Abstract
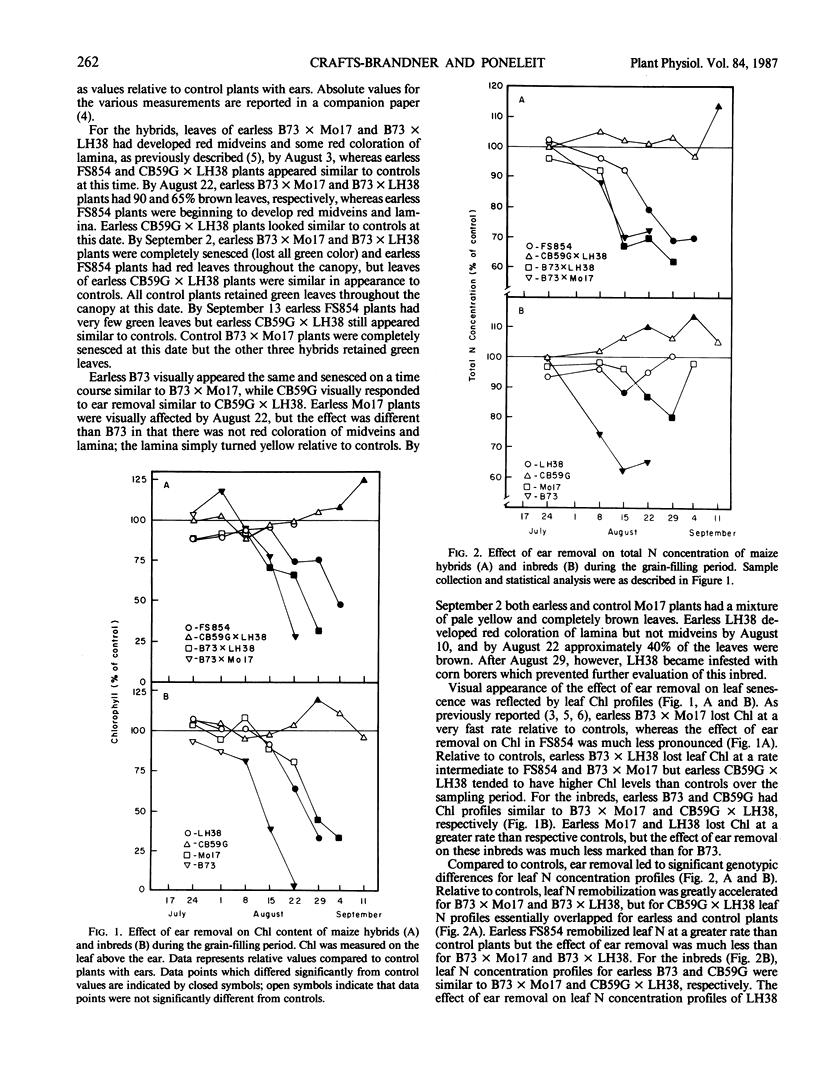
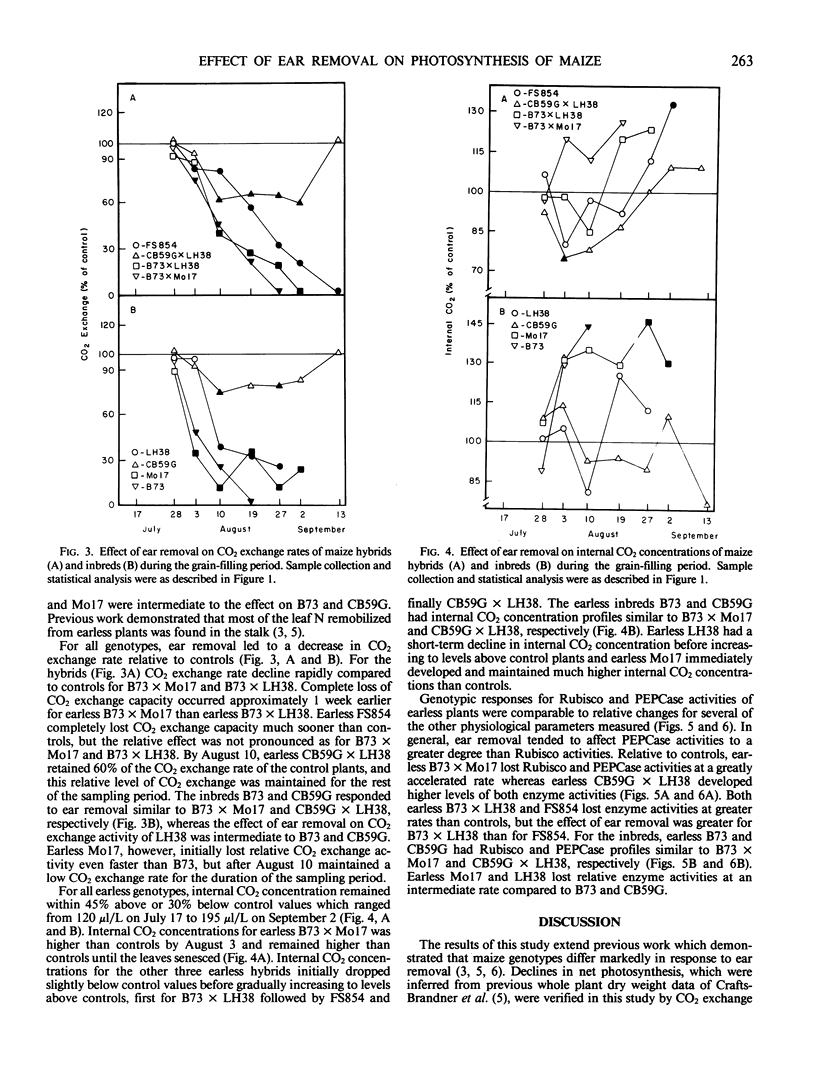
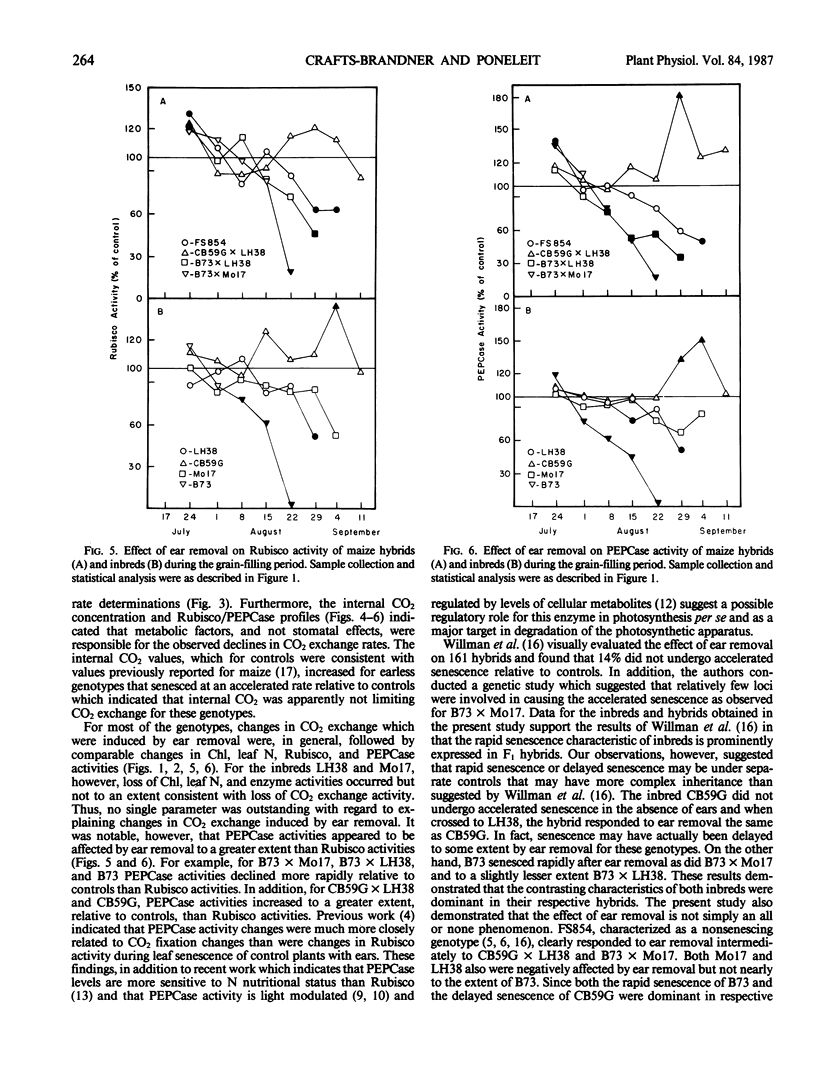
The effects of ear removal on gas exchange traits, chlorophyll, and leaf N profiles, and activities of ribulose 1,5-bisphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase were examined using four maize hybrids (B73 × Mo17, B73 × LH38, FS854, and CB59G × LH38) and four inbred lines (B73, Mo17, LH38, and CB59G) as experimental material. A diverse genotypic response to ear removal was observed which was generally typified by (a) greatly accelerated loss of chlorophyll, leaf N, enzyme activities, and CO2 exchange relative to controls for B73, B73 × Mo17, and B73 × LH38, (b) intermediate rate of decline for leaf constituents for FS854, LH38, and Mo17, or (c) loss of leaf constituents at similar or slower rates than for control plants for CB59G and CB59G × LH38. For all genotypes which had accelerated senescence relative to controls, loss of CO2 exchange activity was correlated with increased internal CO2 concentrations. Thus, it was concluded that metabolic factors and not stomatal effects were responsible for loss of CO2 exchange activity. Loss of chlorophyll, leaf N, and enzyme activities correlated well with loss of CO2 exchange activity only for some of the genotypes. Accelerated leaf senescence in response to ear removal for the inbred line B73 and the hybrids B73 × Mo17 and B73 × LH38, as well as the apparent delayed leaf senescence for the inbred line CB59G and the hybrid CB59G × LH38 show that the contrasting responses to ear removal, rapid versus delayed senescence, can be transmitted as dominant traits to F1 hybrids. The intermediate response by some genotypes, and the dominance of contrasting senescence traits, suggested a relatively complex inheritance for expression of the ear removal response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. C., Weinmann H. Effect of absence of developing grain on carbohydrate content and senescence of maize leaves. Plant Physiol. 1970 Sep;46(3):435–436. doi: 10.1104/pp.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer G. R., Schrader L. E. Relationships between CO(2) Exchange Rates and Activities of Pyruvate,Pi Dikinase and Ribulose Bisphosphate Carboxylase, Chlorophyll Concentration, and Cell Volume in Maize Leaves. Plant Physiol. 1985 Mar;77(3):612–616. doi: 10.1104/pp.77.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen L. E., Below F. E., Hageman R. H. The effects of ear removal on senescence and metabolism of maize. Plant Physiol. 1981 Nov;68(5):1180–1185. doi: 10.1104/pp.68.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Below F. E., Harper J. E., Hageman R. H. Differential Senescence of Maize Hybrids following Ear Removal : I. Whole Plant. Plant Physiol. 1984 Feb;74(2):360–367. doi: 10.1104/pp.74.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Below F. E., Wittenbach V. A., Harper J. E., Hageman R. H. Differential Senescence of Maize Hybrids following Ear Removal : II. Selected Leaf. Plant Physiol. 1984 Feb;74(2):368–373. doi: 10.1104/pp.74.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crafts-Brandner S. J., Poneleit C. G. Carbon dioxide exchange rates, ribulose bisphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase activities, and kernel growth characteristics of maize. Plant Physiol. 1987 Jun;84(2):255–260. doi: 10.1104/pp.84.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Detecting Photoactivation of Phosphoenolpyruvate Carboxylase in C(4) Plants : An Effect of pH. Plant Physiol. 1985 Feb;77(2):300–302. doi: 10.1104/pp.77.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. C., Cowan I. R., Farquhar G. D. Leaf Conductance in Relation to Rate of CO(2) Assimilation: I. Influence of Nitrogen Nutrition, Phosphorus Nutrition, Photon Flux Density, and Ambient Partial Pressure of CO(2) during Ontogeny. Plant Physiol. 1985 Aug;78(4):821–825. doi: 10.1104/pp.78.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]


