Abstract
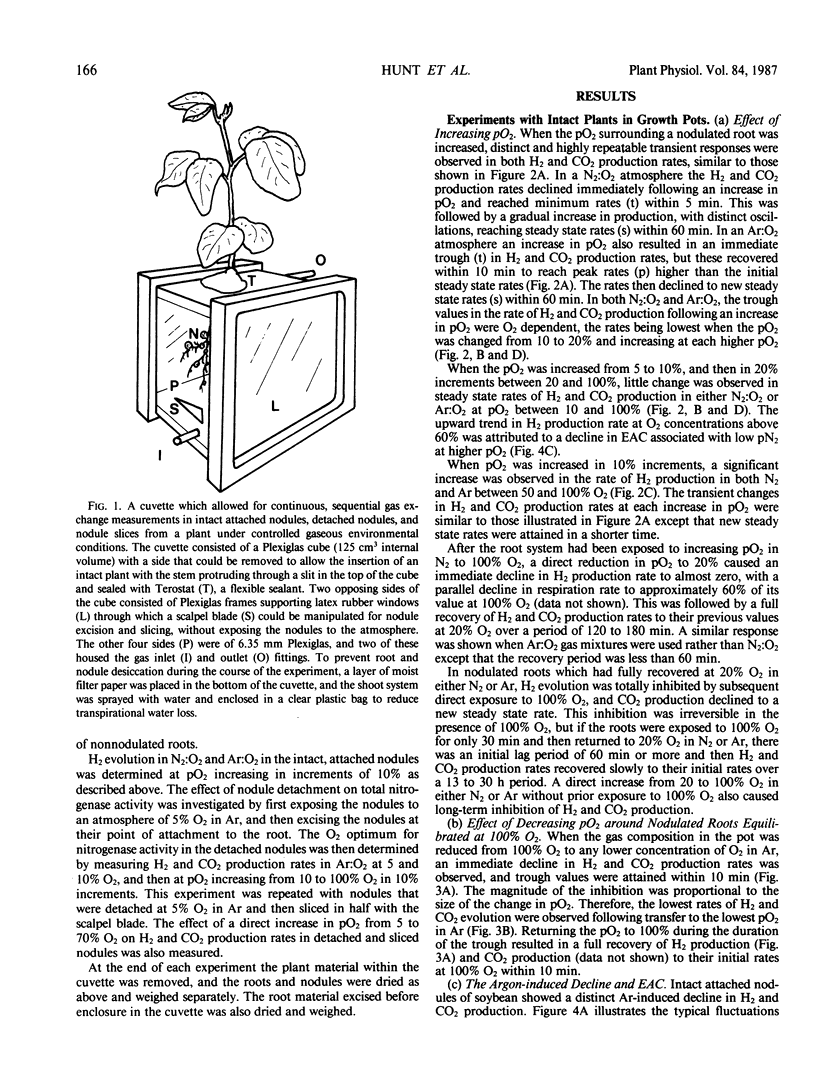
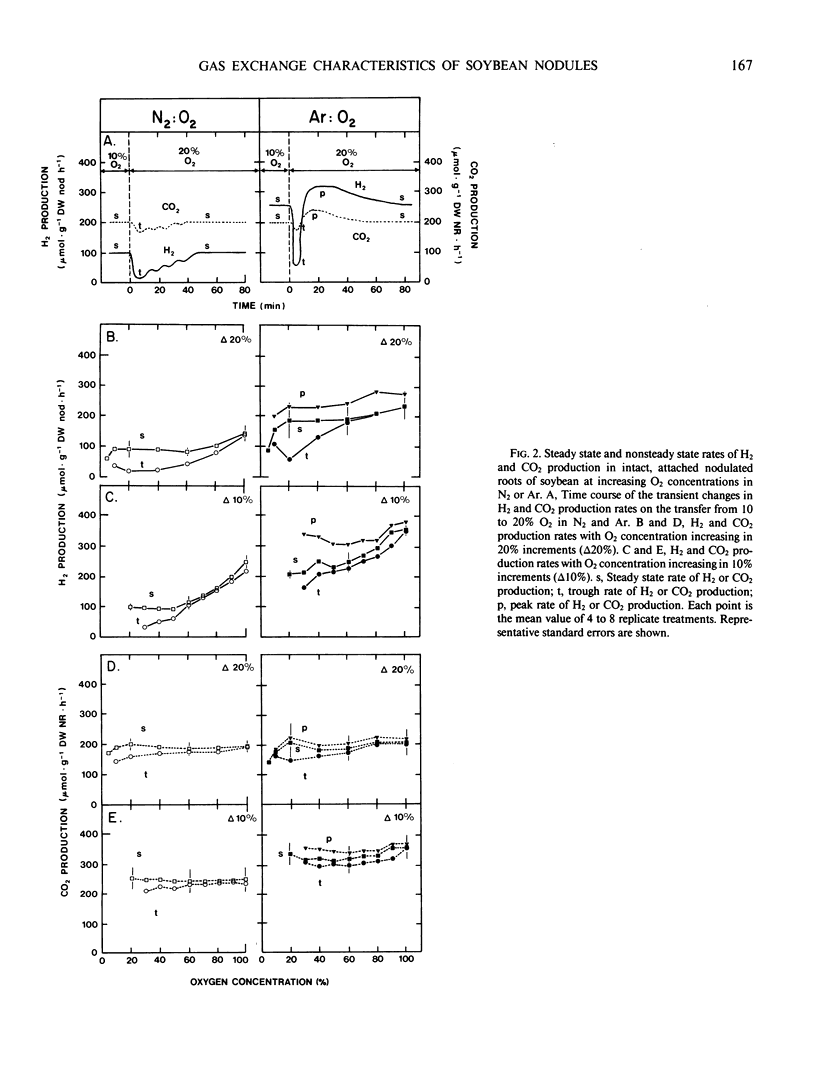
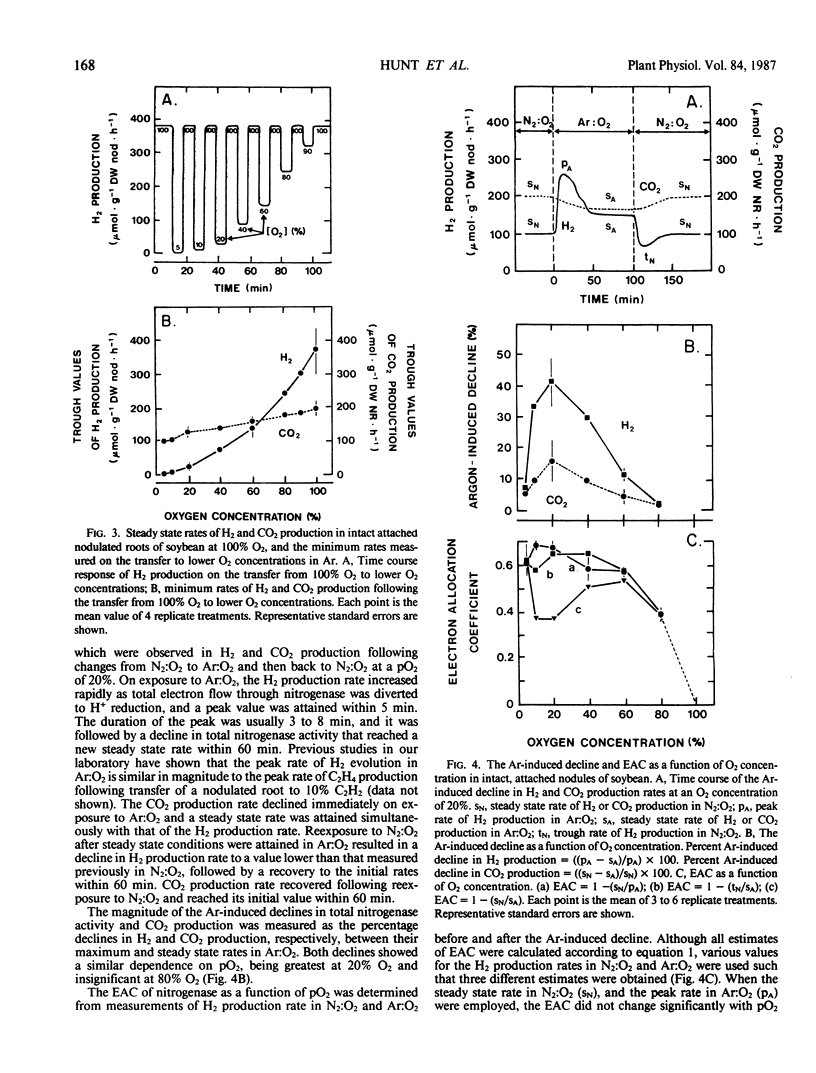
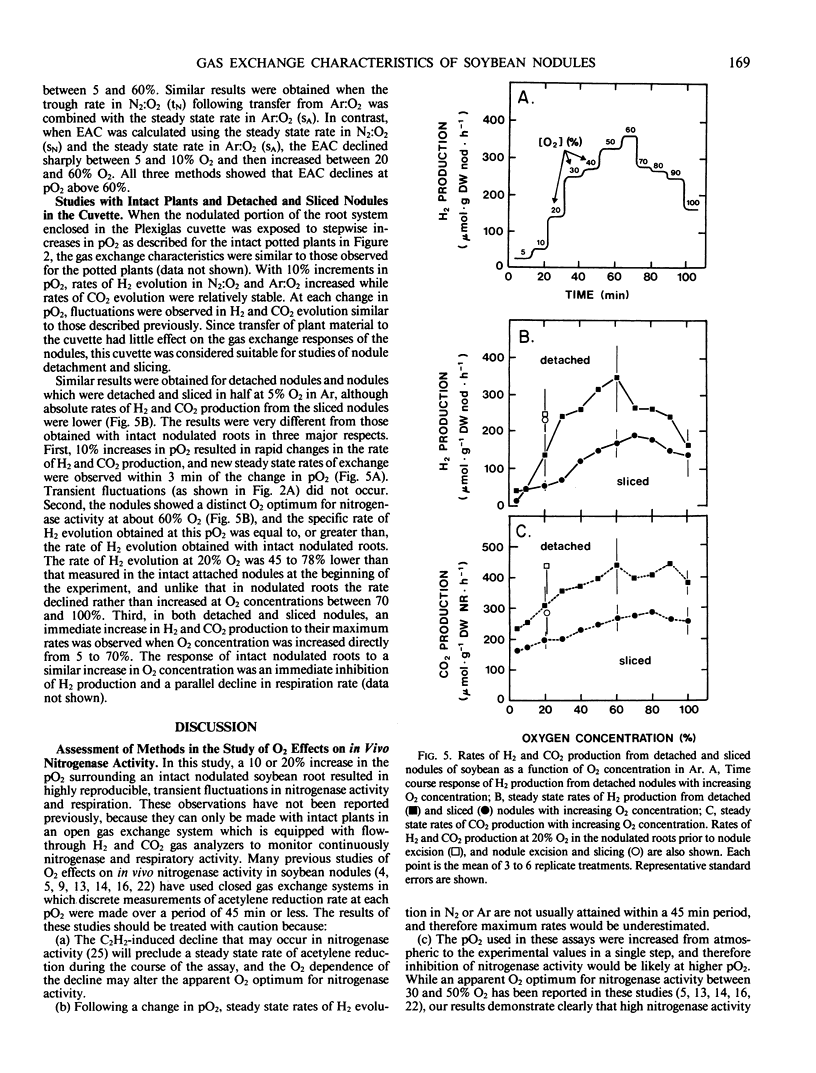
An open gas exchange system was used to monitor the nonsteady state and steady state changes in nitrogenase activity (H2 evolution in N2:O2 and Ar:O2) and respiration (CO2 evolution) in attached, excised, and sliced nodules of soybean (Glycine max L. Merr.) exposed to external pO2 of 5 to 100%. In attached nodules, increases in external pO2 in steps of 10 or 20% resulted in sharp declines in the rates of H2 and CO2 evolution. Recovery of these rates to values equal to or greater than their initial rates occurred within 10 to 60 minutes of exposure to the higher pO2. Recovery was more rapid at higher initial pO2 and in Ar:O2 compared to N2:O2. Sequential 10% increments in pO2 to 100% O2 resulted in rates of H2 evolution which were 1.4 to 1.7 times the steady state rate at 20% O2 in Ar. This was attributed to a relief at high pO2 from the 40% decline in nitrogenase activity that was induced by Ar at a pO2 of 20%. Changes in nodule respiration rate could not account for the nodules' ability to adjust to high external pO2, supporting the hypothesis that soybean nodules have a variable barrier to O2 diffusion which responds slowly (within minutes) to changes in pO2. Nodule excision and slicing resulted in 45 and 78% declines, respectively, in total specific nitrogenase activity at 20% O2. In contrast with the result obtained with intact nodules, subsequent 10% increases in pO2 in Ar:O2 did not result in transient declines in H2 evolution rates, but in the rapid attainment of new steady state rates. Also, distinct optima in nitrogenase activity were observed at about 60% O2. These results were consistent with an increase in the diffusive resistance of the nodule cortex following nodule excision or nodule slicing. This work also shows the importance of using intact plants and continuous measurements of gas exchange in studies of O2 diffusion and nitrogenase activity in legume nodules.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Criswell J. G., Havelka U. D., Quebedeaux B., Hardy R. W. Adaptation of Nitrogen Fixation by Intact Soybean Nodules to Altered Rhizosphere pO(2). Plant Physiol. 1976 Nov;58(5):622–625. doi: 10.1104/pp.58.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevon J. J., Frazier L., Russell S. A., Evans H. J. Respiratory and Nitrogenase Activities of Soybean Nodules Formed by Hydrogen Uptake Negative (Hup) Mutant and Revertant Strains of Rhizobium japonicum Characterized by Protein Patterns. Plant Physiol. 1982 Nov;70(5):1341–1346. doi: 10.1104/pp.70.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edie S. A., Phillips D. A. Effect of the host legume on acetylene reduction and hydrogen evolution by Rhizobium nitrogenase. Plant Physiol. 1983 May;72(1):156–160. doi: 10.1104/pp.72.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Hanus F. J., Russell S. A., Evans H. J. Determination of the hydrogenase status of individual legume nodules by a methylene blue reduction assay. Appl Environ Microbiol. 1985 Aug;50(2):537–539. doi: 10.1128/aem.50.2.537-539.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Weagle G. E., Canvin D. T. A highly sensitive, flow through h(2) gas analyzer for use in nitrogen fixation studies. Plant Physiol. 1984 Jul;75(3):582–585. doi: 10.1104/pp.75.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson T. G., Peterson J. B., Larue T. A. Effect of supra-ambient oxygen on nitrogenase activity (c(2)h(2)) and root respiration of soybeans and isolated soybean bacteroids. Plant Physiol. 1983 Jul;72(3):695–700. doi: 10.1104/pp.72.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Layzell D. B. Carbon and nitrogen assimilation and partitioning in soybeans exposed to low root temperatures. Plant Physiol. 1986 Jan;80(1):249–255. doi: 10.1104/pp.80.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


