, N-dansylaziridine, decreased after freezing. In contrast, acclimated wheat seedlings which showed increased freezing tolerance, as indicated by survival and ion leakage, suffered almost no effects from the freeze thaw treatment as determined by measurements of membrane microviscosity, phospholipid content, protein content, or danzylaziridine fluorescence. An examination of membranes isolated from frozen tissue showed that most of the changes occurred during the freezing and not during the thawing phase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochov A., Halevy A. H. Microviscosity of plasmalemmas in rose petals as affected by age and environmental factors. Plant Physiol. 1978 May;61(5):812–815. doi: 10.1104/pp.61.5.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov H., Shinitzky M. Vertical displacement of membrane proteins mediated by changes in microviscosity. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4526–4530. doi: 10.1073/pnas.73.12.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. K., Andrews C. J., Stanley K. P., Ji-Yin-Gao Physiological and metabolic responses of winter wheat to prolonged freezing stress. Plant Physiol. 1985 May;78(1):207–210. doi: 10.1104/pp.78.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna T., McKersie B. D., Stinson R. H. Association between Membrane Phase Properties and Dehydration Injury in Soybean Axes. Plant Physiol. 1984 Nov;76(3):759–762. doi: 10.1104/pp.76.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Singh J., Miller R. W. Spin-Probe Studies during Freezing of Cells Isolated from Cold-Hardened and Nonhardened Winter Rye : MOLECULAR MECHANISM OF MEMBRANE FREEZING INJURY. Plant Physiol. 1982 Jun;69(6):1423–1428. doi: 10.1104/pp.69.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs G. W., Litman B. J. Microviscosity of the hydrocarbon region of the bovine retinal rod outer segment disk membrane determined by fluorescent probe measurements. Biochemistry. 1976 Jun 29;15(13):2766–2772. doi: 10.1021/bi00658a009. [DOI] [PubMed] [Google Scholar]
- Uemura M., Yoshida S. Studies on Freezing Injury in Plant Cells : II. Protein and Lipid Changes in the Plasma Membranes of Jerusalem Artichoke Tubers during a Lethal Freezing in Vivo. Plant Physiol. 1986 Jan;80(1):187–195. doi: 10.1104/pp.80.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Chemical and Biophysical Changes in the Plasma Membrane during Cold Acclimation of Mulberry Bark Cells (Morus bombycis Koidz. cv Goroji). Plant Physiol. 1984 Sep;76(1):257–265. doi: 10.1104/pp.76.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]


