Abstract
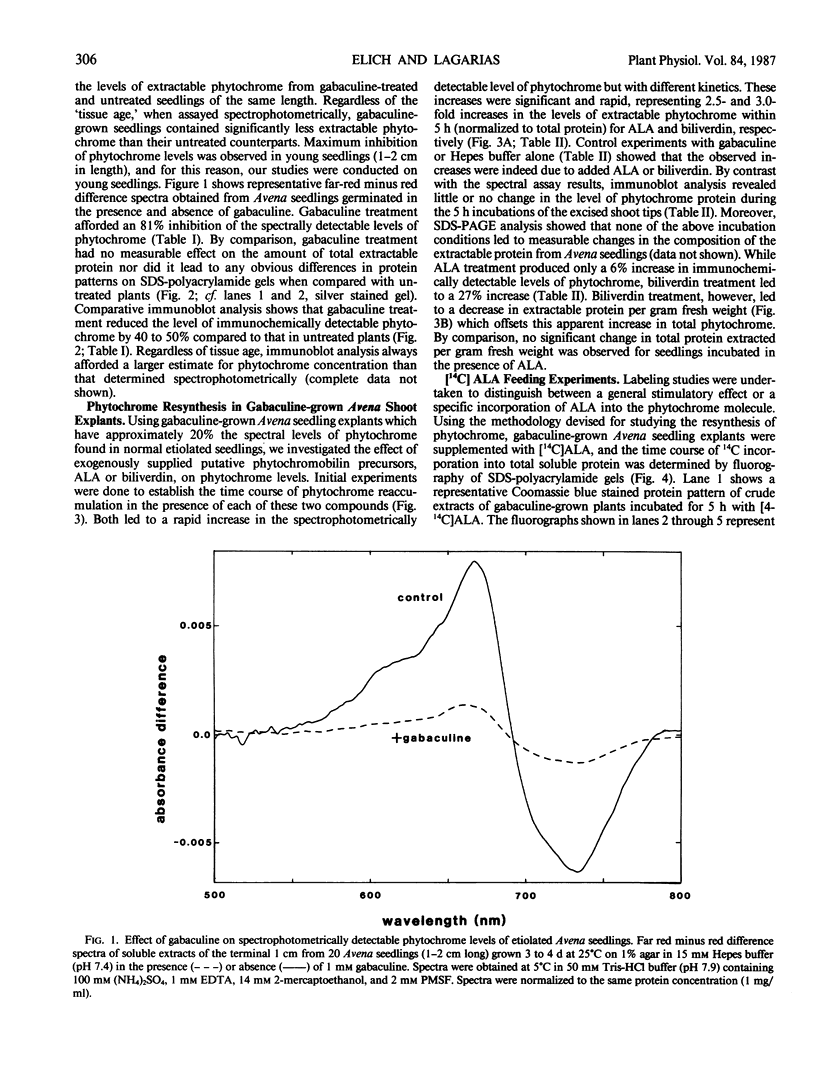
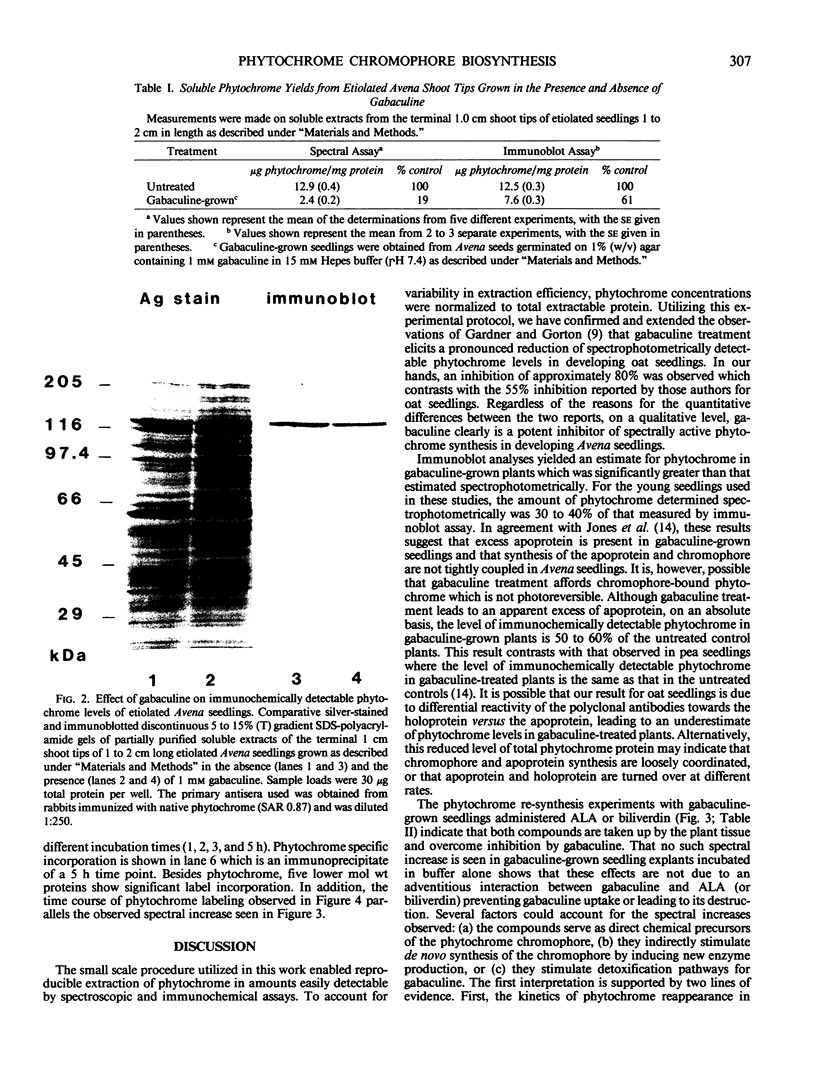
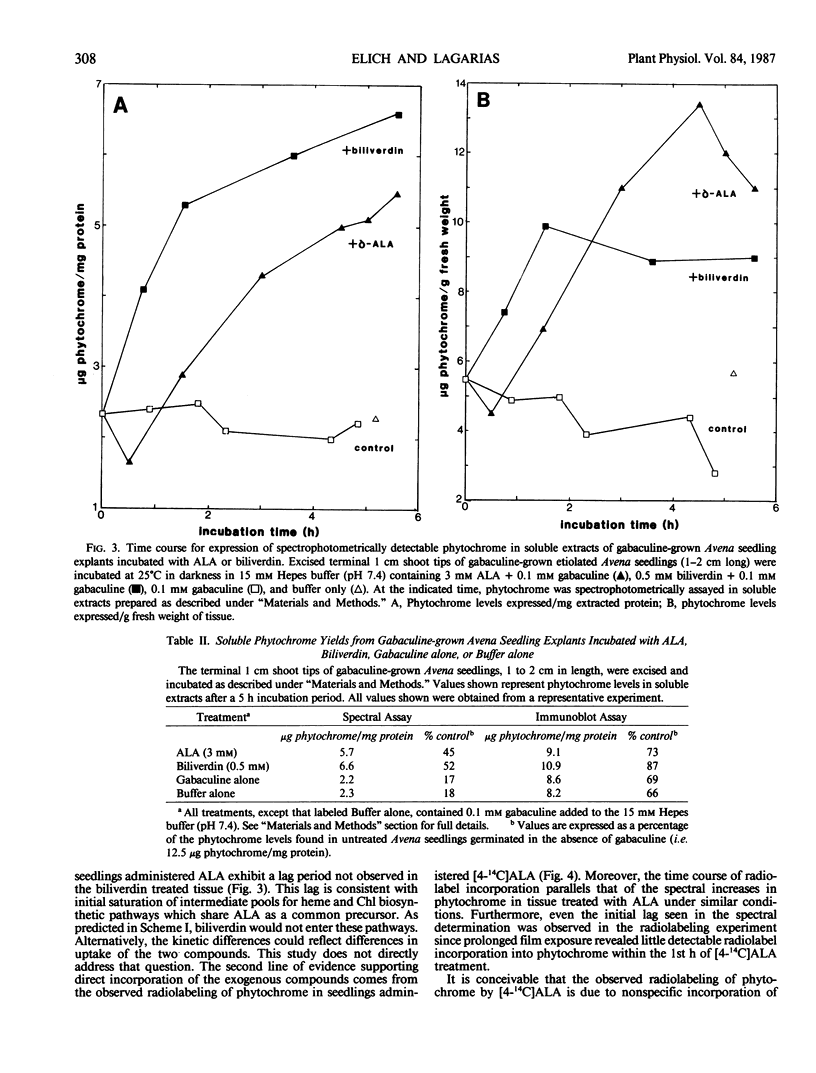
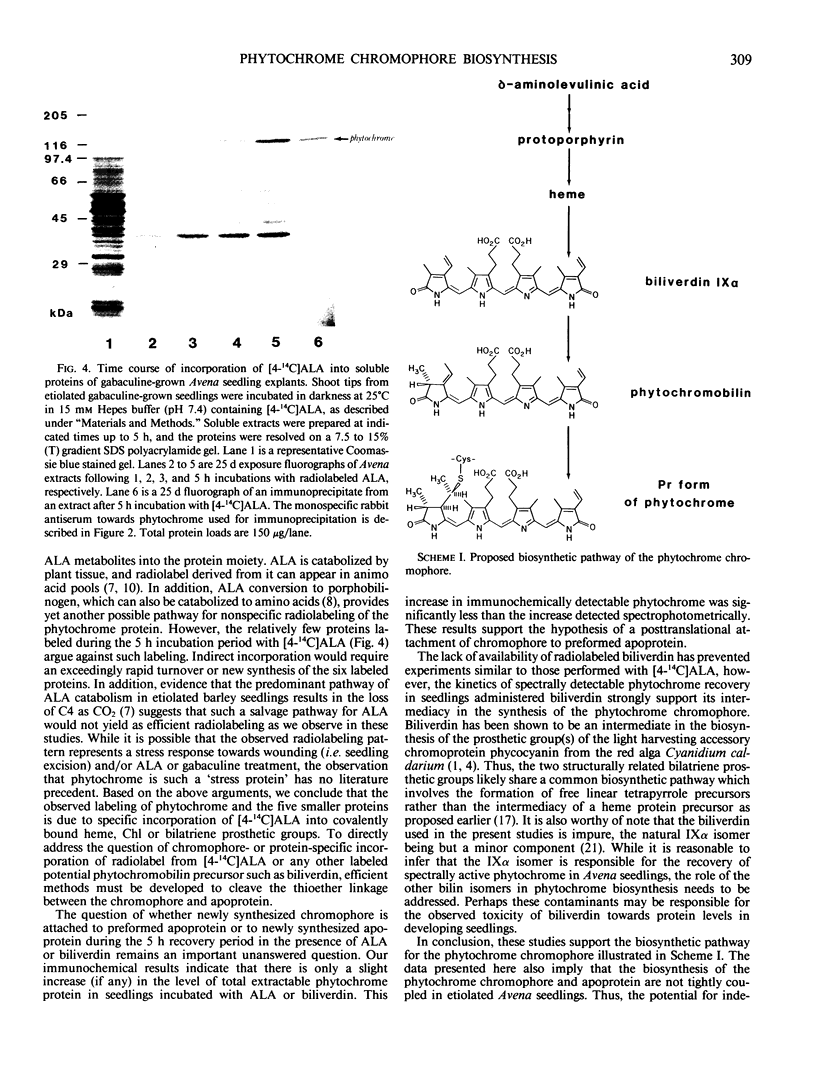
Etiolated Avena sativa L. seedlings grown in the presence of gabaculine (5-amino-1,3-cyclohexadienylcarboxylic acid) contained reduced levels of phytochrome as shown by spectrophotometric and immunochemical assays. Photochromic phytochrome levels in gabaculine-grown plants were estimated to be 20% of control plants, while immunoblot analysis showed that the phytochrome protein moiety was present at approximately 50% of control levels. Gabaculine-grown seedlings administered either 5-aminolevulinic acid or biliverdin exhibited a rapid increase of spectrophotometrically detectable phytochrome. Phytochrome concentrations estimated immunochemically did not similarly increase throughout treatment with either compound. Similar experiments with 5-amino[4-14C] levulinic acid showed radiolabeling of phytochrome with kinetics that paralleled the spectrally detected increase. These results are consistent with (a) the intermediacy of both 5-aminolevulinic acid and biliverdin in the biosynthetic pathway of the phytochrome chromophore and (b) the lack of coordinate regulation of chromophore and apoprotein synthesis in Avena seedlings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Cornejo J. Biosynthesis of phycocyanobilin from exogenous labeled biliverdin in Cyanidium caldarium. Arch Biochem Biophys. 1983 Nov;227(1):279–286. doi: 10.1016/0003-9861(83)90372-7. [DOI] [PubMed] [Google Scholar]
- Briggs W. R., Siegelman H. W. Distribution of Phytochrome in Etiolated Seedlings. Plant Physiol. 1965 Sep;40(5):934–941. doi: 10.1104/pp.40.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B., Holroyd J. A., Vernon D. I. Biosynthesis of phycobiliproteins. Incorporation of biliverdin into phycocyanin of the red alga Cyanidium caldarium. Biochem J. 1984 May 1;219(3):905–909. doi: 10.1042/bj2190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J. X., Meller E., Gassman M. L. Catabolism of 5-Aminolevulinic Acid to CO(2) by Etiolated Barley Leaves. Plant Physiol. 1982 Jan;69(1):19–22. doi: 10.1104/pp.69.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J. X., Meller E., Gassman M. L. Catabolism of porphobilinogen by etiolated barley leaves. Plant Physiol. 1982 Mar;69(3):602–608. doi: 10.1104/pp.69.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Gorton H. L. Inhibition of phytochrome synthesis by gabaculine. Plant Physiol. 1985 Mar;77(3):540–543. doi: 10.1104/pp.77.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Li C. H. Reduction of sulfoxides in peptides and proteins. Methods Enzymol. 1983;91:549–559. doi: 10.1016/s0076-6879(83)91050-9. [DOI] [PubMed] [Google Scholar]
- Jones A. M., Allen C. D., Gardner G., Quail P. H. Synthesis of phytochrome apoprotein and chromophore are not coupled obligatorily. Plant Physiol. 1986 Aug;81(4):1014–1016. doi: 10.1104/pp.81.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagarias J. C. Bile pigment--protein interactions. Coupled oxidation of cytochrome c. Biochemistry. 1982 Nov 9;21(23):5962–5967. doi: 10.1021/bi00266a036. [DOI] [PubMed] [Google Scholar]
- Lagarias J. C., Mercurio F. M. Structure function studies on phytochrome. Identification of light-induced conformational changes in 124-kDa Avena phytochrome in vitro. J Biol Chem. 1985 Feb 25;260(4):2415–2423. [PubMed] [Google Scholar]
- Litts J. C., Kelly J. M., Lagarias J. C. Structure-function studies on phytochrome. Preliminary characterization of highly purified phytochrome from Avena sativa enriched in the 124-kilodalton species. J Biol Chem. 1983 Sep 25;258(18):11025–11031. [PubMed] [Google Scholar]
- McDonagh A. F., Palma L. A. Preparation and properties of crystalline biliverdin IX alpha. Simple methods for preparing isomerically homogeneous biliverdin and [14C[biliverdin by using 2,3-dichloro-5,6-dicyanobenzoquinone. Biochem J. 1980 Aug 1;189(2):193–208. doi: 10.1042/bj1890193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Bangerter F. W. The irreversible inhibition of mouse brain gamma-aminobutyric acid (GABA)-alpha-ketoglutaric acid transaminase by gabaculine. J Am Chem Soc. 1976 Oct 13;98(21):6762–6764. doi: 10.1021/ja00437a090. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Skinner M. K., Griswold M. D. Fluorographic detection of radioactivity in polyacrylamide gels with 2,5-diphenyloxazole in acetic acid and its comparison with existing procedures. Biochem J. 1983 Jan 1;209(1):281–284. doi: 10.1042/bj2090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]