Abstract
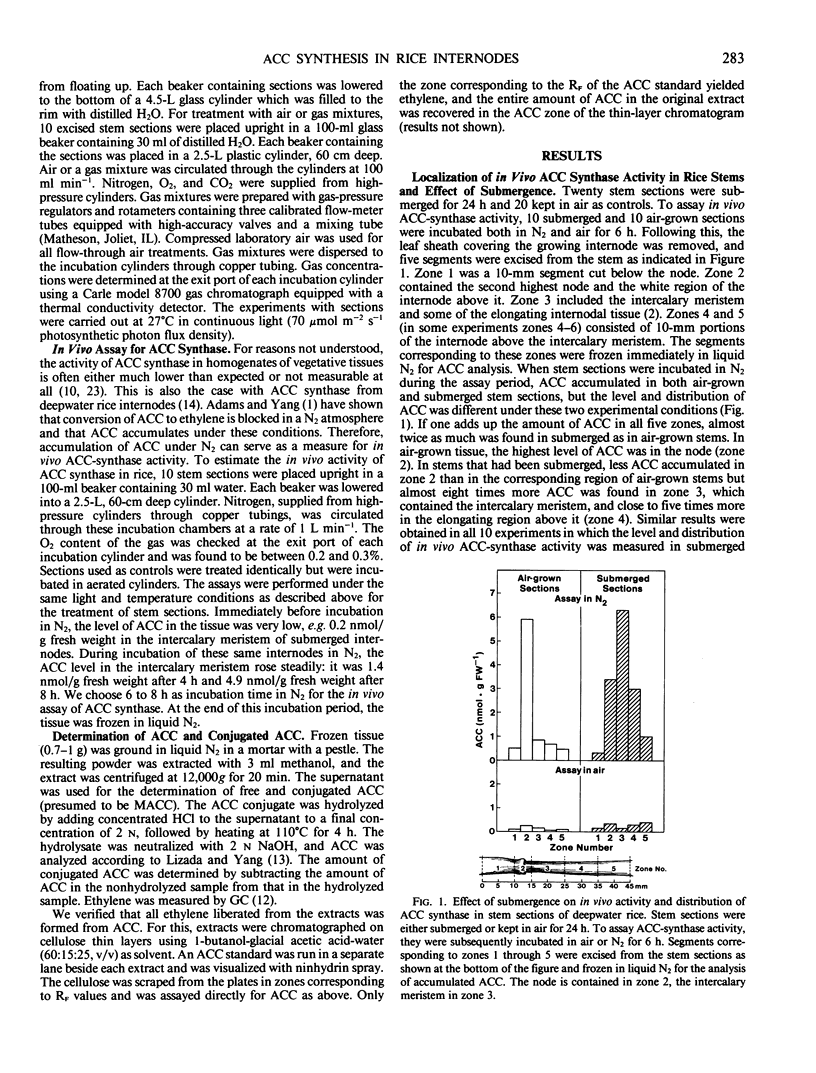
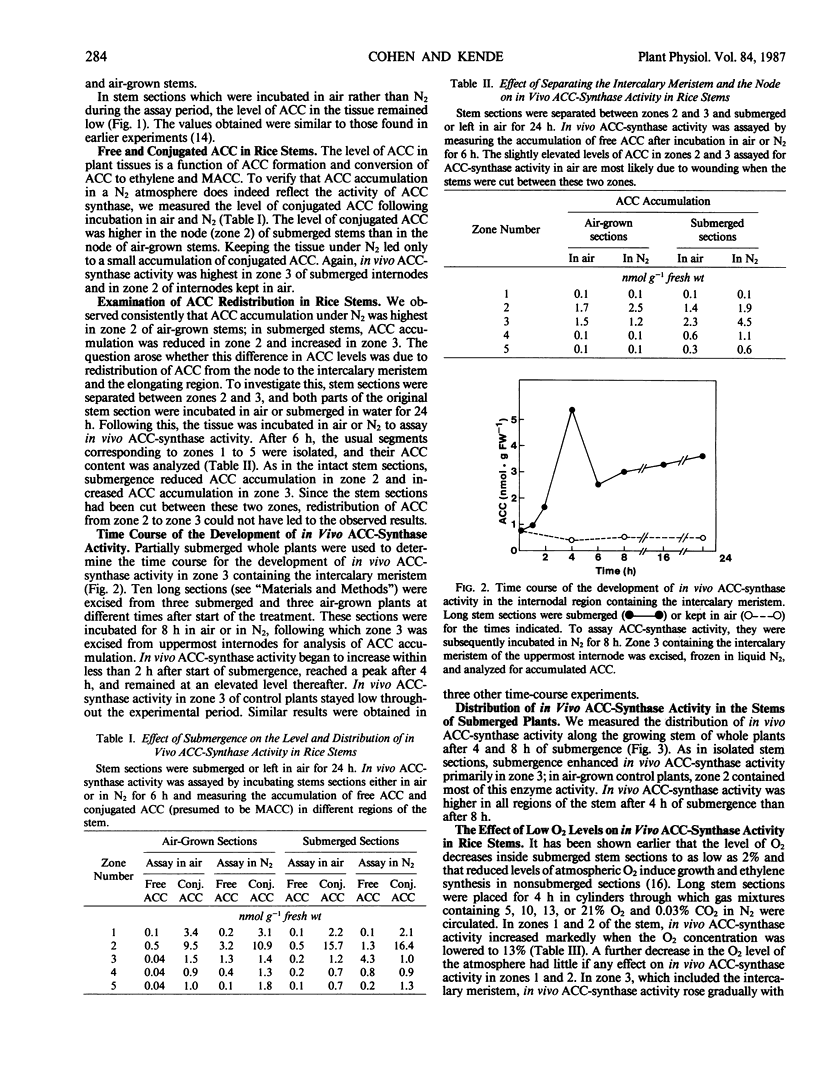
Inasmuch as the activity of 1-aminocyclopropane-1-carboxylate (ACC) synthase cannot be measured in homogenates of deepwater rice internodes (Oryza sativa L.), we have employed an in vivo assay to determine the activity of this enzyme. This assay is based on the accumulation of ACC in tissue kept under N2. Submergence of whole plants or stem sections containing the uppermost, developing internode enhances the in vivo activity of ACC synthase in the stem. This stimulation of in vivo ACC-synthase activity is especially pronounced in the region of the internode containing the intercalary meristem and the elongation zone above it. Enhancement of in vivo ACC-synthase activity is evident after 2 hours of submergence and shows a peak after 4 hours. Reduced levels of atmospheric O2, which promote ethylene synthesis and growth in internodes of deepwater rice, also enhance the in vivo activity of ACC synthase. Our results are consistent with the hypothesis that induction of ACC-synthase activity at low partial O2 pressures is among the first biochemical events leading to internodal growth in deepwater rice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Dilley D. R. Effects of root anaerobiosis on ethylene production, epinasty, and growth of tomato plants. Plant Physiol. 1978 Apr;61(4):506–509. doi: 10.1104/pp.61.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Hsiao T. C., Yang S. F. Inhibition of ethylene synthesis in tomato plants subjected to anaerobic root stress. Plant Physiol. 1982 Nov;70(5):1503–1507. doi: 10.1104/pp.70.5.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith K. A., Raskin I., Kende H. A comparison of the submergence response of deepwater and non-deepwater rice. Plant Physiol. 1986 Feb;80(2):479–482. doi: 10.1104/pp.80.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Métraux J. P., Kende H. The role of ethylene in the growth response of submerged deep water rice. Plant Physiol. 1983 Jun;72(2):441–446. doi: 10.1104/pp.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I., Kende H. Role of gibberellin in the growth response of submerged deep water rice. Plant Physiol. 1984 Dec;76(4):947–950. doi: 10.1104/pp.76.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]


