ABSTRACT
Acute exacerbation (AE) of interstitial pneumonia (IP) shows poor prognosis, due to the typical histological pattern of diffuse alveolar damage superimposed upon lung fibrosis. The previous reports comparing clinical features between AE of idiopathic interstitial pneumonias (IIPs) and those of IPs with known etiology are limited. We retrospectively compared clinical parameters including age, sex, Charlson Comorbidity Index score (CCIS), blood biomarkers at diagnosis of AE, treatment, and 3-month mortality between patients with AE of IIPs and collagen vascular disease-associated interstitial pneumonia (CVD-IP). We assessed 85 patients, comprising 66 patients with AE of IIPs (78%) and 19 patients with AE of CVD-IP (22%). The least absolute shrinkage and selection operator regression selected CCIS (hazard ratio, 1.281; 95% confidence interval, 1.055–1.556; P = 0.012) and log serum lactate dehydrogenase (LDH) (hazard ratio, 6.267; 95% confidence interval, 2.172–18.085; P < 0.001) as significant predictors of 3-month mortality among these patients. Also, the adjusted survival curves using sex, CCIS, and serum LDH showed no significant differences between these two groups. In conclusion, among AE patients, CCIS and serum LDH level may be more important prognostic factors for 3-month mortality rather than two classification of IP subtypes: IIPs and CVD-IP.
Key Words: acute exacerbation, Charlson Comorbidity Index score, collagen vascular disease-associated interstitial pneumonia, idiopathic interstitial pneumonia, lactate dehydrogenase
INTRODUCTION
Subtypes of underlying interstitial pneumonia (IP) comprise idiopathic interstitial pneumonias (IIPs), including idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP), and etiology-known IPs such as collagen vascular disease (CVD) and chronic hypersensitivity pneumonia. Acute exacerbation (AE) is generally accepted to occur not only in IIPs, but also etiology-known IPs.1-3 The prognosis of AE of IP is poor, which the histological pattern typically involves diffuse alveolar damage superimposed upon lung fibrosis without obvious clinical causes like fluid overload, left heart failure, or pulmonary embolism.4-8 Patients with AE of IPF have reportedly shown in-hospital mortality rates in excess of 50%.9-11 In the retrospective cohort study including IPF and non-IPF patients, overall survival rates for AE were 67% at 30 days, 43% at 60 days, and 40% at 90 days after admission.12 Other reports have shown mortality rates for AE of collagen vascular disease-associated IP (CVD-IP) ranging from 34% to 83%.3,13
From the above, the prognosis of AE of IP is highly variable, and may be affected not only by the subtype of underlying IP, but also by various background factors including age, sex, comorbidities, and triggers of AE onset such as infection, drugs, and surgery. Especially, evaluation of comorbidities appears important for determining the prognosis of IP. Several studies have reported significant negative impacts of arteriosclerosis, other cardiovascular diseases, congestive heart failure, and lung cancer among patients with IPF.14,15 In previous reports, we have proposed that the Charlson Comorbidity Index score (CCIS), which reflects the presence and severity of comorbidities, also exerts a significant impact on treatment prognosis for AE-IP patients.16,17 The accuracy of prediction of disease prognosis may be improved by considering comorbidities (CCIS) among patients with subtypes of IP during AE.
This retrospective cohort study aimed to clarify prognostic factors in the AE of IP patients and compared treatment prognosis according to the etiology of IP, including idiopathic disease and CVD.
MATERIALS AND METHODS
Study location and patients
This retrospective cohort study involved patients hospitalized between 2014 and 2018 at Yokohama City University Hospital and Yokohama City University Medical Center. Medical data were assessed for 85 patients with acute or subacute IIPs presenting respiratory failure which required steroid pulse therapy, including AE of IPF and NSIP, acute interstitial pneumonia (AIP), cryptogenic organizing pneumonia (COP), or AE of CVD-IP. Patients who had not received steroid pulse therapy were excluded. From the previous studied for the assessment of prognostic indicators in patients with AE of IPs, we collected for clinical data at the time of starting steroid pulse therapy including age, sex, diagnosis of IP, CCIS, blood parameters (partial pressure of oxygen in arterial blood/fraction of inspired oxygen [PaO2/FiO2], Krebs von den Lungen-6 [KL-6; normal <500 U/mL], lactate dehydrogenase [LDH; normal, <225 U/L]), high-resolution computed tomography (HRCT) scores (including ground-glass opacity (GGO) and honeycomb scores) as assessed independently by two pulmonologists and one radiologists, and treatment regimens (sivelestat Na hydrate, anticoagulation therapy, steroid use before and after steroid pulse therapy, and immunosuppressants).17-20 We compared the extracted data between acute and subacute IIPs and AEs of CVD-IP.
Diagnosis of IP
Subtypes of IIP were confirmed from physical, serological, HRCT, and lung pathological findings, in accordance with the official statement for IIPs.1,21 Patients for whom lung biopsy could not be performed due to severe hypoxemia were diagnosed based on the HRCT classification.1,21 The diagnosis of CVD-IP was confirmed by physical, serological, and HRCT findings consistent with IP, and lung biopsy was performed to exclude other pulmonary diseases. AE of IP was defined as: worsening of hypoxemia reflecting severely impaired gas exchange; worsening of dyspnea; new appearance of alveolar infiltration on radiography; and absence of alternative etiologies including pneumothorax, pulmonary embolism, infection, or heart failure.2-5
Statistical analyses
Data were statistically analyzed using JMP12 (SAS Institute Inc, Cary, NC, USA) and are expressed as medians with 25th–75th percentiles or numbers and percentages. Groups were compared using Wilcoxon rank-sum tests. Survival curves were generated using the Kaplan-Meier method and compared using log-rank tests. Candidate variables for predictors of 3-month mortality were selected based on previous studies and were evaluated the statistical significance using the least absolute shrinkage and selection operator (Lasso) regression.17,18 Differences showing values of P < 0.05 were considered significant.
Study approval
This research was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board at Yokohama City University Hospital (approval no. B171100003). In this retrospective study, consent for participation was obtained by disclosing the clinical study with a description of the opt-out process (https://www.yokohama-cu.ac.jp/amedrc/ethics/ethical/fuzoku_optout.html). The severely ill or deeply sedated condition of AE of IP patients precluded obtaining informed consent from the patients themselves. Written informed consent was therefore obtained from the relatives or legal guardians of patients.
RESULTS
Patient characteristics
Table 1 shows the characteristics of the study participants. The 85 patients included 66 diagnosed with acute or subacute IIPs, including AE of IPF in 37 and other IIPs in 29 (AE of idiopathic NSIP, n = 12; AIP, n = 12; COP, n = 5). The remaining 19 patients have AE of CVD-IP. The CVD-IP involved rheumatoid arthritis in 9 cases, vasculitis in 5, polymyositis/dermatomyositis in 4, and Sjögren syndrome in 1. Causes of AE were idiopathic in 61 cases, infection in 5, and aspiration in 2. All patients had been treated with steroid pulse therapy. Tables 2 and 3 show the clinical differences between patients with IIP and CVD-IP. No significant differences were evident in 3- or 6-month survival rates between groups, or in clinical parameters other than incidence of male sex and immunosuppressant use before pulse therapy.
Table 1.
Patient characteristic
| Characteristic | Total patients (N = 85) |
| Age, years | 76 (71–82) |
| Male, sex | 62 (73) |
| CCIS | 2 (1–3.5) |
| Diagnosis of AE | |
| IIP | |
| IPF | 37 (44) |
| Non-IPF IIP | 29 (34) |
| CVD-IP | 19 (22) |
| Blood biomarkers | |
| PaO2/FiO2 ratio | 268 (177–308) |
| LDH, IU/L | 279 (235–387) |
| KL-6, U/mL | 925 (565–1569) |
| HRCT scores | |
| Honeycomb score | 2 (0–6) |
| GGO score | 10 (7–13) |
| Treatments | |
| PSL before pulse | 19 (22) |
| PSL pulse | 85 (100) |
| PSL after pulse | 73 (86) |
| Anticoagulant | 17 (20) |
| Neutrophil elastase inhibitor | 8 (9) |
| Immunosuppressant before pulse | 5 (6) |
| Immunosuppressant pulse | 4 (5) |
| Immunosuppressant after pulse | 18 (21) |
| Outcome | |
| 3-month mortality | 19 (22) |
| 6-month mortality | 23 (27) |
AE: acute exacerbation
CCIS: Charlson Comorbidity Index score
CVD-IP: collagen vasculitis disease-associated interstitial pneumonia
GGO: ground-glass opacity
HRCT: high-resolution computed tomography
IIP: idiopathic interstitial pneumonia
IPF: idiopathic pulmonary fibrosis
KL-6: Krebs von den Lungen-6
LDH: lactate dehydrogenase
PaO2/FiO2 ratio: partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen
PSL: prednisolone
Results are shown as medians with 25th – 75th percentiles or numbers (%). PaO2/FiO2 ratio was able to be calculated for 84 patients.
Table 2.
Comparison of baseline data between patients with AE of IIP and AE of CVD-IP
| Characteristic |
AE of IIP
(N = 66) |
AE of CVD-IP
(N = 19) |
P value |
| Age, years | 76 (71–82) | 77 (73–79) | 0.920 |
| Male sex | 52 (79) | 10 (53) | 0.029 |
| CCIS | 2 (1–4) | 2 (1–3) | 0.898 |
| Blood biomarkers | |||
| PaO2/FiO2 ratio | 266 (183–309) | 296 (148–325) | 0.662 |
| LDH, IU/L | 286 (237–412) | 251 (231–340) | 0.094 |
| KL-6, U/mL | 944 (527–1765) | 870 (588–1210) | 0.587 |
| HRCT scores | |||
| Honeycomb score | 1 (0–5.3) | 3 (0–7) | 0.156 |
| GGO score | 10 (6.8–13) | 10 (8–13) | 0.836 |
AE: acute exacerbation
CCIS: Charlson Comorbidity Index score
CVD-IP: collagen vasculitis disease-associated interstitial pneumonia
GGO: ground-glass opacity
HRCT: high-resolution computed tomography
IIPs: idiopathic interstitial pneumonia
KL-6: Krebs von den Lungen-6
LDH: lactate dehydrogenase
PaO2/FiO2 ratio: partial pressure of oxygen in arterial blood/fraction of inspiratory oxygen
Results are shown as medians with 25th – 75th percentiles or numbers (%).
Table 3.
Comparison of treatment and outcomes between patients with AE of IIP and AE of CVD-IP
| Characteristic |
AE of IIP
(n = 66) |
AE of CVD-IP
(n = 19) |
P values |
| Treatments | |||
| PSL before pulse | 12 (18) | 7 (37) | 0.085 |
| PSL pulse | 66 (100) | 19 (100) | 1.000 |
| PSL after pulse | 55 (83) | 18 (95) | 0.208 |
| Anticoagulant | 14 (21) | 3 (16) | 0.603 |
| Neutrophil elastase inhibitor | 8 (12) | 0 (0) | 0.111 |
| Immunosuppressant before pulse | 2 (3) | 3 (16) | 0.040 |
| Immunosuppressant pulse | 3 (5) | 1 (5) | 0.896 |
| Immunosuppressant after pulse | 10 (15) | 8 (42) | 0.011 |
| Outcomes | |||
| 3-month mortality | 17 (26) | 2 (11) | 0.160 |
| 6-month mortality | 20 (30) | 3 (16) | 0.210 |
AE: acute exacerbation
CVD-IP: collagen vasculitis disease-associated interstitial pneumonia
IIP: idiopathic interstitial pneumonia
PSL: prednisolone
Results are shown as medians with 25th - 75th percentiles or numbers (%).
Logistic Lasso regression
The variables of age, male (vs female) sex, IIP (vs CVD-IP), serum LDH, serum KL-6, PaO2/FiO2 ratio, CCIS, and honeycomb and GGO scores were assessed using logistic Lasso regression (Table 4). CCIS (odds ratio [OR], 1.281; 95% confidence interval [CI], 1.055–1.556; P = 0.012) and log serum LDH (OR, 6.267; 95%CI, 2.172–18.085; P < 0.001) were significant predictors of 3-month mortality. On the other hand, IP diagnosis of IIP or CVD-IP was not significant.
Table 4.
The least absolute shrinkage and selection operator regression for primary predictors of 3-month mortality
| Variable | Hazard ratio | 95% Confidence interval | P values |
| IIP vs CVD-IP | 0.931 | 0.198–4.382 | 0.928 |
| CCIS | 1.281 | 1.055–1.556 | 0.012 |
| Sex (vs female) | 6.587 | 0.844–51.393 | 0.072 |
| Log serum LDH | 6.267 | 2.172–18.085 | < 0.001 |
IIP: idiopathic interstitial pneumonias
CCIS: Charlson Comorbidity Index score
CVD-IP: collagen vasculitis disease-associated interstitial pneumonia
LDH: lactate dehydrogenase
Survival curves of patients with AE of IIPs and AE of CVD-IP
The enrolled patients comprised 19 patients with CVD-IPs and 66 patients with IIPs. A tendency was seen toward a difference in 3-month prognosis among these groups, but the result was not significant (P = 0.314) (Fig. 1). Using the model including sex, CCIS, serum LDH, adjusted survival curves of patients with AE of IIPs and AE of CVD-IPs provided similar results (Fig. 2).
Fig. 1.
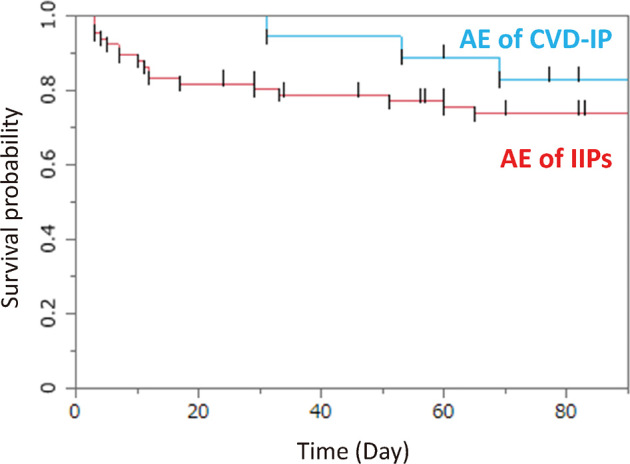
Survival curves for patients with AE of IIP and AE of CVD-IP
The enrolled patients comprised 19 patients with CVD-IPs and 66 patients with IIPs. A tendency was seen, but no significant difference in 3-month prognosis was identified among groups (P = 0.314).
AE: acute exacerbation
IIP: idiopathic interstitial pneumonia
CVD-IP: collagen vascular disease-associated interstitial pneumonia
Fig. 2.
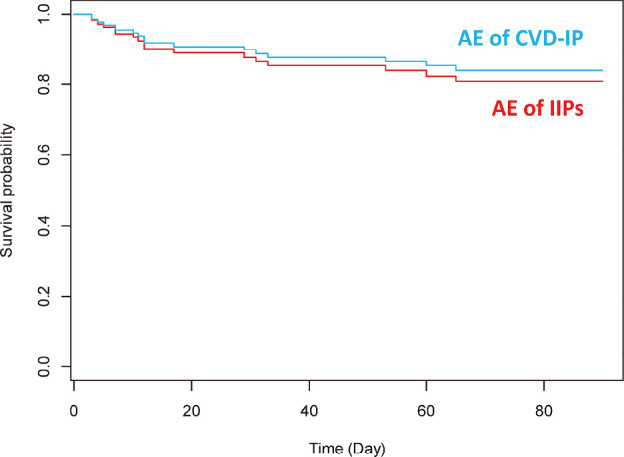
Adjusted survival curves for patients with AE of IIP and AE of CVD-IP using the model including sex, CCIS, and serum LDH
Using the model including sex, CCIS, and serum LDH, adjusted survival curves for patients with AE of IIP or AE of CVD-IP proved to be similar among patients with CVD-IP and those with IIP.
AE: acute exacerbation
IIP: idiopathic interstitial pneumonia
CCIS: Charlson Comorbidity Index score
CVD-IP: collagen vascular disease-associated interstitial pneumonia
LDH: lactate dehydrogenase
DISCUSSION
The previous reports comparing clinical features between AE of IIPs and those of IPs with known etiology are limited. The purpose of this study was to evaluate the clinical course, outcomes, and prognostic factors for AE of IIP and AE of CVD-IP and we found that no differences in 3-month survival rates after AE were seen between these two groups.
Factors contributing to survival in both groups were CCIS and log serum LDH at the start of treatment. The CCIS represents a total score for 19 comorbidities weighted according to severity. The CCIS was developed to assess the risk of death from comorbidities and has been widely applied as a prognostic indicator for patients with colorectal cancer, advanced non-small cell lung cancer, and acute myocardial infarction.19 Although few reports have examined the relationship between IP and comorbidities, patients with comorbidities such as cardiovascular disease (atherosclerosis, valvular disease, arrhythmia, dilated cardiomyopathy, etc) and lung cancer in IPF show significantly lower survival rates.14,15 We have also reported that CCIS has a significant impact on the prognosis of IP patients with or without AE.16 Serum LDH levels reflect the degree of active lung inflammation and direct lung cell damage, and higher levels reflect the disease activity in AE. As a result, serum LDH can be an important prognostic factor for AE-IP patients.15,22,23 In the present study, we found that comorbidities and serum LDH levels strongly influenced 3-month survival in both IIP and CVD-IP patients after the onset of AE. Measuring both clinical parameters is important in determining the treatment and prognosis of patients with AE-IP.
Various studies have reported on differences in prognosis according to the type of IP. In the stable phase of pleuroparenchymal fibroelastosis, a subtype of IP, no difference in survival was evident between idiopathic and secondary forms.24 No difference in prognosis was seen between IPF and unclassifiable IIP after the onset of AE (log-rank, P = 0.681).25 In a study of 15 cases showing AE of CVD-IP, the 90-day survival rate (33%) was similar to that of IIP (44%, P = 0.44).13 Similar to previous reports, our study found no difference in 3-month survival rates between IIP and CVD-IP after the onset of AE. A certain number of patients died early after the onset of AE in both groups, but subsequent clinical courses were similar. No difference in survival was seen according to treatment modality, nor was any improvement seen from immunosuppressive agents or neutrophil elastase inhibitors. On the other hand, the results in this study are likely to depend on how many cases of IPF were included in the IIPs group, because Miyashita K et al reported that AE of IP other than IPF might have a better prognosis than AE of IPF.26 Interestingly, in this study, the clinical characteristics including age, serum LDH, serum KL-6, PaO2/FiO2 ratio, CCIS, HRCT scores, and survival curves between AE of IP other than IPF and AE of IPF were statistically similar (supplement figure), however, we have to perform the validation study to compare the disease outcomes including much more cases diagnosed with IPF, IP other than IP, and CVD-IP.
A combined approach from various clinical parameters has been proposed to obtain more accurate prognostic information. Several studies have investigated the prediction of 3-month survival in IP-AE. Kishaba et al reported that a composite scoring system based on serum LDH level, KL-6 level, ratio of partial pressure of oxygen to partial pressure of inspiratory oxygen, and range of abnormal findings on HRCT was prognostic.18 Murohashi et al reported sex, serum LDH level, and CCIS as useful.17 From the above, it is extremely important to establish the scoring model that includes parameters that have been reported as important including CCIS, serum LDH or KL-6, oxygenation, and HRCT findings) and conduct large-scale validation in the future.
Several limitations to this study must be kept in mind. First, this study was retrospective in design and had the limitations of small sample size. To ensure the reproducibility of results, re-validation in a more extensive study with a larger number of study subjects is needed. Second, the clinical diagnoses of patients enrolled in the CVD-IP were heterogeneous. Third, histopathological evaluations were not performed in some patients after the onset of AE because of severe respiratory failure.
In conclusion, the present study found no significant difference in 3-month survival rates of IIP and CVD-IP after AE onset. Also, among AE patients, CCIS and serum LDH level may be more important prognostic factors for 3-month mortality rather than two classification of IP subtypes: IIPs and CVD-IP.
DECLARATION OF COMPETING INTEREST
The authors declare that they have no conflicts of interest with respect to this study.
Supplementary Materials
Survival curves for patients with AE of IIP other than IPF and AE of IPF
Abbreviations
- AE
acute exacerbation
- CCIS
Charlson Comorbidity Index score
- CVD
collagen vascular disease
- CVD-IP
collagen vascular disease-associated interstitial pneumonia
- HRCT
high-resolution computed tomography
- IIPs
idiopathic interstitial pneumonias
- IP
interstitial pneumonia
- IPF
idiopathic pulmonary fibrosis
- KL-6
Krebs von den Lungen-6
- LDH
lactate dehydrogenase
REFERENCES
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed]
- 2.Park IN, Kim DS, Shim TS, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132(1):214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed]
- 3.Suda T, Kaida Y, Nakamura Y, et al. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103(6):846–853. doi: 10.1016/j.rmed.2008.12.019. [DOI] [PMC free article] [PubMed]
- 4.Hyzy R, Huang S, Myers J, Flaherty K, Martinez F. Acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2007;132(5):1652–1658. doi: 10.1378/chest.07-0299. [DOI] [PubMed]
- 5.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed]
- 6.Ferguson ND, Frutos-Vivar F, Esteban A, et al. Acute respiratory distress syndrome: underrecognition by clinicians and diagnostic accuracy of three clinical definitions. Crit Care Med. 2005;33(10):2228–2234. doi: 10.1097/01.CCM.0000181529.08630.49. [DOI] [PubMed]
- 7.Daniels CE, Yi ES, Ryu JH. Autopsy findings in 42 consecutive patients with idiopathic pulmonary fibrosis. Eur Respir J. 2008;32(1):170–174. doi: 10.1183/09031936.00176307. [DOI] [PubMed]
- 8.Oda K, Ishimoto H, Yamada S, et al. Autopsy analyses in acute exacerbation of idiopathic pulmonary fibrosis. Respir Res. 2014;15(1):109. doi: 10.1186/s12931-014-0109-y. [DOI] [PMC free article] [PubMed]
- 9.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27(1):143–150. doi: 10.1183/09031936.06.00114004. [DOI] [PubMed]
- 10.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed]
- 11.Al-Hameed FM, Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11(2):117–122. doi: 10.1155/2004/379723. [DOI] [PubMed]
- 12.Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open. 2013;3(7):e002971. doi: 10.1136/bmjopen-2013-002971. [DOI] [PMC free article] [PubMed]
- 13.Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration. 2012;83(1):20–27. doi: 10.1159/000329893. [DOI] [PubMed]
- 14.Kreuter M, Ehlers-Tenenbaum S, Palmowski K, et al. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS One. 2016;11(3):e0151425. doi: 10.1371/journal.pone.0151425. [DOI] [PMC free article] [PubMed]
- 15.Kakugawa T, Sakamoto N, Sato S, et al. Risk factors for an acute exacerbation of idiopathic pulmonary fibrosis. Respir Res. 2016;17(1):79. doi: 10.1186/s12931-016-0400-1. [DOI] [PMC free article] [PubMed]
- 16.Yagyu H, Murohashi K, Hara Y, et al. Clinical utility of a composite scoring system including Charlson Comorbidity Index score in patients with interstitial lung disease. J Thorac Dis. 2020;12(10):5774–5782. doi: 10.21037/jtd-20-1302. [DOI] [PMC free article] [PubMed]
- 17.Murohashi K, Hara Y, Saigusa Y, et al. Clinical significance of charlson comorbidity index as a prognostic parameter for patients with acute or subacute idiopathic interstitial pneumonias and acute exacerbation of collagen vascular diseases-related interstitial pneumonia. J Thorac Dis. 2019;11(6):2448–2457. doi: 10.21037/jtd.2019.05.46. [DOI] [PMC free article] [PubMed]
- 18.Kishaba T, Tamaki H, Shimaoka Y, Fukuyama H, Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192(1):141–149. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed]
- 20.Ooi GC, Mok MY, Tsang KWT, et al. Interstitial lung disease in systemic sclerosis. Acta Radiol. 2003;44(3):258–264. doi: 10.1034/j.1600-0455.2003.00058.x. [DOI] [PubMed]
- 21.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed]
- 22.DeRemee RA. Serum Lactic Dehydrogenase Activity and Diffuse Interstitial Pneumonitis. JAMA. 1968;204(13):1193–1195. doi: 10.1001/jama.1968.03140260033015. [DOI] [PubMed]
- 23.Hara Y, Shinkai M, Kanoh S, et al. Arterial Carboxyhemoglobin Measurement Is Useful for Evaluating Pulmonary Inflammation in Subjects with Interstitial Lung Disease. Intern Med. 2017;56(6):621–626. doi: 10.2169/internalmedicine.56.7418. [DOI] [PMC free article] [PubMed]
- 24.Oda T, Sekine A, Tabata E, Iwasawa T, Takemura T, Ogura T. Comparison of Clinical Characteristics and Outcomes between Idiopathic and Secondary Pleuroparenchymal Fibroelastosis. J Clin Med. 2021;10(4):846. doi: 10.3390/jcm10040846. [DOI] [PMC free article] [PubMed]
- 25.Enomoto N, Naoi H, Aono Y, et al. Acute exacerbation of unclassifiable idiopathic interstitial pneumonia: comparison with idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2020;14:1753466620935774. doi: 10.1177/1753466620935774. [DOI] [PMC free article] [PubMed]
- 26.Miyashita K, Kono M, Saito G, et al. Prognosis after acute exacerbation in patients with interstitial lung disease other than idiopathic pulmonary fibrosis. Clin Respir J. 2021;15(3):336–344. doi: 10.1111/crj.13304. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival curves for patients with AE of IIP other than IPF and AE of IPF


