Abstract
Background
Inappropriate polypharmacy is a particular concern in older people and is associated with negative health outcomes. Choosing the best interventions to improve appropriate polypharmacy is a priority, so that many medicines may be used to achieve better clinical outcomes for patients. This is the third update of this Cochrane Review.
Objectives
To assess the effects of interventions, alone or in combination, in improving the appropriate use of polypharmacy and reducing medication‐related problems in older people.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL and two trials registers up until 13 January 2021, together with handsearching of reference lists to identify additional studies. We ran updated searches in February 2023 and have added potentially eligible studies to 'Characteristics of studies awaiting classification'.
Selection criteria
For this update, we included randomised trials only. Eligible studies described interventions affecting prescribing aimed at improving appropriate polypharmacy (four or more medicines) in people aged 65 years and older, which used a validated tool to assess prescribing appropriateness. These tools can be classified as either implicit tools (judgement‐based/based on expert professional judgement) or explicit tools (criterion‐based, comprising lists of drugs to be avoided in older people).
Data collection and analysis
Four review authors independently reviewed abstracts of eligible studies, and two authors extracted data and assessed the risk of bias of the included studies. We pooled study‐specific estimates, and used a random‐effects model to yield summary estimates of effect and 95% confidence intervals (CIs). We assessed the overall certainty of evidence for each outcome using the GRADE approach.
Main results
We identified 38 studies, which includes an additional 10 in this update. The included studies consisted of 24 randomised trials and 14 cluster‐randomised trials. Thirty‐six studies examined complex, multi‐faceted interventions of pharmaceutical care (i.e. the responsible provision of medicines to improve patients' outcomes), in a variety of settings. Interventions were delivered by healthcare professionals such as general physicians, pharmacists, nurses and geriatricians, and most were conducted in high‐income countries. Assessments using the Cochrane risk of bias tool found that there was a high and/or unclear risk of bias across a number of domains. Based on the GRADE approach, the overall certainty of evidence for each pooled outcome ranged from low to very low.
It is uncertain whether pharmaceutical care improves medication appropriateness (as measured by an implicit tool) (mean difference (MD) ‐5.66, 95% confidence interval (CI) ‐9.26 to ‐2.06; I2 = 97%; 8 studies, 947 participants; very low‐certainty evidence). It is uncertain whether pharmaceutical care reduces the number of potentially inappropriate medications (PIMs) (standardised mean difference (SMD) ‐0.19, 95% CI ‐0.34 to ‐0.05; I2 = 67%; 9 studies, 2404 participants; very low‐certainty evidence). It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PIM (risk ratio (RR) 0.81, 95% CI 0.68 to 0.98; I2 = 84%; 13 studies, 4534 participants; very low‐certainty evidence). Pharmaceutical care may slightly reduce the number of potential prescribing omissions (PPOs) (SMD ‐0.48, 95% CI ‐1.05 to 0.09; I2 = 92%; 3 studies, 691 participants; low‐certainty evidence), however it must be noted that this effect estimate is based on only three studies, which had serious limitations in terms of risk of bias. Likewise, it is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PPO (RR 0.50, 95% CI 0.27 to 0.91; I2 = 95%; 7 studies, 2765 participants; very low‐certainty evidence).
Pharmaceutical care may make little or no difference to hospital admissions (data not pooled; 14 studies, 4797 participants; low‐certainty evidence). Pharmaceutical care may make little or no difference to quality of life (data not pooled; 16 studies, 7458 participants; low‐certainty evidence). Medication‐related problems were reported in 10 studies (6740 participants) using different terms (e.g. adverse drug reactions, drug‐drug interactions). No consistent intervention effect on medication‐related problems was noted across studies. This also applied to studies examining adherence to medication (nine studies, 3848 participants).
Authors' conclusions
It is unclear whether interventions to improve appropriate polypharmacy resulted in clinically significant improvement. Since the last update of this review in 2018, there appears to have been an increase in the number of studies seeking to address potential prescribing omissions and more interventions being delivered by multidisciplinary teams.
Keywords: Aged, Humans, Drug-Related Side Effects and Adverse Reactions, Hospitalization, Pharmaceutical Services, Polypharmacy, Quality of Life
Plain language summary
A review of the ways healthcare professionals can make sure older people are given suitable medicines
What is the aim of this review?
The aim of this Cochrane Review was to find out whether any approaches can improve the use of suitable medicines in older people. Researchers collected and analysed all relevant studies to answer this question and included 38 trials in the review.
Key messages
Taking medicine to treat symptoms of chronic illness and to prevent worsening of disease is common in older people. However, taking too many medicines can cause harm. Following our analyses, we are uncertain whether the interventions we studied improve the correct use of medicines. We need more and better research to consider these issues.
What was studied in the review?
This review examines studies in which healthcare professionals have taken action to make sure that older people are receiving the most effective and safest medicines for their illness. Actions taken included providing a service, known as pharmaceutical care. This involves promoting the correct use of medicines by identifying, preventing and resolving medication‐related problems. Another strategy that we were interested in was using computerised decision support. This involves a program on the doctor’s computer that aids the selection of appropriate treatment(s) or strategies ‐ and can involve different healthcare professionals working together.
What are the main results of the review?
The review authors found 38 relevant trials from 19 countries that involved 18,073 older people. These studies compared interventions aiming to improve the appropriate use of many medicines with usual care. It is uncertain whether the interventions improved the correct use of medicines. After analysing all the studies, we were not able to conclude that the interventions improved the appropriateness of medicines (based on scores assigned by expert professional judgement) or reduced the number of potentially inappropriate medicines (medicines in which the harms outweigh the benefits). We were also not able to say whether the interventions reduced the proportion of patients with one or more potentially inappropriate medication or reduced the proportion of patients with one or more potential prescribing omission (cases where a useful medicine has not prescribed). This is because of the quality of the evidence. However, compared to the last update of this review, there were more studies focusing on potential prescribing omissions and more studies involving a number of healthcare professionals working together. In addition, we found that the interventions may lead to little or no difference in hospital admissions or quality of life.
What are the limitations of the evidence?
The quality of the studies was low and there were substantial differences in the patient populations, how the appropriateness of medications was measured and the interventions that were delivered.
How up‐to‐date is this review?
Review authors searched for studies that had been published up to January 2021.
Summary of findings
Summary of findings 1. Pharmaceutical care compared with usual care for older people receiving polypharmacy.
| Patient or population: older people receiving polypharmacy Settings: community, nursing home, hospital Intervention: pharmaceutical care Comparison: usual care | ||||||
| Outcomes | Effect estimate | Relative effect (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Usual care | Pharmaceutical care | |||||
|
Medication appropriateness (as measured by an implicit tool; lower scores are better) From baseline to follow‐up Follow‐up: 0 to 6 months |
Medication appropriateness (as measured by an implicit tool) across control groups ranged from ‐0.49 to 20 | Medication appropriateness (as measured by an implicit tool) in the intervention groups was 5.66 lower (9.26 to 2.06 lower) | — | 947 (8 studies) | ⊕⊝⊝⊝ very low a,b,c,d |
MAI was used as an implicit tool in the pooled studies. Heterogeneity: I2 = 97%, P = 0.00001. It is uncertain whether pharmaceutical care improves medication appropriateness because the certainty of the evidence is very low. |
| Potentially inappropriate medications | ||||||
| The number of potentially inappropriate medications (PIMs) Follow‐up: 0 to 12 months | — | Standardised mean difference§ in the intervention groups was 0.19 SD lower (0.05 to 0.34 lower) | — | 2404 (9 studies) | ⊕⊝⊝⊝ very lowa,b,c | The STOPP and Beers criteria, and the Meds 75+ database, were used as explicit tools in the pooled studies. It is uncertain whether pharmaceutical care reduces the number of PIMs because the certainty of this evidence is very low. |
|
The proportion of patients with one or more potentially inappropriate medication (PIM) Follow‐up: 0 to 12 months |
435 per 1000 | 83 fewer per 1000 (from 139 fewer to 9 fewer) | RR 0.81 (0.68 to 0.98) | 4534 (13 studies) |
⊕⊝⊝⊝ very lowa,b,c | The STOPP and Beers criteria were used as explicit tools in the pooled studies. Heterogeneity: I2 = 84%, P = < 0.00001. It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PIM because the certainty of this evidence is very low. |
| Potential prescribing omissions | ||||||
| The number of potential prescribing omissions (PPOs) Follow‐up: 0 to 12 months | — | Standardised mean difference§ in the intervention groups was 0.48 SD lower (from 1.05 lower to 0.09 higher) | — | 691 (3 studies) |
⊕⊝⊝⊝ very lowa,c,d | START and ACOVE were used as explicit tools in the pooled studies. Heterogeneity: I2 = 92%, P < 0.00001. Pharmaceutical care may slightly reduce the number of PPOs, however this finding is uncertain due to very serious design limitations with implications in terms of selection bias, performance bias and risk of contamination bias. |
|
The proportion of patients with one or more potential prescribing omission (PPO) Follow‐up: 0 to 24 months |
559 per 1000 | 280 fewer per 1000 (from 408 fewer to 50 fewer) | RR 0.50 (0.27 to 0.91) | 2765 (7 studies) |
⊕⊝⊝⊝ very lowa,b,c | START and ACOVE were used as explicit tools in the pooled studies. Heterogeneity: I² = 95%, P < 0.00001. It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PPO because the certainty of this evidence is very low. |
|
Hospital admissions Follow‐up: 1 to 12 months |
Only 2 out of 14 studies reported a reduction in hospital admissions; the others found little or no difference between groups. | — | 4797 (14 studies) | ⊕⊕⊝⊝ lowa,c |
— | |
|
Quality of life Follow‐up: 3 to 12 months |
Six studies reported some changes in QoL; 10 found no changes. | — | 7458 (16 studies) | ⊕⊕⊝⊝ lowa,c |
— | |
| GRADE Working Group grades of evidence High: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different‡ is low. Moderate: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different‡ is moderate. Low: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different‡ is high. Very low: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different‡ is very high. ‡Substantially different = a large enough difference that it might affect a decision | ||||||
ACOVE: Assessing Care of the Vulnerable Elderly, CI: confidence interval, MAI: Medication Appropriateness Index, PIMs: Potentially Inappropriate Medications, PPOs: Potential prescribing omissions, QoL: quality of life; RR: risk ratio, SD: standard deviation; STOPP: Screening Tool of Older People’s potentially inappropriate Prescriptions, START: Screening Tool to Alert to Right Treatment
§The standardised mean difference was used in cases where a range of tools were used to generate the pooled effect estimate.
aWe downgraded the evidence due to risk of bias. For medication appropriateness, this was downgraded by one level. For the number of PIMs, the proportion of patients with one or more PIM, the number of PPOs and the proportion of patients with one or more PPO, the evidence was downgraded by two levels.
bWe downgraded the evidence by one level due to indirectness.
cWe downgraded the evidence by two levels due to inconsistency in the results that could not be fully explained.
dWe downgraded the evidence by one level due to imprecision: CIs were wide and/or crossed the line of no effect.
Background
Prescribing for older people is complex because of factors such as increased life expectancy, age‐related changes in body composition and multiple morbidities (the presence of two or more chronic health conditions), and also because prescribing guidelines recommend more than one drug for certain long‐term diseases (Cadogan 2016a; Guthrie 2015; Hughes 2014).
Finding the balance between aggressively treating diseases and avoiding medication‐related harm is a critical objective for healthcare professionals, yet has proven challenging to achieve in clinical practice (Steinman 2007). This review updates the previous Cochrane Review of Interventions to improve the appropriate use of polypharmacy for older people (Rankin 2018a), which concluded that despite the potential to reduce inappropriate prescribing, it was unclear whether interventions to improve appropriate polypharmacy in older people resulted in clinically significant improvements.
Polypharmacy refers to the use of multiple medicines. The term itself has been the subject of much discussion but no standard definition is used consistently (Cadogan 2016a; King's Fund 2013; Stewart 1990). In a systematic review of definitions of polypharmacy (Masnoon 2017), 138 definitions were noted, ranging from two to 11 or more medicines, with five or more daily the most common. For the purpose of this update of the review, we defined it as 'the concomitant ingestion of four or more medicines'. However, in recognition of the fact that the number of medicines used to define polypharmacy is arbitrary, the focus of the interventions of interest to this review is the appropriateness of the medications prescribed for older people and not the specific number of medicines taken.
Polypharmacy is common in older people, conventionally defined as those aged 65 years and older, as this age group is often subject to multimorbidity (defined as two or more chronic conditions) (Barnett 2012), such as cardiovascular disease and diabetes that require multiple medicines for treatment and prophylaxis. In England, data from October to December 2019 show that 8.4 million people (14.9%) were taking five or more medicines each day, and 3.8 million (6.8%) were taking eight or more medicines daily ‐ although it is not possible to state what proportion of these examples of polypharmacy is inappropriate (Department of Health and Social Care 2021).
In the United States of America (USA), the prevalence of polypharmacy in older people has increased over time, and data indicate that approximately 39% of older people in the USA take five or more medicines (Kantor 2015). Data from The Irish Longitudinal Study on Ageing have identified polypharmacy in 27% of the older population using the same definition (McGarrigle 2017). Although prevalence estimates in older people vary across countries, polypharmacy in older people is recognised as a widespread global issue (Stewart 2017). Consequently, older people use a disproportionate quantity of health service resources. For example, in terms of medicines, in 2016, patients aged 60 and older accounted for 23% of the population in England and were dispensed 61.0% of all prescription items (Information Centre 2017).
It is widely recognised that prescribing guidelines typically focus on single diseases and when applied to complex multimorbid patients, often fail to provide information on how to prioritise treatment recommendations and can act as a driving force for polypharmacy (Hughes 2012). In a qualitative study designed to investigate how doctors plan treatment for complex multi‐morbid patients (Schuttner 2022), the results from 23 interviews with physicians revealed many factors including making decisions in line with habit, working within their organisation’s structures and boundaries, collaborating with other members of the care team and working towards an overall goal for care. Some of the findings were deemed useful strategies to use to approach complex cases.
Inappropriate prescribing in the context of older people can be defined as the prescribing of "medications or medication classes that should generally be avoided in persons 65 years or older because they are either ineffective or they pose unnecessarily high risk for older persons and a safer alternative is available" (Beers 1991). The term ‘potentially inappropriate prescribing (PIP)’ encompasses potentially inappropriate medicines (PIMs) and potential prescribing omissions (PPOs) (see Appendix 1 for acronyms used in this review). A PIM is a medicine that could potentially lead to a significant risk of adverse drug events (ADEs) and arises from prescribing practices such as continuing therapy for longer than necessary or recommended in prescribing guidelines. The American Geriatrics Society (AGS) Beers Criteria for PIM use in Older Adults are updated regularly and detail PIMs that should be avoided. The most recent publication presented 70 modifications to the previous list including new medications and drug‐drug interactions (DDIs) (AGS 2019). A PPO involves the omission of a medication that is clinically indicated for disease treatment or prevention (O'Connor 2012). Although polypharmacy is often clinically indicated and beneficial in specific conditions (e.g. hypertension, diabetes mellitus) and patient populations (e.g. patients with multimorbidity), it also poses risks of medication‐related harm and safety risks to patients. A medication‐related problem is described as “an event or circumstance involving a patient’s drug treatment that actually, or potentially, interferes with the achievement of an optimal outcome” and includes adverse drug reactions and drug interactions (Simonson 2005). Polypharmacy in older people has been associated with PIP and negative health outcomes, including an increased risk of hospital admissions, ADEs and mortality (Cahir 2010). In a study to investigate the link between polypharmacy in people aged at least 85 years and all‐cause mortality (Davies 2022), each additional medication prescribed was associated with a 3% increase in mortality. The chance of medication‐related problems (such as adverse drug reactions and DDIs) occurring increases in older age, in part because the ageing process reduces the efficiency of the body’s organs in eliminating drugs (Mangoni 2003). A large study of community‐dispensed prescribing in Scotland (between 1995 and 2010) showed that the proportion of older adults prescribed more than five medicines and with potentially serious DDIs had more than doubled to 13% in 2010 (Guthrie 2015). It is known that the number of medicines prescribed is predictive of the number of drug interactions likely to occur (Gallagher 2001). Poor understanding of causes of certain disorders makes prescribing drug combinations more difficult and treating poorly understood diseases may increase the risk for inappropriate prescribing (Werder 2003). The association between PIMs and PPOs and functional disability (using the World Health Organization Disability Assessment Schedule 2.0 (WHODAS)) among older adults was investigated in a cross‐sectional analysis of a randomised comparative effectiveness trial (Salm 2022). Among 461 patients, PIMs and PPOs were significantly associated with an increase in WHODAS‐score, however no significant link was found between WHODAS‐score and number of medications. The authors commented that their results showed a relationship between inappropriate prescribing and functional disability in older adults who are at risk of further declining health.
Despite the recognised potential for medication safety risks in older people, recent cohort studies have challenged previous assumptions that polypharmacy is hazardous and associated with poor clinical outcomes (Appleton 2014; Guthrie 2015). For example, an analysis of Scottish primary care data linked to hospital discharge data highlighted the limitations of crude measures of polypharmacy (i.e. the number of medicines prescribed) as quality indicators or predictors of hospital admissions when patients’ clinical context is not taken into consideration (Appleton 2014). The findings showed that patients prescribed an increased number of cardiovascular medicines were more likely to experience unplanned hospital admissions. However, when the analysis was adjusted to account for clinical factors such as non‐cardiovascular morbidity and drug burden, no evidence of an increase in non‐cardiovascular admissions with increasing numbers of cardiovascular medicines was found. Another study examined medication‐related quality of life (MRQoL) in older adults with multiple morbidities (Jennings 2022). Scores were low, which indicated that patients had good MRQoL and results also indicated that the presence of PIMs was not found to be associated with poorer QoL.
Greater use of the term ‘appropriate polypharmacy’ has thus been advocated, which refers to ‘prescribing for an individual with complex or multiple conditions where medicine use has been optimised and prescribing is in accordance with best evidence’ (Cadogan 2016; King's Fund 2013). In assessing older patients’ prescriptions, it is important to consider whether each drug has been prescribed appropriately or inappropriately, both individually and in the context of the whole prescription (Aronson 2006). Improving appropriate polypharmacy involves encouraging use of the correct drugs under appropriate conditions to treat the right diseases. In certain circumstances, this may include the removal of unnecessary drugs or those with no valid clinical indication and the addition of useful ones. Therefore, interventions that seek solely to reduce the number of prescribed medicines fail to consider polypharmacy in its entirety. PPOs are also highly prevalent in older populations and have been shown to be associated with polypharmacy, whereby the probability of under‐prescription increases with the number of medicines prescribed (Galvin 2014).
These findings may be explained by the unwillingness of general practitioners (GPs) to prescribe additional drugs for patients with polypharmacy (for reasons such as complexity of drug regimens, fear of ADEs and DDIs, and poor adherence) (Kuijpers 2007). This so‐called treatment/risk paradox or risk/treatment mismatch is seen when patients with the highest risk of complications are determined to have the lowest probability of receiving the recommended medications (Ko 2004; Lee 2005).
Differentiating between 'many' medicines (appropriate polypharmacy) and 'too many' medicines (inappropriate polypharmacy) is a prescriber's dilemma, and choosing the best interventions aimed at ensuring appropriate polypharmacy remains a challenge for healthcare practitioners and organisations.
Description of the condition
The causes of inappropriate polypharmacy are multifactorial (Stewart 2017), and for the purpose of this review we have focused on interventions that have targeted PIMs, PPOs, or both, using validated instruments or screening tools such as a validated list of medicines considered inappropriate for older people (AGS 2012; Beers 1991; Fick 2003; King's Fund 2013), a list of clinically significant criteria for potentially inappropriate prescribing in older people (Gallagher 2008; O'Mahony 2015) or the Medication Appropriateness Index (MAI) (Hanlon 1992). These screening tools can be classified as either implicit (judgement‐based) or explicit (criterion‐based) tools (Kaufmann 2014; O'Connor 2012). Implicit tools, such as MAI (Appendix 2) and the Assessment of Underutilization of Medication (AOU) tool (Jeffery 1999), are judgement‐based indicators of prescribing quality that are applied by clinicians to a patient’s prescription. Explicit tools, such as Beers’ criteria (Appendix 2) and Screening Tool of Older Person's Prescriptions (STOPP)/Screening Tool to Alert doctors to the Right Treatment (START) criteria (Gallagher 2008; O'Mahony 2015), are usually developed from literature reviews, expert opinion and consensus exercises. The criteria typically comprise lists of drugs to be avoided or added in older people.
Description of the intervention
Improvement in appropriate polypharmacy can be achieved through a wide range of interventions (e.g. educational programmes for prescribers or consumers; medication review clinics and specific prescribing audits; prescribing incentive schemes and regulatory interventions). Interventions that reduce the risk of medication‐related problems are important to consider (Fick 2008). These may be provided by healthcare professionals, educators, policy‐makers and healthcare service planners. Previously, interventions targeting polypharmacy in older people have often focused on reducing the number of medicines prescribed (Rollason 2003), based on the assumption that polypharmacy is harmful. However, by focusing solely on the number of prescribed medicines, these interventions have failed to consider inappropriate prescribing in its entirety. As noted above, inappropriate prescribing is not restricted to over‐prescribing, but also encompasses mis‐prescribing (i.e. incorrect prescribing of a necessary drug) and under‐prescribing (i.e. prescribing omissions).
Methods recommended in previous intervention studies include use of computer data entry and feedback procedures, which have been shown to decrease polypharmacy and drug‐drug interactions (Werder 2003); visual identification of medicines; continuous medication review and thorough patient education to optimise polypharmacy (Fulton 2005). More recently, complex interventions have included training of health professionals and the delivery of individualised medication reviews to patients (Del Cura‐Gonzalez 2022; McCarthy 2022).
This review seeks to identify evidence regarding which types of interventions can improve appropriate polypharmacy in older people.
How the intervention might work
Interventions to improve appropriate polypharmacy are likely to achieve the following outcomes.
Improvement in medication appropriateness (as measured by an implicit tool).
Reduction of inappropriately prescribed medication (as measured by an explicit tool).
Reduction of prescribing omissions (as measured by an explicit tool) by promoting prescribing of evidence‐based therapy where clinically indicated.
Computerised decision support (CDS) aimed at prescribers, whereby electronic alerts are produced to guide the prescriber to the right treatment, has been successful in reducing inappropriate prescribing for older people (Yourman 2008).
Pharmaceutical care is the responsible provision of drug therapy for the purpose of achieving definitive outcomes that improve a patient’s quality of life (Hepler 1990). Pharmaceutical care reflects a systematic approach that ensures patients receive the correct medicines, at an appropriate dose, for appropriate indications. It involves pharmacists moderating drug management in collaboration with physician, patient and carer (Hepler 1990). Pharmacist‐led interventions such as medication review, co‐ordinated transition from hospital to long‐term care facility and pharmacist consultations with patients and physicians have been shown to effectively reduce inappropriate prescribing and ADEs (Hanlon 1996; Kaur 2009). Multi‐disciplinary case conferences involving GPs, geriatricians, pharmacists and residential care staff, wherein individual patient cases are discussed, have reduced the use of inappropriate medications in residential care (Crotty 2004a).
While polypharmacy interventions have been shown to reduce inappropriate prescribing and improve medication adherence, their effect on clinical outcomes is less clear (Ali 2022). In a review of systematic reviews of interventions to improve polypharmacy among adults with multiple morbidities, the authors concluded that better understanding of the characteristics and implementation of these complex interventions is needed (Ali 2022).
Why it is important to do this review
A systematic review may help to identify how we can improve appropriate polypharmacy in older people. Inappropriate prescribing for older people is both highly prevalent and commonly associated with polypharmacy (Bradley 2012; Cahir 2010). It is important that the current available evidence be identified and appraised, so that interventions that are effective in managing disease with appropriate polypharmacy may be identified and put into practice. This is an update of the Cochrane Review (Rankin 2018a).
Objectives
To assess the effects of interventions, alone or in combination, in improving the appropriate use of polypharmacy and reducing medication‐related problems in older people.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials and cluster‐randomised trials meeting the Effective Practice and Organisation of Care (EPOC) specification (EPOC 2017). While the previous version of this review included non‐randomised trials (Rankin 2018a), we decided to focus on randomised and cluster‐randomised trials only in an effort to maximise the quality of evidence.
We classified trials eligible for inclusion according to the degree of certainty that random allocation was used to form comparison groups in the trial. If study author(s) stated explicitly that groups compared in the trial were established by random allocation, we classified the trial as a randomised trial and included it in the review. If study author(s) did not state explicitly that the trial was randomised, we excluded it.
Types of participants
The review included studies of people aged 65 years and older, who had more than one long‐term medical condition and were receiving polypharmacy (classified as four or more medicines). Medications were counted based on what was reported in the trials, which generally did not differentiate between formulation type. This included a prescribed medication (one that is scheduled or part of a repeat prescription, and does not include over‐the‐counter and herbal products) and included studies targeting patient groups in which polypharmacy was common practice, such as patients with Parkinson’s disease or diabetes. We considered trials for inclusion if they included a majority (80% or more) of participants aged 65 years and older, or if the mean age of study participants was over 65 years. If studies included both older and younger people, we included them if we were able to extract relevant data. We contacted study authors to check the availability of relevant data.
We excluded studies in which the intervention focused on people with a single long‐term medical condition or who were receiving short‐term polypharmacy, for example those who were terminally ill or were receiving cancer chemotherapy.
Types of interventions
We examined all types of interventions aimed at improving appropriate polypharmacy in any setting (such as pharmaceutical care) compared with usual care or the control group (as defined by the study). We included all uni‐faceted interventions, for example those targeted solely at drug prescriptions, and multi‐faceted interventions, such as specialist clinics involving comprehensive geriatric assessment. We included studies of interventions for which the target was polypharmacy across all ages, provided results for those aged 65 years and older were available separately. We examined all types of interventions as set out by the most recent EPOC taxonomy of health systems interventions (EPOC 2015; EPOC 2016), which directly or indirectly affected prescribing and were aimed at improving appropriate polypharmacy. These included the following:
Implementation strategies (previously categorised as professional interventions), defined as interventions designed to bring about changes in healthcare organisations, the behaviour of healthcare professionals or the use of health services by healthcare recipients, such as educational programmes aimed at prescribers.
Delivery arrangements (previously categorised as organisational interventions), defined as changes in how, when and where healthcare is organised and delivered, and who delivers healthcare, such as skill‐mix changes, pharmacist‐led medication review services or specialist clinics, information and communication technology (ICT) interventions such as clinical decision support systems or use of risk screening tools.
Financial arrangements (previously categorised as financial interventions), defined as changes in how funds are collected, insurance schemes, how services are purchased, and the use of targeted financial incentives or disincentives, such as incentive schemes for changes in prescribing practice.
Governance arrangements (previously categorised as regulatory interventions), defined as rules or processes that affect the way in which powers are exercised, particularly with regard to authority, accountability, openness, participation and coherence, such as changes in government policy or legislation affecting prescribing.
Types of outcome measures
Inappropriate prescribing measured by validated tools (such as Beers criteria (Fick 2003), MAI (Hanlon 1992), STOPP/START criteria (Gallagher 2008; O'Mahony 2015) or Assessing Care of Vulnerable Elderly (ACOVE) (Wenger 2001)) was the main outcome measure considered in the review, as in previous iterations of the review. We excluded studies in which medication appropriateness was determined solely by expert opinion (i.e. no measures/tools were used).
Primary outcomes
The primary outcomes of interest for this review were the following.
Medication appropriateness (as measured by an implicit, i.e. judgement‐based, tool, e.g. MAI (Hanlon 1992) or a defined subset of criteria from a validated instrument) (Appendix 2).
Potentially inappropriate medications (as defined by a validated explicit, i.e. criteria‐based, tool, e.g. STOPP criteria (Gallagher 2008; O'Mahony 2015), which could consist of the number of potentially inappropriate medications and/or the proportion of patients with one or more potentially inappropriate medication) (Appendix 2).
Potential prescribing omissions (as defined by a validated explicit tool, e.g. START criteria (Gallagher 2008; O'Mahony 2015)), which could consist of the number of potential prescribing omissions and/or the proportion of patients with one or more potential prescribing omission.
Hospital admissions (including all‐cause hospital admissions and unplanned hospital readmissions).
Secondary outcomes
Secondary outcomes included the following.
Medication‐related problems, for example adverse drug reactions and drug‐drug interactions (DDIs).
Adherence to medication.
Quality of life (as assessed by a validated method).
Search methods for identification of studies
The Information Specialist for the EPOC group updated the searches and searched the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects (DARE) for related systematic reviews, as well as the databases listed below for primary studies. Searches were originally conducted in May 2016, with an updated search conducted in February 2018. An updated search for the current review was undertaken in January 2021. The search strategy for the 2021 search is detailed in Appendix 3.
The Health Technology Assessment Database and NHS Economic Evaluation Database (NHS EED) were searched for the previous update of this review (February 2018). NHS EED ceased adding new records after 2014.
Databases
Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 1) in the Cochrane Library
MEDLINE Ovid (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations) (1946 to 13 January 2021)
Embase Ovid (1974 to 13 January 2021)
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1980 to 13 January 2021)
Trial registries
Two trials registers were searched on 7 February 2018 and an updated search was completed in January 2021.
International Clinical Trials Registry Platform (ICTRP), World Health Organization (WHO) (www.who.int/ictrp/en)
ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov)
Search strategies comprised keywords and, when available, controlled vocabulary such as MeSH (medical subject headings). All databases were searched for articles indexed from 2018 onwards. Two methodological search filters were used to limit retrieval to appropriate study designs. No language restrictions were applied.
We ran a new search in February 2023 and have added potentially eligible studies published between January 2021 and February 2023 to Characteristics of studies awaiting classification.
Searching other resources
We reviewed the reference lists of relevant systematic reviews (Appendix 4).
We contacted the authors of relevant studies and reviews to ask that they clarify reported published information or to seek unpublished results/data.
We contacted researchers with expertise relevant to the review topic or to EPOC interventions.
We conducted cited reference searches on studies selected for inclusion in this review, related reviews and other relevant citations as listed on the Institute for Scientific Information (ISI) Web of Science/Web of Knowledge.
Data collection and analysis
We carried out data collection and analyses as below.
Selection of studies
For this update, four review authors (JC, HB, DCB and MA) independently screened titles and abstracts identified in the searches to assess which studies met the inclusion criteria for the review. At this stage, we excluded papers that did not meet the inclusion criteria. If uncertainty or disagreement arose at this stage, we obtained full‐text articles and assessed them independently to determine whether they met the previously defined inclusion criteria. Any remaining disagreement or uncertainty was resolved by consensus through discussion with another review author (CH).
Data extraction and management
Two review authors (JC and MA) independently extracted details of articles included in this update, including study design, study population, intervention, usual care (or control group), outcome measures used and length of follow‐up data, using a specially designed data extraction form based on the EPOC template (EPOC 2017). We contacted study authors to ask for missing information or clarification. We used information from the data extraction forms to guide the extraction of numerical data for meta‐analysis in Review Manager 4.13 (RevMan 2022).
We presented data from randomised trials using the format suggested in the EPOC Working Paper on presentation of data (EPOC 2017). We extracted outcome data at the last time point reported to assess enduring effects of the intervention.
Assessment of risk of bias in included studies
Two review authors (JC and MA) independently assessed the internal validity of each study included in this update and resolved discrepancies by discussion. Any remaining disagreement was resolved by discussion with CH.
We used the Cochrane tool for assessing risk of bias (Higgins 2011), based on six standard criteria: adequate sequence generation, concealment of allocation, blinding of participants and personnel, blinded or objective assessment of primary outcome(s), adequately addressed incomplete outcome data, freedom from selective reporting and freedom from other risks of bias (including contamination). We reported all included studies in the risk of bias tables.
Measures of treatment effect
We measured the effect of the intervention by referencing published tools (e.g. implicit, judgement‐based tools such as the MAI (Hanlon 1992) and/or explicit, criterion‐based tools such as the Beers criteria (AGS 2019; Fick 2003)) used to assess inappropriate prescribing as outlined above. We reported outcomes for each study in natural units. When baseline results were available from studies, we reported means and standard deviation (SD) values for the change from baseline for intervention and control groups (or usual care). When baseline results were not available, we reported postintervention means and SD values and/or the proportion of patients with one or more PIMs or PPOs for intervention and control groups (usual care). We analysed data using RevMan 4.13.
We planned to perform an assessment of evidence on the theoretical basis underpinning the interventions. For example, if studies reported that interventions were based on the Theory of Diffusion (Rogers 2003), then we planned to pool data across these studies, where appropriate, in order to develop a cumulative evidence base for the theory in question. Where possible, instead of subgrouping outcomes according to the specific tool (i.e. STOPP versus Beers), we pooled studies under the broad descriptions of medication appropriateness (as measured by an implicit tool), PIMs (which consists of the number of PIMs and/or the proportion of patients with one or more PIM) and PPOs (which consists of the number of PPOs and/or the proportion of patients with one or more PPO).
Unit of analysis issues
We critically examined the methods of analysis of all study types. When studies with a unit of analysis error were identified, we re‐analysed the data excluding such studies (sensitivity analysis).
For new cluster‐randomised controlled trials included in this update, we considered whether clustering had been taken into account in the trials. If not, we estimated effective sample sizes by calculating the study's design effect using an intracluster correlation coefficient (ICC) as reported in the trials. If the ICC was not reported, we used an ICC from similar trials. For dichotomous data, the number of participants and the number experiencing the event/outcome were divided by the same design effect. For continuous data, we only calculated the effective sample size and did not alter means or standard deviations (Higgins 2022, chapter 23.1.4).
Dealing with missing data
We assessed the methods used in each included study to deal with missing data. Any study with a differential loss to follow‐up between groups greater than 20% was excluded from meta‐analysis.
Assessment of heterogeneity
We examined and interpreted heterogeneity using the I2 statistic and the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% may represent considerable heterogeneity) (Higgins 2022).
Assessment of reporting biases
We assessed reporting bias by scrutinising study results using the risk of bias tables provided in RevMan 4.13. We examined funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias.
Data synthesis
We pooled the results of studies in a meta‐analysis using a random‐effects model if at least two studies were comparable in terms of participants, interventions and outcomes. We pooled outcome data on the basis of whether included studies had used an implicit (judgement‐based) or explicit (criterion‐based) tool to measure inappropriate prescribing. We presented results with 95% CIs, and estimates when different scales were used to report the same dichotomous outcomes (e.g. the proportion of patients with one or more potentially inappropriate prescriptions) as risk ratios (RRs). We used standardised mean differences (SMDs) in meta‐analyses when different scales were used to report the same continuous outcome.
Subgroup analysis and investigation of heterogeneity
We grouped studies and described them according to type of intervention, setting and study design, and we planned to perform an assessment of evidence on the theoretical basis underpinning the interventions. For example, if studies reported that interventions were based on the Theory of Diffusion (Rogers 2003), then we planned to pool data across these studies, where appropriate, in order to develop a cumulative evidence base for the theory in question. Where possible, instead of subgrouping outcomes according to the specific tool (i.e. STOPP versus Beers), we pooled studies under the broad descriptions of medication appropriateness (as measured by an implicit tool), potentially inappropriate medications (which consists of the number of potentially inappropriate medications and/or the proportion of patients with one or more potentially inappropriate medications), and potential prescribing omissions (which consists of the number of potential prescribing omissions and/or the proportion of patients with one or more potential prescribing omissions).
Our plan to pool studies under the broad descriptions of medication appropriateness, PIMs and PPOs is outlined in the section Measures of treatment effect.
Sensitivity analysis
We performed a sensitivity analysis for pooled results based on methodological quality to assess the overall effect. We excluded studies with a unit of analysis error from the meta‐analysis.
Summary of findings and assessment of the certainty of the evidence
We graded our confidence in the evidence by creating a summary of findings table, using the approach recommended by the GRADE Working Group and guidance developed by EPOC (EPOC 2017b; Guyatt 2008). We included the most important outcomes, which were: medication appropriateness (as measured by an implicit tool), the number of PIMs, the proportion of patients with one or more PIM, the number of PPOs, the proportion of patients with one or more PPO, hospital admissions and quality of life. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), along with GRADE worksheets, to assess the certainty of evidence (GRADEpro GDT 2015). Two review authors (JC, CC) independently assessed the certainty of evidence for each outcome. We have presented the certainty of evidence for each outcome in GRADE tables (Table 1; Appendix 5).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; and Characteristics of studies awaiting classification.
Results of the search
We updated the electronic searches and identified 2811 potentially relevant citations (Figure 1). After six duplicates were removed, we screened titles and abstracts and retrieved 68 studies for full‐text review.
1.
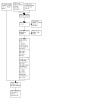
Study flow diagram.
Of these, 22 studies were identified as ongoing, one was already included in the review and one was a brief report. This left nine studies for inclusion in the updated review.
We identified three additional studies: two from searching references used in systematic reviews and one study previously identified as ongoing, which was published in July 2021. The two studies identified through systematic reviews were excluded due to not using a validated measure of prescribing appropriateness. This left 10 studies to include in this update of the review.
Included studies
In total, we identified 38 eligible studies, of which 10 were newly included in this update. Where data from the studies that were added to the review could not be included in any form of meta‐analysis, narrative descriptions of results are presented. Details are provided in the Characteristics of included studies table and are briefly summarised below.
Study design
The included studies consisted of 24 randomised trials (Auvinen 2021; Basger 2015; Bladh 2011; Bucci 2003; Campins 2017; Coronado‐Vazquez 2019; Crotty 2004b; Curtin 2020; Dalleur 2014; Frankenthal 2014; Fried 2017; Gallagher 2011; Haag 2016; Hanlon 1996; Michalek 2014; Milos 2013; O'Mahony 2020; Olsson 2012; Schmader 2004; Shim 2018; Spinewine 2007; Syafhan 2021; Taylor 2003; Wehling 2016), and 14 cluster‐randomised trials (Blum 2021; Boersma 2019; Clyne 2015; Crotty 2004a; Garcia‐Gollarte 2014; Franchi 2016; Koberlein‐Neu 2016; Muth 2016; Muth 2018; Pitkala 2014; Romskaug 2020; Strauven 2019; Tamblyn 2003; Thyrian 2017).
Settings
Of the 19 studies conducted in hospital settings (7916 participants), five were conducted in hospital outpatient clinics (Boersma 2019; Bucci 2003; Hanlon 1996; Schmader 2004; Shim 2018). One was conducted at the hospital/home care interface (Crotty 2004b), and 13 in an inpatient setting (Basger 2015; Bladh 2011; Blum 2021; Curtin 2020; Dalleur 2014; Franchi 2016; Gallagher 2011; Haag 2016;Michalek 2014; O'Mahony 2020; Olsson 2012; Spinewine 2007; Wehling 2016).
Thirteen studies were conducted in primary care settings (15,740 participants) (Campins 2017; Clyne 2015; Coronado‐Vazquez 2019; Fried 2017; Koberlein‐Neu 2016; Milos 2013; Muth 2016; Muth 2018; Romskaug 2020; Syafhan 2021; Tamblyn 2003; Taylor 2003; Thyrian 2017).
One study was carried out among patients in home‐delivered care settings (512 participants) (Auvinen 2021), and five took place in nursing homes (3562 participants) (Crotty 2004a; Frankenthal 2014; Garcia‐Gollarte 2014; Pitkala 2014; Strauven 2019).
All studies reported trials that were confined to a single setting.
The included studies were carried out in 18 high‐income countries: Australia (three studies), Belgium (five studies), Canada (two studies), England (one study), Finland (two studies), Germany (six studies), Hong Kong (one study), Iceland (one study), Ireland (five studies), Israel (one study), Italy (two studies), the Netherlands (two studies), Northern Ireland (one study), Norway (one study), Scotland (one study), Spain (three studies), Sweden (three studies), Switzerland (one study) and the USA (five studies), and one upper middle‐income country: Malaysia (one study) (World Bank 2022).
Participants
A total of 18,073 participants were included in this review, most of whom were female (53.6%) and had a mean age of 79.1 years. Ethnicity was reported in six studies (1398 participants), and in four of these most participants were white (Haag 2016; Hanlon 1996; Schmader 2004; Taylor 2003); in one study 7.25% were non‐English speaking (Crotty 2004b) and in one most (63.8%) were Chinese (Shim 2018). All study participants had more than one long‐term medical condition, which included asthma, diabetes, dyslipidaemia, hypertension, cardiovascular disease (including congestive heart failure) and dementia. On average, participants were receiving more than four medicines at baseline. Data pertaining to the number of medicines prescribed at baseline were available for 36 of the 38 studies (15,010 participants), and showed that participants were prescribed on average 8.98 medicines at baseline.
Interventions
In all cases, interventions were classified as either delivery arrangements (Auvinen 2021; Basger 2015; Bladh 2011; Blum 2021; Boersma 2019; Bucci 2003; Crotty 2004b; Curtin 2020; Fried 2017; Haag 2016; Koberlein‐Neu 2016; Michalek 2014; Milos 2013; Muth 2016; Muth 2018; Olsson 2012; O'Mahony 2020; Schmader 2004; Shim 2018; Spinewine 2007; Thyrian 2017), implementation strategies (Franchi 2016; Garcia‐Gollarte 2014), or both (Campins 2017; Clyne 2015; Coronado‐Vazquez 2019; Crotty 2004a; Dalleur 2014; Frankenthal 2014; Gallagher 2011; Hanlon 1996; Pitkala 2014; Romskaug 2020; Strauven 2019; Syafhan 2021; Tamblyn 2003; Taylor 2003; Wehling 2016) (see Types of interventions for definitions). However, within these broad intervention categories, there were relatively small numbers of studies examining the outcomes of interest. Hence, as outlined in the Methods, analysis was driven by the way in which prescribing appropriateness was judged.
Complexity and variability
Thirty‐six studies examined complex, multi‐faceted interventions of pharmaceutical care in a variety of settings. One uni‐faceted study examined computerised decision support (CDS) provided to GPs in their own practices (Tamblyn 2003); one tested a medication withdrawal plan among hospital patients (Curtin 2020). Pharmaceutical care was commonly provided by pharmacists working closely with other healthcare professionals in a variety of settings. In hospital settings, pharmacists worked as part of a multi‐disciplinary team in outpatient clinics (Bucci 2003; Hanlon 1996; Schmader 2004; Shim 2018), in inpatient services on hospital wards as a clinical pharmacy service (Basger 2015; Bladh 2011; Blum 2021; Dalleur 2014; Franchi 2016; Gallagher 2011; Haag 2016; Michalek 2014; Olsson 2012; O'Mahony 2020; Spinewine 2007; Wehling 2016), or as part of the hospital discharge process (Crotty 2004b). In community settings, pharmaceutical care services, including medication reviews, patient interviews and counselling, were provided by different healthcare professionals. This included pharmacists working in community‐based family medicine clinics (Taylor 2003), or within primary care centres (Campins 2017; Milos 2013), general practices (Clyne 2015;Fried 2017; Koberlein‐Neu 2016; Syafhan 2021), nurses/healthcare assistants (Muth 2016; Muth 2018; Thyrian 2017), and with physicians and nurses in home care settings (Auvinen 2021). In nursing homes, interventions involved multi‐disciplinary case conferences combined with staff education provided by pharmacists (Crotty 2004a), medication reviews by the study pharmacists and discussed with the chief physician (Frankenthal 2014), training sessions for staff (Garcia‐Gollarte 2014; Pitkala 2014), and a combination of educational workshops and medication reviews conducted by pharmacists, physicians and nurses (Strauven 2019).
Health professionals' input
Physicians delivered the intervention via a computerised support programme in one study (Tamblyn 2003); in another doctors and nurses delivered the intervention using a web‐based prescribing tool (Boersma 2019); family doctors and nurses used a paper‐based decision tool to conduct the intervention in another study in primary care (Coronado‐Vazquez 2019). Only doctors were involved in intervention delivery in two studies, one among hospital inpatients (Curtin 2020) and another in primary care (Romskaug 2020). In all other studies, structured processes were used to develop recommendations for improving the appropriateness of prescribing to prescribers.
In 19 studies, the pharmacist(s) conducted an independent medication review using participant notes (Auvinen 2021; Bladh 2011; Campins 2017; Crotty 2004a; Crotty 2004b;Koberlein‐Neu 2016; Milos 2013), together with participants during a face‐to‐face encounter (Basger 2015; Bucci 2003; Frankenthal 2014; Hanlon 1996; Schmader 2004; Shim 2018; Spinewine 2007; Syafhan 2021; Tamblyn 2003; Taylor 2003); in a face‐to‐face encounter also involving a GP and nurse (Strauven 2019), or during a medication therapy management (MTM) consultation over the telephone (Haag 2016).
Following medication reviews, recommendations were discussed with a multi‐disciplinary team during case conferences (Auvinen 2021; Crotty 2004a; Crotty 2004b), sent to patients' own GPs or consultants (Basger 2015; Bladh 2011; Blum 2021; Campins 2017; Frankenthal 2014;Milos 2013; Syafhan 2021), or discussed with prescribers and followed up by written recommendations (Hanlon 1996) from multi‐disciplinary team members at the same outpatient clinic (Bucci 2003), or during inpatient ward rounds (Spinewine 2007). In eight studies, medicine reviews were undertaken by a doctor (Boersma 2019; Clyne 2015; Coronado‐Vazquez 2019; Curtin 2020; Fried 2017; Muth 2016; Muth 2018; Wehling 2016); in two studies, medication reviews were carried out by a doctor and pharmacist (Blum 2021; O'Mahony 2020); and in one, a geriatrician undertook medication reviews and discussed them with a family physician who also followed up the patients (Romskaug 2020). In three studies, nurses were asked to identify potential medication‐related problems and bring these to the attention of the consulting physician (Pitkala 2014), or conduct prescription reviews (Thyrian 2017), which were sent to the study physician (Olsson 2012). In one study, the pharmacist was an integral member of the multi‐disciplinary team (Schmader 2004) and contributed to the pharmaceutical care aspect of participants' care plans at the point of decision‐making. In one study, a nurse informed patients about changes to their drug regimens and changes were implemented if the patients accepted them (Auvinen 2021).
In four studies, participants' medication lists were screened by a geriatrician (Dalleur 2014), or by the primary research physician upon admission to hospital (Gallagher 2011; Garcia‐Gollarte 2014; Michalek 2014), and oral and written recommendations outlining appropriate prescribing changes were then provided to the attending physicians. In the Dalleur 2014 study, no pharmacist was available to collaborate with the inpatient geriatric consultation team owing to lack of resources within the hospital.
Education
Participant education was provided as part of the pharmaceutical care intervention in four studies in which the intervention was conducted face‐to‐face, and these participants were given 'directive guidance' and specialised medication scheduling tools (e.g. monitored dosage systems) to encourage adherence to their prescribed medication regimens (Bucci 2003; Hanlon 1996; Spinewine 2007; Taylor 2003). Directive guidance describes pharmaceutical care activities such as provision of information about medications, their administration and their adverse effects (Bucci 2003). In one study, patients received information leaflets during the medicines reviews, describing potentially inappropriate prescribing (PIP) and alternative treatment options (pharmacological and non‐pharmacological) (Clyne 2015). In another, patients met with a pharmacist in a pharmacy and received education on their medications, how to use them and the importance of adherence (Shim 2018).
Shared decision‐making was central to two studies (Blum 2021; Coronado‐Vazquez 2019). Each involved a consultation between a doctor and the patient at which medications, potential risks and options were discussed, and a mutual consensus on the patient's medication regimen reached.
Education was provided to prescribers and other healthcare professionals included in the multi‐disciplinary team as part of the intervention in 14 studies (Auvinen 2021; Bucci 2003; Clyne 2015; Crotty 2004a; Crotty 2004b; Franchi 2016; Garcia‐Gollarte 2014; Hanlon 1996; O'Mahony 2020; Pitkala 2014; Spinewine 2007; Strauven 2019; Syafhan 2021; Wehling 2016). This occurred at case conferences, during ward rounds, as part of workshops, or when evidence‐based information and answers to specific medication‐related queries were presented. In two studies in which the pharmacist was part of a multi‐disciplinary team, no educational intervention was specified in the methodology (Schmader 2004; Taylor 2003).
Timing of intervention delivery
The timing of provision of the intervention was variable. Interventions were delivered over a period of time, for example during the hospital inpatient stay and at discharge (Bladh 2011; Franchi 2016; Haag 2016; Michalek 2014; Schmader 2004; Spinewine 2007), or over several clinic visits and over several months on an ongoing basis (Tamblyn 2003). Interventions were also delivered at the time of an event, for example following hospital admission (Blum 2021; Curtin 2020; Dalleur 2014; Gallagher 2011; O'Mahony 2020), at discharge from hospital (Basger 2015), during attendance at outpatient clinics (Boersma 2019; Bucci 2003; Hanlon 1996; Schmader 2004; Taylor 2003), at nursing home visits (Crotty 2004a; Strauven 2019), at hospital discharge to a nursing home (Crotty 2004b), home visit by a nurse (Auvinen 2021; Olsson 2012), or GP visit (Campins 2017; Clyne 2015; Fried 2017; Muth 2016; Muth 2018). In one study, the intervention was delivered during a consultation at a pharmacy (Shim 2018); in another, it was conducted in the patient's own home or at the hospital outpatient geriatric unit, depending on suitability, and follow‐up visits were done by a geriatrician, family physician, by telephone, or via the home nursing service (Romskaug 2020).
In studies for which details of intervention administration were provided, interventions were most commonly administered during a single episode of care (Bucci 2003; Crotty 2004a; Curtin 2020; Hanlon 1996; Tamblyn 2003; Taylor 2003). Interventions were implemented over varying durations, ranging from three months (Curtin 2020), five or six months (Auvinen 2021; Bucci 2003; Coronado‐Vazquez 2019; Romskaug 2020; Shim 2018; Syafhan 2021), one year (Blum 2021; Boersma 2019; Frankenthal 2014; Koberlein‐Neu 2016; Strauven 2019), to three years and three months (Schmader 2004). Further details of the interventions are detailed in the Characteristics of included studies tables.
Outcomes
The first primary outcomes of interest in this review were medication appropriateness (as measured by an implicit tool), potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs). Validated assessments of appropriateness reported in all included studies were measured independently by pharmacists, geriatricians or the research team, who had access to participants' charts and medication records. Time between delivery of the intervention and follow‐up outcome measurement varied from immediately postintervention (e.g. post hospital discharge or clinic visit) (Michalek 2014; Schmader 2004; Spinewine 2007; Tamblyn 2003; Wehling 2016) to at least one month (Bucci 2003), eight weeks (Crotty 2004b) or two months (Blum 2021; Shim 2018; Syafhan 2021), 12 weeks (O'Mahony 2020) or three months (Basger 2015; Boersma 2019; Crotty 2004a; Curtin 2020; Garcia‐Gollarte 2014), 16 weeks (Romskaug 2020), six months (Auvinen 2021; Clyne 2015; Coronado‐Vazquez 2019; Gallagher 2011), eight months (Strauven 2019), up to one year (Dalleur 2014; Franchi 2016; Hanlon 1996; Pitkala 2014; Taylor 2003), and up to two years (Frankenthal 2014).
Fourteen studies measured medication appropriateness (as measured by an implicit tool, i.e. judgement‐based); all used the Medication Appropriateness Index (MAI) (Boersma 2019; Bucci 2003; Crotty 2004a; Crotty 2004b; Gallagher 2011; Hanlon 1996; Muth 2016; Muth 2018; Romskaug 2020; Schmader 2004; Shim 2018; Spinewine 2007; Syafhan 2021; Taylor 2003). One study measured medication appropriateness based on STOPP/START and Beers criteria (Coronado‐Vazquez 2019), but this was not suitable for inclusion in the meta‐analysis. Eight studies reported MAI as a change from baseline and 10 studies reported postintervention scores. One study reported the MAI score in terms of the number of prescriptions with inappropriate medications; this was unsuitable for inclusion in the meta‐analysis (Taylor 2003).
Twenty‐three studies measured PIMs (Auvinen 2021; Bladh 2011; Blum 2021; Boersma 2019; Campins 2017; Clyne 2015; Coronado‐Vazquez 2019; Dalleur 2014; Franchi 2016; Frankenthal 2014; Fried 2017; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Koberlein‐Neu 2016; Milos 2013; Olsson 2012; Pitkala 2014; Schmader 2004; Spinewine 2007; Strauven 2019; Tamblyn 2003; Thyrian 2017). These studies used a range of explicit (criterion‐based) tools, including Beers criteria (Coronado‐Vazquez 2019; Franchi 2016; Pitkala 2014; Schmader 2004; Spinewine 2007), Screening Tool of Older Person’s Prescriptions (STOPP) criteria (Blum 2021; Boersma 2019; Campins 2017; Clyne 2015; Coronado‐Vazquez 2019; Dalleur 2014; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016), Tool to Reduce Inappropriate Medication (TRIM) recommendations (Fried 2017), the drug‐specific quality indicators established by the Swedish National Board of Health and Welfare (Bladh 2011; Milos 2013; Olsson 2012), the PRISCUS criteria (Koberlein‐Neu 2016; Thyrian 2017) and the Meds 75+ Database (Auvinen 2021), which were measured at varying time points ranging from at the point of inpatient discharge to 24 months follow‐up. Nine studies reported the number of PIMs, as identified using Beers criteria (Pitkala 2014; Schmader 2004; Spinewine 2007) and STOPP criteria (Clyne 2015; Garcia‐Gollarte 2014), STOPP and Beers (Coronado‐Vazquez 2019), the PRISCUS criteria (Koberlein‐Neu 2016) and the Meds 75+ Database (Auvinen 2021). One study reported the number of PIM changes per patient using STOPP criteria (Boersma 2019) and another reported the number of events and percentage using STOPP (Blum 2021).
Thirteen studies reported the proportion of patients with one or more PIM, as identified using Beers criteria (Pitkala 2014; Spinewine 2007), the STOPP criteria (Blum 2021; Boersma 2019; Clyne 2015; Dalleur 2014; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016), the drug‐specific quality indicators established by the Swedish National Board of Health and Welfare (Milos 2013), TRIM recommendations (Fried 2017) or the PRISCUS criteria (Thyrian 2017).
One study used the McLeod criteria and reported the rate of inappropriate medications prescribed per physician visit postintervention (Tamblyn 2003).
Potential prescribing omissions (PPOs) or under‐use of medication were reported in 10 studies (Blum 2021; Boersma 2019; Coronado‐Vazquez 2019; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Schmader 2004; Spinewine 2007; Strauven 2019), and both were reported as postintervention scores. The only implicit tool used was the Assessment of Under‐utilisation of Medication (AUM) instrument (Gallagher 2011; Jeffery 1999; Schmader 2004). Nine studies used explicit tools, including the seven process measures from the full range of Assessing Care of Vulnerable Elderly (ACOVE) criteria (Spinewine 2007) and the Screening Tool to Alert doctors to the Right Treatment (START) criteria (Blum 2021; Boersma 2019; Coronado‐Vazquez 2019; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014;Haag 2016; Strauven 2019). All but two studies using an explicit tool reported the proportion of patients with one or more PPOs, which were measured at varying time points ranging from at the point of inpatient discharge to 24 months follow‐up. Two studies reported mean and median values respectively for PPOs at baseline but follow‐up values were not reported (Coronado‐Vazquez 2019; Strauven 2019).
Four other studies reported results in the form of combined PIM and PPO indicators/scores (Basger 2015; Michalek 2014; Strauven 2019; Wehling 2016). One study measured appropriateness using the prescribing appropriateness criteria‐set for application in older Australians (Basger 2012) and reported changes in the number of criteria met (Basger 2015). This method uses a combination of both explicit and implicit tools to measure appropriateness. Two studies used the Fit for The Aged (FORTA) criteria (Kuhn‐Thiel 2014), to evaluate the appropriateness of medications in terms of unnecessary, inappropriate or harmful medications and drug omissions (Michalek 2014; Wehling 2016). In the Michalek 2014 study, this was the number of drugs within each FORTA classification (i.e. FORTA drug labels ranged from A (indispensable), B (beneficial), C (questionable) to D (avoid)), while the Wehling 2016 study reported the summated FORTA score postintervention along with the change in FORTA score postintervention. Strauven 2019 reported two outcomes, A and B, relating to medication appropriateness. Outcome A was achieved when at least one PIM or PPO that had been present at baseline had been solved by the end of the study. Outcome B was achieved when no new PIMs or PPOs were present at the end of the study compared to baseline. PIP was measured using the STOPP‐START criteria and Beers' criteria through an algorithm in the study database, which was programmed to detect PIMs and PPOs.
No other validated criteria (e.g. Zhan criteria) were reported.
The other primary outcome of interest in this review was hospital admissions (including unplanned hospital admissions). Fourteen studies measured hospital admissions by examining hospital records at varying time points postintervention (Blum 2021; Campins 2017; Crotty 2004b; Curtin 2020; Franchi 2016; Frankenthal 2014; Gallagher 2011; Haag 2016; Muth 2018; Romskaug 2020; Spinewine 2007; Strauven 2019; Syafhan 2021; Taylor 2003), ranging from eight weeks (Crotty 2004b; Spinewine 2007), one to three months (Curtin 2020; Haag 2016) and six months to one year (Blum 2021; Campins 2017; Franchi 2016; Frankenthal 2014; Gallagher 2011; Muth 2018; Romskaug 2020; Strauven 2019; Syafhan 2021; Taylor 2003).
The secondary outcomes of interest in this review were medication‐related problems (i.e. drug interactions, adverse drug reactions (ADRs)), adherence to medication and quality of life. Medication‐related problems were measured in 10 studies and were reported as medication misadventures (defined as iatrogenic incidents that occur as a result of error, immunological response or idiosyncratic response and are always unexpected or undesirable to the participant) (Taylor 2003), potential drug‐drug interaction (DDI) and/or potentially severe DDI (Auvinen 2021; Blum 2021; Franchi 2016), postintervention adverse drug events (ADEs) (Crotty 2004b; Hanlon 1996; Schmader 2004; Wehling 2016), adverse drug reactions (ADRs) (O'Mahony 2020) or medication related problems (MRPs) (Syafhan 2021).
Adherence to medication was measured in six studies (Campins 2017; Haag 2016; Muth 2016; Muth 2018; Shim 2018; Taylor 2003), three studies used the Morisky‐Green test (Campins 2017; Muth 2016; Muth 2018), one study used an adapted Morisky Medication Adherence Scale (MMAS) (Haag 2016), one used the Malaysian Medication Adherence Scale (MALMAS) (Shim 2018), one used the Medication Adherence Scale (MMAS‐8) (Blum 2021), one used the Medication Adherence Report Scale (MARS) (Syafhan 2021), one study assessed adherence to medication via participant self‐report (Taylor 2003), and one was unclear in its methodology (Coronado‐Vazquez 2019). Adherence to medications was assessed at varying time points postintervention medication, ranging from 30 days (Haag 2016), six to nine months (Campins 2017; Coronado‐Vazquez 2019; Muth 2018; Shim 2018; Syafhan 2021) to one year (Blum 2021; Muth 2016; Taylor 2003).
Quality of life (QoL) was assessed in 16 studies. Researchers used the Medical Outcomes Study 36‐item Short Form health survey (SF‐36) in three studies (Basger 2015; Hanlon 1996; Taylor 2003), the Medical Outcomes Study 12‐item Short‐Form Health Survey (SF‐12) in one study (Frankenthal 2014), the EuroQol‐ED (EQ‐5D) in eight studies (Bladh 2011; Blum 2021; Campins 2017; Muth 2016; Muth 2018; Olsson 2012; O'Mahony 2020; Syafhan 2021), the 15‐dimensional instrument of health‐related quality of life (15D) in two studies (Pitkala 2014; Romskaug 2020), the Quality of Life in Alzheimer Disease instrument in one study (Thyrian 2017) and the QUALIDEM (an instrument completed by health professionals that measures QoL in people at all stages of dementia; Ettema 2007) and ICECAP‐O (a measure of QoL not confined to health, which was designed for use in the economic evaluation of health and social care interventions in older adults; Coast 2008) instruments in one study (Curtin 2020). Quality of life was assessed at varying time points postintervention, ranging from three months (Basger 2015; Curtin 2020), 12 weeks (O'Mahony 2020), six to nine months (Bladh 2011; Campins 2017; Muth 2018; Romskaug 2020; Syafhan 2021) to one year (Blum 2021; Frankenthal 2014; Hanlon 1996; Muth 2016; Olsson 2012; Pitkala 2014; Taylor 2003; Thyrian 2017).
Excluded studies
We have presented a small sample of the total excluded publications in the Characteristics of excluded studies table. Many studies were excluded because they did not meet the criteria for inclusion in our review, such as study design, not using a validated measure of medication appropriateness or not focusing on polypharmacy. The sample we have presented are examples of those that seemed appropriate for inclusion but on closer inspection were rejected for reasons such as not all patients in the study were prescribed polypharmacy.
Studies awaiting classification
The Studies awaiting classification section contains studies identified for potential inclusion in the review following a search conducted in February 2023.
Ongoing studies
We have described ongoing studies identified during completion of the review and provided details such as primary author, research question(s) and methods and outcome measures, together with an estimate of the reporting date, in the Characteristics of ongoing studies table appended to this review.
Risk of bias in included studies
Details of the risk of bias are presented in Figure 2 and Figure 3 and in the Characteristics of included studies tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Twenty‐two trials reported adequate sequence generation (Auvinen 2021; Boersma 2019; Bucci 2003; Campins 2017; Clyne 2015; Crotty 2004a; Crotty 2004b; Curtin 2020; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Hanlon 1996; Milos 2013; Muth 2018; O'Mahony 2020; Pitkala 2014; Romskaug 2020; Schmader 2004; Shim 2018; Strauven 2019; Syafhan 2021; Thyrian 2017), and 18 reported concealment of allocation (Bladh 2011; Blum 2021; Campins 2017; Clyne 2015; Crotty 2004a; Crotty 2004b; Curtin 2020; Frankenthal 2014; Gallagher 2011; Haag 2016; Koberlein‐Neu 2016; O'Mahony 2020; Michalek 2014; Milos 2013; Pitkala 2014; Romskaug 2020; Strauven 2019; Wehling 2016).
Blinding
In 19 studies, blinded measurement of outcomes had taken place to ensure that primary outcome assessors had no knowledge of the intervention received by participants (Blum 2021; Bucci 2003; Clyne 2015; Crotty 2004b; Curtin 2020; Dalleur 2014; Franchi 2016; Frankenthal 2014; Gallagher 2011; Haag 2016; Hanlon 1996; Muth 2016; O'Mahony 2020; Pitkala 2014; Romskaug 2020; Schmader 2004; Shim 2018; Tamblyn 2003; Wehling 2016). Blinding of participants and personnel had taken place to ensure there was no performance bias in six studies (Garcia‐Gollarte 2014; Michalek 2014; Muth 2016; Olsson 2012; O'Mahony 2020; Pitkala 2014).
Incomplete outcome data
Incomplete outcome data were adequately addressed in 25 studies. In one study, 864 participants were randomly assigned but only 834 were included in the analysis, and no intention‐to‐treat analysis was reported (Schmader 2004). Therefore, it was unclear whether all outcome data were included.
In one study, missing data meant that the difference in the secondary outcomes could not be analysed (between baseline and three‐month follow‐up: 62.9% of MMSE data were missing), 28.2% of Katz‐ADL data were missing and 24.2% of Fried criteria data were missing; at one year follow‐up 47.9% of mortality data were missing and could not be analysed (Boersma 2019). In one study, EQ‐5D‐5L was planned to be reported as part of a cost utility analysis, but other aspects of this analysis were not reported, such as quality‐adjusted life years (QALYs) (O'Mahony 2020). In Romskaug 2020, test results of some secondary outcomes were not reported, apart from the statistical results, while in Syafhan 2021, a secondary outcome measure listed in the study’s entry in the clinical trials website (clinicaltrials.gov), namely patient laboratory data, was not reported. In another study, the methods section stated that data collection would take place at baseline, middle and end of study, but results were presented for baseline and end only (Strauven 2019).
Selective reporting
We considered six studies at high risk of reporting bias (Koberlein‐Neu 2016; O'Mahony 2020; Spinewine 2007; Strauven 2019; Syafhan 2021; Thyrian 2017). In the Spinewine 2007 study, the authors failed to report one of the secondary outcomes: medications taken.
Other potential sources of bias
Contamination bias occurs when members of the control group are inadvertently exposed to the intervention, thus potentially minimising differences in outcomes between the two groups (Higgins 2011). Participants in 11 studies were protected from contamination (Blum 2021; Boersma 2019; Clyne 2015; Coronado‐Vazquez 2019; Crotty 2004a; Michalek 2014; Muth 2018; Pitkala 2014, Romskaug 2020; Strauven 2019;Thyrian 2017). In 15 studies it was unclear whether protection against contamination had been provided (Basger 2015; Curtin 2020; Dalleur 2014; Franchi 2016; Frankenthal 2014; Fried 2017; Gallagher 2011; Garcia‐Gollarte 2014; Milos 2013; Muth 2016; Olsson 2012; Schmader 2004; Shim 2018; Syafhan 2021; Tamblyn 2003), and 12 studies were determined to have high risk of contamination (Auvinen 2021; Bladh 2011; Bucci 2003; Campins 2017; Crotty 2004b; Haag 2016; Hanlon 1996; Koberlein‐Neu 2016; O'Mahony 2020; Spinewine 2007; Taylor 2003; Wehling 2016). Contamination bias is an important limitation for this review, where, in some studies, for example, a pharmacist involved in the provision of pharmaceutical care to members of the intervention group may have inadvertently modified the treatment of those in the control group as a result of having knowledge of the intervention. The possible influence of contamination bias should be considered when the results of this review are interpreted.
A funnel plot of postintervention estimates of the proportion of patients with one or more PIM showed little evidence of publication bias (Figure 4).
4.

Funnel plot of comparison: Postintervention analysis: The proportion of patients with one or more potentially inappropriate medication
Effects of interventions
See: Table 1
Key findings are summarised in Table 1.
There was a lack of certainty regarding the effects of pharmaceutical care interventions included in this review on inappropriate prescribing (medication appropriateness as measured by an implicit tool, the number of PIMs, the proportion of patients with one or more PIM, the number of PPOs and the proportion of patients with one or more PPO) (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6).
1.1. Analysis.

Comparison 1: Postintervention analysis, Outcome 1: Medication appropriateness (as measured by an implicit tool)
1.2. Analysis.

Comparison 1: Postintervention analysis, Outcome 2: Medication appropriateness (as measured by an implicit tool) (excluding Crotty 2004a)
1.3. Analysis.

Comparison 1: Postintervention analysis, Outcome 3: Number of potentially inappropriate medications
1.4. Analysis.

Comparison 1: Postintervention analysis, Outcome 4: Proportion of patients with one or more potentially inappropriate medication
1.5. Analysis.

Comparison 1: Postintervention analysis, Outcome 5: Number of potential prescribing omissions
1.6. Analysis.

Comparison 1: Postintervention analysis, Outcome 6: Proportion of patients with one or more potential prescribing omission
Hospital admissions, as reported in 14 studies, were reduced in two studies with 12 finding little or no difference between intervention and control (or usual care) groups.
No consistent intervention effect on medication‐related problems was observed across the 10 studies that evaluated this outcome; these problems were reported in terms of medication‐related problems (Syafhan 2021), ADEs (Blum 2021; Crotty 2004b; Hanlon 1996; O'Mahony 2020; Schmader 2004; Wehling 2016), medication misadventures (Taylor 2003), potential DDIs or potentially severe DDIs (Auvinen 2021; Blum 2021; Franchi 2016).
Improvement in adherence to medication was demonstrated in three studies (Coronado‐Vazquez 2019; Shim 2018; Taylor 2003), while the other six studies found little or no difference (Blum 2021; Campins 2017; Haag 2016; Muth 2016; Muth 2018; Syafhan 2021).
Quality of life (QoL) was assessed by 16 studies, with six reporting some changes (Blum 2021; Curtin 2020; O'Mahony 2020; Pitkala 2014; Romskaug 2020; Syafhan 2021), and 10 reporting no changes in QoL (Bladh 2011; Basger 2015; Campins 2017; Frankenthal 2014; Hanlon 1996; Muth 2016; Muth 2018; Olsson 2012; Taylor 2003;Thyrian 2017).
Based on the GRADE approach (Guyatt 2008), we deemed the overall certainty of the body of evidence for each primary outcome for which data were included in a meta‐analysis to be low or very low, which means that the confidence in the effect estimates is very limited. Although each study included in the meta‐analyses was of a randomised design, and, where assessed, no evidence of publication bias was found (Figure 4), we downgraded the certainty of the body of evidence for each outcome based on other GRADE considerations (i.e. study limitations, consistency of effect, imprecision, indirectness) (Appendix 5).
Primary outcome results
Medication appropriateness (as measured by an implicit tool)
It is uncertain whether pharmaceutical care improves medication appropriateness (as measured by an implicit tool) because the certainty of this evidence is very low (8 studies, 947 participants). Six studies reported medication appropriateness using an implicit (judgement‐based) assessment tool (Bucci 2003; Crotty 2004a; Muth 2016; Romskaug 2020; Shim 2018; Syafhan 2021), and further unpublished data were received from the authors of two studies (Crotty 2004b; Spinewine 2007). All of these studies used the Medication Appropriateness Index (MAI) as the implicit tool. Comparison of medication appropriateness (as measured by an implicit tool) from baseline to follow‐up between the intervention group and the usual care group is shown in Analysis 1.1. Overall, a greater improvement in medication appropriateness (as measured by an implicit tool) post‐intervention was seen in the intervention group compared with the control group (mean difference (MD) ‐5.66, 95% confidence interval (CI) ‐9.26 to ‐2.06; I2 = 97%; 8 studies, 947 participants; Analysis 1.1). Marked heterogeneity between studies was noted (97%). Crotty 2004a reported a unit of analysis error; nursing homes were the unit of randomisation, but the analysis was conducted at the participant level. A sensitivity analysis excluding Crotty 2004a showed a similar improvement in medication appropriateness (as measured by an implicit tool) in favour of the intervention group (MD ‐5.97, 95% CI ‐10.08 to ‐1.85; I2 = 97%; 876 participants; Analysis 1.2).
We downgraded the certainty of the body of evidence for medication appropriateness (as measured by an implicit tool) to very low. The evidence was downgraded by one level due to study limitations/risk of bias and imprecision, and indirectness of evidence, and by two levels due to inconsistency of effect.
We identified very serious design limitations with implications in terms of selection bias, performance bias, reporting bias and risk of contamination bias in several studies. We deemed Spinewine 2007 to have high risk of bias in terms of selection bias (allocation concealment), performance bias, detection bias, contamination bias and selective reporting, which resulted in the downgrading of the certainty of evidence.
In terms of indirectness, some studies answered a restricted version of the research question, as a validated assessment of under‐prescribing was not included as part of the overall assessment of inappropriate prescribing. Therefore, these interventions did not directly target appropriate polypharmacy. Additionally, we identified evidence of inconsistency (I2 = 97%), as well as imprecision in the effect estimate, whereby the 95% CI was wide and/or crossed the line of no effect. We did not assess publication bias due to there being fewer than 10 studies.
Potentially inappropriate medications (PIMs) (including the number of PIMs and the proportion of patients with one or more PIM)
Pooled data from nine studies showed that the number of PIMs was lower in the intervention group participants compared with usual care group participants postintervention (standardised mean difference (SMD) ‐0.19, 95% CI ‐0.34 to ‐0.05; I2 = 67%; 9 studies, 2404 participants; Analysis 1.3) (Auvinen 2021; Bladh 2011; Clyne 2015; Coronado‐Vazquez 2019; Garcia‐Gollarte 2014; Koberlein‐Neu 2016; Pitkala 2014; Schmader 2004; Spinewine 2007). The numbers of PIMs were determined using explicit (criterion‐based) assessment tools, including Screening Tool of Older Person’s Prescriptions (STOPP) (version 1: Gallagher 2008; a version adapted for Spanish context by Delgado Silveiro et al (2009) as used by Coronado‐Vazquez 2019), and Beers (1997 version: Beers 1997; 2003 version: Fick 2003; 2012 version: Coronado‐Vazquez 2019), PRISCUS criteria (Holt 2010), the drug‐specific quality indicators established by the Swedish National Board of Health and Welfare (Fastbom 2015), and the Meds 75+ database (Auvinen 2021). However, it is uncertain whether pharmaceutical care reduces the number of PIMs because the certainty of this evidence is very low. The Olsson 2012 study reported the number of drug‐risk indicators per patient according to the drug‐specific quality indicators established by the Swedish National Board of Health and Welfare, and the Campins 2017 study reported the proportion of patients with at least one drug discontinuation based on STOPP criteria. The latter two studies were not included in the meta‐analyses as we were unable to extract the data required to enable comparison with the other studies.
Thirteen studies reported the proportions of patients with one or more PIM before and after intervention (Blum 2021; Boersma 2019; Clyne 2015; Dalleur 2014; Franchi 2016; Frankenthal 2014; Fried 2017; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Milos 2013; Spinewine 2007; Thyrian 2017). The proportions of patients with one or more PIM were determined using explicit (criterion‐based) assessment tools, including STOPP (version 1: Gallagher 2008; version 2: O'Mahony 2015), and Beers (1997 version: Beers 1997 and 2012 version: AGS 2012; and 2019 version: AGS 2019) (Appendix 2), the Tool to Reduce Inappropriate Medication (TRIM) recommendations based on Beers (2012 version: AGS 2012) and STOPP criteria (version 1: Gallagher 2008), PRISCUS (Holt 2010) and the drug‐specific quality indicators established by the Swedish National Board of Health and Welfare (Fastbom 2015). Pooled data from 13 studies showed that improvements were reported in the proportion of intervention patients with one or more PIM, compared to the control group participants, between baseline and discharge (risk ratio (RR) 0.81, 95% CI 0.68 to 0.98; I2 = 84%; 13 studies, 4534 participants; Analysis 1.4). There was considerable heterogeneity among the 13 trials (heterogeneity: Tau2 = 0.08; Chi² = 73.08, df = 12 (P < 0.00001); I² = 84%). It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PIM because the certainty of this evidence is very low.
We downgraded the certainty of the body of evidence for the proportion of patients with one or more PIM to very low. We identified very serious design limitations with implications in terms of selection bias, performance bias and risk of contamination bias in several studies. We deemed Spinewine 2007 to have high risk of bias in terms of selection bias (allocation concealment), performance bias, detection bias, contamination bias and selective reporting, which resulted in the downgrading of the certainty of evidence. We downgraded the certainty of evidence due to indirectness, as some studies answered a restricted version of the research question, as a validated assessment of under‐prescribing was not included as part of the overall assessment of inappropriate prescribing. Therefore, interventions did not directly target appropriate polypharmacy. Additionally, we identified evidence of inconsistency (I2 = 84%) as well as imprecision in the effect estimate, whereby the 95% CI was wide and/or crossed the line of no effect, which resulted in the downgrading of the certainty of evidence.
We assessed publication bias for studies that assessed the proportion of patients with one or more PIM, however little evidence of this was found (Figure 4).
Potential prescribing omissions (PPOs) (including the number of PPOs and the proportion of patients with one or more PPO)
Pooled data from three studies showed that the number of PPOs was lower in the intervention group participants compared with usual care group participants postintervention (SMD ‐0.48, 95% CI ‐1.05 to 0.09; I2 = 92%; 3 studies, 691 participants; Analysis 1.5) (Coronado‐Vazquez 2019; Garcia‐Gollarte 2014; Spinewine 2007). The number of PPOs was determined using explicit (criterion‐based) assessment tools, including Assessing Care of the Vulnerable Elderly (ACOVE) (version 1: Wenger 2001) and START (version 1: Gallagher 2008). Pharmaceutical care may slightly reduce the number of PPOs, but this is uncertain (very low‐certainty evidence).
We downgraded the certainty of the body of evidence for the number of PPOs to very low. We identified very serious design limitations with implications in terms of selection bias, performance bias and risk of contamination bias, which were were high or unclear in Spinewine 2007, and unclear in Garcia‐Gollarte 2014. Risks of selection bias, performance bias and attrition bias were high or unclear in Coronado‐Vazquez 2019. We deemed Spinewine 2007 to have high risk of bias in terms of selection bias (allocation concealment), performance bias, detection bias, contamination bias and selective reporting, which resulted in the downgrading of the certainty of evidence.
Seven studies also reported the proportion of patients with one or more PPO (Blum 2021; Boersma 2019; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Spinewine 2007). The proportions of patients with one or more PPO were determined using explicit (criterion‐based) assessment tools, including START (version 1: Gallagher 2008; version 2: O'Mahony 2015), and ACOVE (version 1: Wenger 2001). The proportion of patients in the intervention group with one or more PPO was lower than for those in the control group (RR 0.50, 95% CI 0.27 to 0.91; I2 = 95%; 7 studies, 2765 participants; Analysis 1.6). There was considerable heterogeneity among the seven trials (heterogeneity: Tau2 = 0.58; Chi² = 124.32, df = 6 (P < 0.00001); I² = 95%). It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PPO because the certainty of this evidence is very low.
We downgraded the certainty of the body of evidence for the proportion of patients with one or more PPO to very low due to very serious design limitations with implications in terms of selection bias, performance bias and risk of contamination bias in several studies. We deemed Spinewine 2007 to have high risk of bias in terms of selection bias (allocation concealment), performance bias, detection bias, contamination bias and selective reporting, which resulted in the downgrading of the certainty of evidence. We identified evidence of inconsistency (I2 = 95%), which resulted in the downgrading of the certainty of evidence.
Hospital admissions
Fourteen studies measured hospital admissions postintervention (Blum 2021; Campins 2017; Crotty 2004b; Curtin 2020; Franchi 2016; Frankenthal 2014; Gallagher 2011; Haag 2016; Muth 2018; Romskaug 2020; Spinewine 2007; Strauven 2019; Syafhan 2021; Taylor 2003; 4797 participants).
Hospital admissions were reduced in two studies postintervention (Crotty 2004b; Taylor 2003), with the remaining 12 finding little or no difference between intervention and usual care groups.
While two of the new studies included in this review found no significant difference in hospital admissions between intervention and control groups, notable results were presented (Strauven 2019; Syafhan 2021). Strauven 2019 found that the median number of days per hospitalisation of nursing home residents was significantly lower in the intervention group compared to the control group (intervention 7.0 days (interquartile range (IQR) 0 to 11), control 8.0 days (IQR 3 to 15.8); ratio 0.578, 95% CI 0.366, 0.913, P = 0.0203). Syafhan 2021 found a significant decrease in unplanned hospital admissions in the intervention group during the six‐month study period compared to the previous six months (total number six months pre‐study = 40, mean 0.2 ± SD ‐0.51; total number six‐month follow‐up = 21, mean 0.1 ± SD 0.40; P = 0.023).
Taylor 2003 reported a reduction in both the number of hospital admissions (P value = 0.003) and the number of emergency department visits (P value = 0.044) during the intervention year compared with preintervention. Crotty 2004b reported less hospital usage among participants who received the intervention and were still alive at eight weeks postintervention compared with usual care group participants (risk ratio (RR) 0.38, 95% CI 0.15 to 0.99). However, analysis of all participants including deaths and losses to follow‐up showed similar hospital usage in the intervention and control groups (‐9 (16.7%) with intervention versus ‐15 (26.8%) with control; RR 0.58, 95% CI 0.28 to 1.21).
Because of differences in the methods used to measure hospital admissions and the presentation of results, a meta‐analysis was not possible for studies reporting hospital admissions. Despite the absence of a meta‐analysis, we carried out a GRADE assessment using the available narrative summaries (Table 1).
Secondary outcome results
Medication‐related problems (e.g. adverse drug reactions (ADRs), drug‐drug interactions (DDIs))
Medication‐related problems were reported in 10 studies (Auvinen 2021; Blum 2021; Crotty 2004b; Franchi 2016; Hanlon 1996; O'Mahony 2020; Schmader 2004; Syafhan 2021; Taylor 2003; Wehling 2016; 6740 participants) using different terms. A number of studies gave details concerning how medication‐related problems were identified and measured, such as via hospital records (Wehling 2016), patient self‐report during closeout telephone interviews (Hanlon 1996), reviewing the adverse event narrative using Naranjo’s algorithm (Schmader 2004), using software to detect DDIs, such as INTERcheck® (Franchi 2016), the SFINX drug‐drug interaction database (Auvinen 2021), SafeScript (O'Mahony 2020) and a statistical package, developed to examine patient data, which was based on a validated consensus list published in 2021 (Blum 2021).
No consistent intervention effect on medication‐related problems was noted across studies. Five studies reported medication‐related problems as adverse drug events (ADEs) (Blum 2021; Crotty 2004b; Hanlon 1996; Schmader 2004; Wehling 2016), while O'Mahony 2020 measured adverse drug reactions (ADRs). Schmader 2004 showed that the risk of a serious ADE was reduced (RR 0.65, 95% CI 0.45 to 0.93; P value = 0.02) in a geriatric outpatient clinic compared with usual outpatient care; however, little or no difference in the risk of an ADE was noted when all types of ADEs were considered (RR 1.03, 95% CI 0.86 to 1.23; P value = 0.75). Wehling 2016 showed that the total number of adverse drug reactions (ADRs) of specific geriatric relevance (incidence of falls, confusion, nausea, dizziness, obstipation, diarrhoea, dyspnoea, cardiac decompensation, angina pectoris and renal failure) were significantly reduced by implementation of the FORTA‐based intervention (P value < 0.05). Blum 2021, Crotty 2004b, Hanlon 1996 and O'Mahony 2020 showed little or no difference between the proportions of intervention and usual care group participants with ADEs/ADRs at follow‐up. Franchi 2016 also reported no decrease in the prevalence of at least one potential DDI (odds ratio (OR) 0.67, 95% CI 0.34 to 1.28) and potentially severe DDI (OR 0.86, 95% CI 0.63 to 1.15) at discharge. Taylor 2003 reported medication‐related problems as medication misadventures. Proportions of intervention group (2.8%) and control group (3.0%) participants with at least one medication misadventure at 12 months were similar (P value = 0.73).
Auvinen 2021 reported drug‐drug interactions, specifically the number of patients classified as having interactions that can be handled or should be avoided. While the numbers in each class decreased over the six‐month study period, the difference between intervention and control groups was not significant.
Syafhan 2021 reported a significant decrease in the median number of medication‐related problems from baseline to the third assessment point among 118 intervention patients (baseline 360 MRPs, median 3.0 (2 to 4); third assessment 87 MRPs, median 0.5 (0 to 1); P < 0.001).
Adherence to medication
Nine studies reported adherence to medication. Four studies reported little or no differences in adherence scores between intervention and control groups at follow‐up based on the Morisky‐Green test and adapted Morisky Medication Adherence Scale (Campins 2017; Haag 2016; Muth 2016; Muth 2018). Blum 2021 measured drug compliance and found no significant difference between the intervention and control groups at two and 12 months using the MMAS‐8 (Medication Adherence Scale) for this outcome. Syafhan 2021 used the Medication Adherence Report Scale (MARS) but the only results presented were median scores of 24 for both intervention and control groups throughout the study.
One study (69 participants) reported adherence to medication in terms of compliance scores, calculated through assessment of participants' reports of missed doses (Taylor 2003). Those with medication compliance scores of 80% to 100% increased by 15% at 12 months from a mean (± standard deviation (SD)) of 84.9 ± 6.7% to 100% in the intervention group (33 participants), but the control group (36 participants) did not change from 88.9% ± 5.8% at baseline to 88.9% ± 6.3% at 12 months (P value = 0.115).
Coronado‐Vazquez 2019 measured medication adherence as a subgroup of medication appropriateness and focused on adherence to treatment in the intervention and control groups. While medication appropriateness was assessed by a family physician using START, STOPP and Beers criteria, it is unclear how medication adherence was measured. The authors stated that nurses performed a functional, mental and social assessment, "also determining the level of adherence to the treatment". Reported results showed that in patients with "good" medication adherence, medication appropriateness was 62% in the intervention group and 37.9% in the control group (P = 0.005).
Shim 2018 measured medication adherence at baseline and just after the intervention ended at six months using the Malaysian Medication Adherence Scale (MALMAS), a validated instrument for assessing patients’ medication in Malaysia. In the intervention group, 22 patients (30.1%) had a score representing non‐adherence at six months, and 51 (69.9%) were classified as adherent. In the control group, 54 patients (68.4%) had non‐adherence and 25 (31.6%) had adherence (P < 0.001).
Because of differences in methodology in the measurement of adherence and the expression of results, a meta‐analysis was not possible for studies reporting adherence to medication.
Quality of life (QoL) (as assessed by a validated method)
Sixteen studies assessed QoL using six different scales (EQ‐5D, SF‐36, SF‐12, 15D, QUALIDEM and ICECAP‐O) (Basger 2015; Bladh 2011; Blum 2021; Campins 2017; Curtin 2020; Frankenthal 2014; Hanlon 1996; Muth 2016; Muth 2018; Olsson 2012; O'Mahony 2020; Pitkala 2014; Romskaug 2020; Syafhan 2021; Taylor 2003; Thyrian 2017; 7458 participants).
In the Pitkala 2014 study, there was a decline in QoL (using the 15D) in both the intervention and usual care groups, although the decline was significantly lower in the intervention group (‐0.038 in the intervention group versus ‐0.072 in the usual care group).
Blum 2021 reported an improvement in QoL in both groups, but with a significant improvement within the intervention group at 12 months. Curtin 2020 reported a significant decline in QoL in both groups from baseline to three‐month follow‐up, but no significant difference between groups. In the O'Mahony 2020 study, the change in QoL for all patients was statistically significant, however there was no significant difference between the intervention and usual care groups. Romskaug 2020 found a statistically significant difference between groups at the 16‐week follow‐up point, favouring the intervention group.
No changes in QoL were detected in 10 studies (Bladh 2011; Basger 2015; Campins 2017; Frankenthal 2014; Hanlon 1996; Muth 2016; Muth 2018; Olsson 2012; Syafhan 2021; Taylor 2003; Thyrian 2017). While Syafhan 2021 found no difference between groups, the usual care group demonstrated a statistically significant decline in the visual analogue score (VAS) of the EQ‐5D‐5L questionnaire at follow‐up (the VAS is one of two sections of the EQ‐5D‐5L).
Because of differences in methodology in the measurement of quality of life and the expression of results, a meta‐analysis was not possible for studies reporting quality of life, however we carried out a GRADE assessment using the narrative summaries (Table 1).
It was also not possible to perform an assessment of the evidence on the theoretical basis underpinning the interventions as no studies presented clear descriptions of the theories that had informed the intervention development process.
Discussion
Summary of main results
The addition of 10 studies to this updated review, which now includes 38 studies (four from the previous version of the review were excluded because of our decision to confine the updated review to randomised controlled trials in an effort to maximise the quality of the evidence), represents a marked increase in intervention studies that have been conducted since this review was last published, aimed at improving appropriate polypharmacy in older people. However, these additional 10 studies had little impact on the overall findings of the review. Some of the included studies were limited by their small sample sizes and poor certainty of evidence (as assessed using GRADE).
This review examined outcomes of medication appropriateness (as measured by an implicit tool), PIMs (including number of PIMs and the proportion of patients with one or more PIM) and PPOs (including the number of PPOs and the proportion of patients with one or more PPO).
The standardised mean difference (SMD) is used as a summary statistic in meta‐analyses when the studies assess the same continuous outcome but measure it in a variety of ways. For example, in this review, the number of PIMs was measured using different explicit tools. It is therefore necessary to standardise the results of the studies to a uniform scale before they can be combined. The SMD expresses the size of the intervention effect in each study relative to the variability observed in that study. This would also therefore ameliorate any differences between revised versions of the same scale (i.e. Beers criteria: 1997, 2003, 2012 and 2019 versions).
Analysis of medication appropriateness (as measured by an implicit tool) revealed greater heterogeneity among the included studies (I2 = 97%), largely because of the influence of the results of one study (Spinewine 2007). Overall, medication appropriateness (as measured by an implicit tool) in the intervention group postintervention was greater than that in the control group and indicated an improvement in the appropriateness of the medications prescribed (mean difference ‐5.66, 95% CI ‐9.26 to ‐2.06) (Analysis 1.1). A sensitivity analysis in which Crotty 2004a was removed because of a unit of analysis error showed a change in the effect estimate (mean difference ‐5.97, 95% CI ‐10.08 to ‐1.85) (Analysis 1.2). It is uncertain whether pharmaceutical care improves medication appropriateness (as measured by an implicit tool) because the certainty of this evidence is very low.
When the studies measuring PIMs (i.e. based on the number of PIMs and/or the proportion of patients with one or more PIM), as determined using explicit tools (criterion‐based), were combined, for those measuring the number of PIMs (Auvinen 2021; Bladh 2011; Clyne 2015; Coronado‐Vazquez 2019; Garcia‐Gollarte 2014; Koberlein‐Neu 2016; Pitkala 2014; Schmader 2004; Spinewine 2007) and the proportion of patients with one or more PIM (Blum 2021; Boersma 2019; Clyne 2015; Dalleur 2014; Franchi 2016; Frankenthal 2014; Fried 2017; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Milos 2013; Spinewine 2007; Thyrian 2017), differences between intervention and control groups favoured the intervention group (Analysis 1.3; Analysis 1.4). It is uncertain whether pharmaceutical care reduces the number of PIMs or the proportion of patients with one or more PIM because the certainty of this evidence is very low.
When the studies measuring PPOs (i.e. based on the number of PPOs and/or the proportion of patients with one or more PPO), as determined using explicit tools (criterion‐based), were combined (the number of PPOs: Coronado‐Vazquez 2019; Garcia‐Gollarte 2014; Spinewine 2007; the proportion of patients with one or more PPO: Blum 2021; Boersma 2019; Frankenthal 2014; Gallagher 2011; Garcia‐Gollarte 2014; Haag 2016; Spinewine 2007), there was a reduction in the proportion of patients with one or more PPO in the intervention group compared to the control group. The heterogeneity present in the meta‐analysis may have been due to the fact that the studies employed a number of different measurement instruments (Analysis 1.5; Analysis 1.6). Furthermore, while differences between intervention and control groups in the number of PPOs favoured the intervention group when analysed in the previous version of this review, the addition of one study (Coronado‐Vazquez 2019) caused the pooled estimate to cross the line of no effect. It is uncertain whether pharmaceutical care reduces the proportion of patients with one or more PPO because the certainty of this evidence is very low.
The various tools used to assess inappropriate prescribing in the included studies are surrogate markers of appropriate polypharmacy. As was observed in previous versions of this review, few studies examined clinical outcomes, and this should be addressed in future studies. For example, only 14 studies reported on hospital admissions and 16 on quality of life. However, we were unable to combine these results, as the reporting styles were different across studies.
Overall completeness and applicability of evidence
The types of interventions included in the review were limited. Few trials aimed to improve the skills of the prescriber. Most interventions were pharmaceutical care interventions, which included outreach by pharmacists, screening of automated drug alerts by consultant pharmacists visiting nursing homes and clinical pharmacist interventions in various settings. The interventions were complex and most were multi‐faceted with a range of health professionals involved, however it was surprising that, even among many of the 10 new studies added in this update, little detail was provided on intervention development and content.
Several studies employed computer programs designed to detect PIMs, PPOs or drug‐drug interactions as part of their interventions. While results were disappointing, one study conducted follow‐up qualitative interviews towards the end of patient recruitment with researchers and doctors involved in the study, based on the Theoretical Domains Framework, to explore reasons for adherence or lack thereof to the software program's prompts (O'Mahony 2020). At least four main reasons were discovered, including prescriber “alert fatigue” caused by the computer program often producing recommendations of little relevance to patients who were acutely ill, that the busy, high‐pressure acute hospital environment was not conducive to delivering medication advice in terms of timing and location, the differing level of prescribers’ experience and their views of clinical trials, and patient‐specific issues such as medication preferences and clinical status in hospital. The authors commented that consideration of these could be useful in designing future interventions.
A number of studies stated that shared decision‐making between health professionals and patients was part of their intervention. There were varying degrees of patient involvement in these processes. Coronado‐Vazquez 2019 based their shared decision‐making intervention on a model (Elwyn 2012) comprising a series of steps and, in translating this to medication appropriateness, developed a decision support tool.
Other studies commented that when drug regimen changes had been decided upon by the health professional, they were implemented if the patient accepted them (e.g. Auvinen 2021), suggesting that minimal patient input was sought. It was not clear what happened if the patient did not accept the proposed drug changes.
Collaboration between health professionals was a commonly used framework for interventions, including between geriatricians and family doctors (Romskaug 2020), pharmacists and doctors (Shim 2018; Syafhan 2021), and general practitioners, pharmacists and nurses (Strauven 2019).
Most of the new studies added in this update focused on both stopping inappropriate medications and starting appropriate drugs. This represents progress towards a more holistic approach to prescribing (examining over‐ and under‐treatment) compared to the previous version of this review (Rankin 2018a), in which the focus was often on deprescribing, and detecting inappropriate medications and DDIs. The observed heterogeneity noted in the pooled estimates means that the results of the meta‐analyses should be treated cautiously as the interventions did not seem to work consistently across all studies. In addition, study‐specific factors, such as variation in the quality of studies, may have played a role.
Although the effect of interventions on potentially inappropriate prescribing (PIP) was potentially promising and suggested that some of the interventions described in this review may have helped to improve the appropriateness of polypharmacy, despite observed limitations in the available evidence, the clinical impact of these reductions in inappropriate prescribing is not known. This is partly due to the fact that the predictive validity of many assessment tools has not been established (Cahir 2014).
Furthermore, few rigorously conducted studies have tested interventions and examined clinically relevant outcomes such as hospital admissions or ADEs. Fourteen studies in this review reported hospital admissions postintervention (Blum 2021; Campins 2017; Crotty 2004b; Curtin 2020; Franchi 2016; Frankenthal 2014; Gallagher 2011; Haag 2016; Muth 2018; Romskaug 2020; Spinewine 2007; Strauven 2019; Syafhan 2021; Taylor 2003), however we were unable to pool data due to heterogeneity in terms of outcome assessment and reporting across studies. Eight studies reported that the appropriateness of prescribing improved, as was shown by reductions in PIMs, although the association with hospital admissions was inconsistent (Crotty 2004b; Curtin 2020; Gallagher 2011; Romskaug 2020; Spinewine 2007; Strauven 2019; Syafhan 2021; Taylor 2003). Use of different appropriateness scales in the included studies made it difficult to assess the impact of any change of medication appropriateness on hospital admissions. Similarly, some associations between measures of medication appropriateness and medication‐related problems appeared to exist but were difficult to assess because of variation in scales used to measure outcomes and in reporting methods.
While an updated search was conducted in February 2023 to identify the most recent, relevant studies, we were unable to incorporate them into the analyses due to time constraints and lack of capacity to undertake further data extraction. Potentially eligible studies found in this search are listed in Studies awaiting classification. There are 10 studies awaiting classification ranging in sample size from 68 to 5663 patients.
We are not waiting for any specific study to be published. The next update of this review may take place approximately two years following publication of this version, thus the inclusion of further studies may affect the conclusions of this review.
Quality of the evidence
Evidence of potential bias was found in numerous studies. For example, only 18 studies reported adequate concealment of allocation, and only 11 reported appropriate protection from contamination, both of which may have influenced the effect estimate in these studies and therefore the overall pooled estimate.
Although we identified 38 studies, pooled analyses remain limited. For example, the meta‐analysis based on the number of PPOs per participant comprised just three studies. This limits the value of any pooled effect estimate. Furthermore, as shown in the Table 1, the certainty of evidence presented in this review, as described by the GRADE approach, remains low or very low. Despite confining our review to data from randomised trial designs in the meta‐analyses, the certainty of the body of evidence was subsequently downgraded when each of the GRADE considerations was taken into account (i.e. study limitations, consistency of effect, imprecision, indirectness, publication bias). This limits our confidence in the pooled effect estimates.
Considering study limitations, we downgraded studies due to problems with a range of risk of bias domains, in particular allocation concealment and blinding of participants and personnel. When analysing consistency of effect, we determined that heterogeneity between studies was high in all meta‐analyses, with 95% confidence intervals not overlapping, which led to the decision to downgrade the certainty of evidence by one or two levels. There was also heterogeneity in the interventions and considerable statistical heterogeneity in some analyses.
For imprecision, we downgraded the certainty of evidence by one level in four out of the nine meta‐analyses, due to wide confidence intervals. To judge the indirectness of evidence, we considered whether studies incorporated a validated assessment of under‐prescribing and, if not, they were not a direct assessment of appropriate polypharmacy. We downgraded the certainty of evidence in eight of the nine meta‐analyses for this reason.
Based on observed heterogeneity in the pooled effect estimates, the findings of the meta‐analyses (medication appropriateness (as measured by an implicit tool), the number of PIMs and the proportion of patients with one or more PIM or PPO) should be treated cautiously, as the interventions did not seem to work consistently across all studies. Factors contributing to this heterogeneity could have included variation in type, intensity and duration of interventions, as well as differences in the timing of follow‐up assessments. In addition, study‐specific factors such as variation in study quality may have played a role. However, no systematic approach was used to ensure a consistent level of detail in published reports of the interventions. Other information pertinent to intervention success, such as documentation, communication and intervention pharmacists' level of access to clinical records, was not clearly reported in the papers.
Potential biases in the review process
Our review was conducted using standard Cochrane methodology, based on a thorough literature search. Two review authors in our team screened all search results in order to reduce the risk of missing a study for inclusion. To ensure that the inclusion criteria had been consistently applied, the review authors discussed studies for possible inclusion and if any disagreement remained an additional review author's input was sought. Agreement was reached on all studied included and excluded.
We placed no language restrictions on the search strategy, but all of the included trials were published in English and all but one were conducted in high‐income countries (Shim 2018 was conducted in Malaysia, an upper middle‐income country). We only assessed publication bias for outcomes with more than 10 studies, namely the proportion of patients with one or more PIM, and the funnel plot showed no apparent publication bias.
The number of studies in each category was quite small and findings were very difficult to interpret, therefore studies were pooled. We attempted to conduct a comprehensive search for studies, but the fact that 10 studies have not yet been assessed may be a source of potential bias.
Agreements and disagreements with other studies or reviews
A systematic scoping review to examine interventions designed to optimise prescribing and/or adherence in older adults with cancer had similarities to our review, such as different methods being used to assess prescribing‐related outcomes and adherence, and a lack of rigour and detail concerning intervention development (Murphy 2022).
Mekonnen 2021 examined the association between potentially inappropriate prescribing (PIP) and health‐related and system‐related outcomes in hospital inpatient settings. Just one of the 63 included studies was a randomised controlled trial. Rather than analyse the number of PIPs, which comprised PIMs and PPOs, across studies, this systematic review focused on the association of PIPs with various outcomes such as mortality, length of hospital stay, quality of life and falls. It was found that the occurrence of PIPs during hospital stay had significant associations with health and system‐related outcomes, including hospitalisation due to medication (measured by 12 out of 63 studies), ADEs (reported by 23 studies), functional decline (reported by 12 studies), falls (reported by two studies) and healthcare costs (reported by three studies). No significant association was found between PIPs and all‐cause mortality (measured by 19 studies), or hospital readmissions (measured by 18 studies). The authors commented that the most important finding of their review was that outcomes were mostly related to PIMs because none of the included studies examined the effect of PPOs on adverse drug events, functional decline, falls or cost. Another systematic review aimed to evaluate interventions that were designed to improve prescribing among frail older people in secondary or acute care settings (Saeed 2021). Three randomised controlled trials met the inclusion criteria, and the authors commented that one reason for such a low number could have been because they stipulated that eligible studies had to include a diagnosis of frailty using a validated instrument. All three studies are included in our current review (Curtin 2020; Dalleur 2014; Schmader 2004). These latter studies reported significant improvements in prescribing appropriateness following interventions that involved comprehensive geriatric assessment (Schmader 2004) and deprescribing plans (Curtin 2020; Dalleur 2014). However, no significant changes were found relating to clinical outcomes such as hospital admissions, falls, fractures, quality of life and mortality.
Xu 2021 conducted a systematic review to analyse factors affecting potentially inappropriate prescriptions in older adults in primary care, and also barriers to prescribing optimisation. They included 14 qualitative studies, 34 cross‐sectional and two cohort studies. Clinical factors (including medication count and co‐morbidities) and non‐clinical factors (such as age and sex) associated with potentially inappropriate prescriptions were presented.
The review undertaken by Mucherino 2022 focused on the interventions designed to reduce potentially inappropriate prescriptions and the resulting impact on healthcare costs. While only one study of the 18 included in the review was a randomised controlled trial, the considerable and avoidable economic impact of PIMs was presented.
Authors' conclusions
Implications for practice.
The evidence obtained when the results of the studies were combined is rather weak, and it is uncertain whether interventions provided to improve appropriate polypharmacy, such as pharmaceutical care, resulted in clinically significant improvement. Uncertainty surrounds the effects of such interventions on hospital admissions and medication‐related problems, and it could be argued that these are the critical outcomes for patients. However, the pooled effect estimates suggest some improvements in outcomes such as medication appropriateness and the number of potentially inappropriate medicines (PIMs) but due to limitations with the quality of evidence, uncertainty exists. There was a lack of certainty regarding the effects of pharmaceutical care interventions included in this review on inappropriate prescribing (medication appropriateness, the number of PIMs, the proportion of patients with one or more PIM, the number of potential prescribing omissions (PPOs) and the proportion of patients with one or more PPO).
In previous iterations of this review, several studies focused on reducing the number of medications, rather than on improving the overall appropriateness of prescribing, including under‐prescribing, that is, recommending medications that are clinically indicated yet are currently missing. An increasing number of studies meeting the inclusion criteria included a validated assessment of under‐prescribing; four studies in this updated review assessed under‐prescribing, adding to the six studies reported in the previous version. Furthermore, an increasing number of studies meeting the inclusion criteria also included a measure of quality of life, however only three of the 16 studies reported a benefit.
Given the difficulties involved in applying the results of clinical studies to older people, physicians should carefully consider their sources of evidence and recommendations to find the right balance between avoiding the 'risk/treatment paradox' (high‐risk older patients denied safe medications capable of materially improving survival or quality of life) and avoiding inappropriate use of medications for which risks are likely to outweigh benefits (Scott 2010). It must also be noted that the intervention studies included in this review focused on reducing inappropriate prescribing of prescription medications and over‐the‐counter (OTC) medication use was often not assessed, nor was it specifically examined as part of this review. OTC medication use is common among older patients receiving prescription medications with the potential for drug interactions to occur (Agbabiaka 2017). This should not be overlooked by healthcare professionals when reviewing older patients’ medication use.
Based on the findings of our updated review, it is clear that with the inclusion of more studies from 2018 onwards, there has been increasing emphasis on multi‐disciplinary healthcare teams and collaboration between pharmacists, doctors and nurses, as well as the patient being involved to varying degrees in decision‐making.
However, we are still uncertain about which elements of the intervention processes constitute success in improving appropriate polypharmacy. For example, it cannot be stated with certainty whether it is sufficient to provide the intervention during a single episode of care, or on a daily, weekly or monthly basis, how long the intervention should last and the level of input from each health professional. The 10 studies in 'Studies awaiting classification' may alter the conclusions of the review once assessed.
Implications for research.
The aim of many of the intervention studies included in this review was to reduce harm resulting from inappropriate prescribing and to ensure that older people were prescribed appropriate medications that enhance their quality of life.
Overall, the quality of the studies in this review was poor, and further research should attend to rigour in study design. We found that there was a lack of consistency and reporting of the characteristics and implementation of the interventions.
Uncertainty about which elements of the intervention are critical to successful outcomes needs to be addressed. On the basis of the studies included in this review, this poses challenges, as details of intervention development and delivery were lacking. It is widely recognised that better understanding of the characteristics and implementation of complex interventions is needed (Ali 2022). The methods sections of studies provided little detail on how complex interventions were developed, how trials were designed and how staff were trained in delivery of the intervention. Staff training is important to ensure consistency; the receptiveness of prescribers, patients and staff in various settings will have an impact on the uptake and effectiveness of interventions in older people. Other information pertinent to the success of pharmaceutical care interventions including background practice and culture, documentation, communication and sharing of information, and extent of access to clinical records given to intervention pharmacists was not stated clearly in the papers.
Methods of specifying and reporting complex interventions, as well as their implementation strategies, are necessary to strengthen the evidence base required for interventions to be more effective, implementable and replicable across different settings (Michie 2011; Proctor 2013). Future intervention studies targeting appropriate polypharmacy could benefit from guidance provided by the framework of the Medical Research Council (MRC) on the design of complex interventions (MRC 2008; Skivington 2021). This framework recognises the importance of the initial stage of intervention development, in which evidence and theory are used to inform the selection of relevant components before the intervention is piloted, and the feasibility of delivering it in practice is assessed. These initial stages precede formal evaluations seeking to establish the effectiveness of the intervention. One of the newly included studies in this review (Strauven 2019) noted that the authors followed MRC guidelines (Craig 2008).
Another referred to the MRC guidance (Craig 2008) in their published protocol (Romskaug 2017). Romskaug 2017 commented on the “major challenge” of describing a complex intervention with enough detail and accuracy to enable replication. The authors stated in their protocol that their strategy would be to describe in detail the interventions, especially the changes made to patients’ drug regimens, “to compensate for the necessary degree of pragmatism”. Although a description of the intervention appeared in the final trial paper (Romskaug 2020), it was not clear how the MRC guidance had informed the development of the intervention.
Adequate documentation of intervention development and intervention content as well as the training and background of providers that may be critical to intervention effectiveness is essential for facilitating replication of successful interventions in practice. However, no studies included in this review referred to using available intervention tools reporting, such as the TIDieR (Template for Intervention Description and Replication) checklist (Hoffmann 2014).
The framework of the MRC guidance (Craig 2008; Skivington 2021) also outlines the potential application of qualitative methodologies, such as semi‐structured interviews, to involve users and to gain insights into the processes of change that underlie the intervention. For example, establishing the reasons why not all interventions are accepted may be enlightening and may support research into the development of more successful interventions. There appears to be a ceiling effect (approximately 75%), whereby inappropriate prescribing continues despite the evidence base of interventions (Furniss 2000; Zermansky 2006).
A process evaluation, including qualitative interviews of prescribers, may uncover reasons as to why they did not accept interventions (e.g. timing or appropriateness of provision of the intervention, the expertise of providers). Among the 10 newly included studies in this update, only two conducted a process evaluation (O'Mahony 2020; Strauven 2019). O'Mahony 2020 included qualitative interviews based on the Theoretical Domains Framework to try to understand the underlying mechanisms of their intervention, including adherence to software‐guided advice.
Anrys 2016, who published a protocol for one of the newly included studies in this review (Strauven 2019), commented that to enable policymakers to roll out a larger‐scale intervention it is necessary to know what works and why. Referring to MRC guidance on process evaluations (Moore 2015), Strauven 2019 reported that a detailed process evaluation had been completed alongside the intervention. A brief summary of this evaluation was provided in the main trial paper (Strauven 2019), while the detailed process evaluation indicated that GPs who were open to suggestions from other healthcare professionals (HCPs) were seen to facilitate the implementation of the intervention (Anrys 2016).
Additionally, poor prescribing practice must be explored and understood with the goal of learning how to improve it and how to enhance patient safety by reducing the need for intervention. The importance of these investigations extends beyond the research context alone. Given the high financial expenditure that has been attributed to potentially inappropriate prescribing (PIP) in older people (Bradley 2012; Cahir 2010), it is likely that policy‐makers will continue to be interested in the costs of these types of interventions.
It is important that sufficient detail about the context in which complex interventions are conducted, such as those included in this review, is reported and understood, so the transferability of complex interventions can be assessed (Wells 2012). For example, heterogeneity among older people in relation to differing levels of frailty (Spinewine 2007a) means that translational research and retesting of successful interventions may be necessary in dissemination to new populations, as a population of quite healthy 70‐year‐old people may respond differently to an intervention compared with a group of very frail 92‐year‐old individuals. Skivington 2021 emphasised that detailed reporting of context is critical in understanding implementation; an intervention reported as effective in one situation could be harmful in another, different, setting. The authors added that key features of an intervention’s context are physical, spatial, organisational, social, cultural, political or economic, and that the effects of an intervention can vary dependent on these features.
It is worth noting that only two of the included studies followed participants for longer than 12 months (Frankenthal 2014; Strauven 2019). The newly included studies had a follow‐up period of between three months and 15 months. The lack of evidence of effectiveness of pharmaceutical care interventions may be due in part to inadequate length of follow‐up. Future studies should be longer in duration, to address this issue and to evaluate the longer‐term sustainability of pharmaceutical care interventions in improving the appropriate use of polypharmacy for older people. However, funding constraints for such studies may also be a barrier.
Perhaps most critically, the selection of clinical and humanistic outcomes appropriate for older people (e.g. hospital admissions, adverse drug events (ADEs)) will be important to consider in future studies. Strategies for improving the evidence base for older patient care have been reviewed by Scott 2010. Indeed, a key challenge for interventions aimed at improving appropriate polypharmacy for older people is the selection and reporting of consistent outcomes (i.e. patient‐related or medication‐related outcomes). The Core Outcome Measures for Effectiveness Trials (COMET) initiative was launched to develop and apply core outcome sets (COS), which have been proposed as one method of addressing this problem (Williamson 2017). A COS is an agreed and standardised set of outcomes or outcome domains that should be measured and reported, as a minimum, in all trials in a specific clinical area. Alongside the Core Outcome Set‐STAndards for Reporting (COS‐STAR) guidelines (Kirkham 2016), the development of COSs in a specific health area should facilitate more robust synthesis of evidence in the future. A COS for use in interventions to improve the appropriate use of polypharmacy for older people in primary care is now available (Rankin 2018). However, there has been little uptake of this COS to date. Furthermore, the selection of appropriate outcome measure instruments also needs close attention, particularly for use in the older population where changes may be more difficult to detect.
What's new
| Date | Event | Description |
|---|---|---|
| 11 October 2023 | New search has been performed | We added 10 included studies to the review. We made changes to the pooling of outcome data in the meta‐analysis. The search was updated in February 2023 and we added potentially eligible studies to 'Studies awaiting classification'. |
| 11 October 2023 | New citation required and conclusions have changed | Change to methods (to include only randomised controlled trials), results and conclusions. This is the third update of the review. |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 5, 2012
| Date | Event | Description |
|---|---|---|
| 7 February 2018 | New citation required and conclusions have changed | Change to conclusion. Second update of this review. |
| 7 February 2018 | New search has been performed | Updated searches completed. Twenty new included studies added to the review. Changes made to pooling of outcome data in meta‐analysis. |
Acknowledgements
We would like to acknowledge the valuable input of Alexandra McIlroy (Queen's University Belfast) and Paul Miller (EPOC Group Information Specialist) in the development of the search strategy over the various iterations of this review. We would like to thank all members of the EPOC Group formerly at Newcastle University, UK, led by Professor Martin Eccles, for their kind assistance with preparation of the protocol. We would like to thank Dr Marie C Bradley for authorship on previous versions. We would also like to thank Julia Worswick (EPOC Group) for her assistance and the referees and editors for their contribution to the review.
National Institute for Health Research, via Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Editorial and peer reviewer contributions
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Greg Irving
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article): Helen Wakeford, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision): Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods), Steve McDonald, Cochrane Australia (search), Karen Gainey PhD Candidate, The University of Sydney, Australia (consumer), Demetra Antimisiaris, PharmD, BCGP, Assoc. Professor, University of Louisville, Director U of L Frazier Polypharmacy Program (clinical), Jonas W. Wastesson, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden (clinical), and Kate Wang, RMIT University (clinical).
Appendices
Appendix 1. Acronyms
ACOVE: Assessing Care of Vulnerable Elderly
ADE: adverse drug event
AOU: Assessment of Underutilization of Medication
CDS: computerised decision support
DDI: drug‐drug interaction
GP: general practitioner
MAI: Medication Appropriateness Index
MRQoL: medication‐related quality of life
PIM: potentially inappropriate medicine
PIP: potentially inappropriate prescribing
PPO: potential prescribing omission
QoL: quality of life
START: Screening Tool to Alert doctors to the Right Treatment
STOPP: Screening Tool of Older Person’s Prescriptions
Appendix 2. Tools used to define appropriate medications
The tools used by studies in this review include the following:
The Medication Appropriateness Index (MAI): the MAI was designed to assist physicians and pharmacists in assessing the appropriateness of a medication for a given patient. The MAI requires clinicians to rate 10 explicit criteria to determine whether a given medication is appropriate for an individual. For each criterion, the index has operational definitions, explicit instructions and examples, and the evaluator rates whether the particular medication is "appropriate," "marginally appropriate" or "inappropriate" (Table 3).
1. Medication Appropriateness Index.
| To assess the appropriateness of the drug, please answer the following questions and circle the applicable score. | |||||
| 1. Is there an indication for the drug? Comments: |
1 | 2 | 3 | 9 DK | |
| Indicated | Not indicated | ||||
| 2. Is the medication effective for the condition? Comments: |
1 | 2 | 3 | 9 DK |
|
| Effective | Ineffective | ||||
| 3. Is the dosage correct? Comments: |
1 | 2 | 3 | 9 DK |
|
| Correct | Incorrect | ||||
| 4. Are the directions correct? Comments: |
1 | 2 | 3 | 9 DK |
|
| Correct | Incorrect | ||||
| 5. Are the directions practical? Comments: |
1 | 2 | 3 | 9 DK |
|
| Practical | Impractical | ||||
| 6. Are there clinically significant drug‐drug interactions? Comments: |
1 | 2 | 3 | 9 DK |
|
| Insignificant | Significant | ||||
| 7. Are there clinically significant drug‐disease/condition interactions? Comments: |
1 | 2 | 3 | 9 DK |
|
| Insignificant | Significant | ||||
| 8. Is there unnecessary duplication with other drug(s)? Comments: |
1 | 2 | 3 | 9 DK |
|
| Necessary | Unnecessary | ||||
| 9. Is the duration of therapy acceptable? Comments: | 1 | 2 | 3 | 9 DK |
|
| Acceptable | Unacceptable | ||||
| 10. Is this drug the least expensive alternative compared with others of equal utility? Comments: |
1 | 2 | 3 | 9 DK |
|
| Least expensive | Most expensive | ||||
DK: don't know
The Beers criteria: these are consensus explicit criteria used to enhance safe medication use in older adults when precise clinical information is lacking (see Table 4; Table 5; Table 6; Table 7; Table 8). The Beers criteria are based on expert consensus developed through an extensive literature review with a bibliography and a questionnaire evaluated by nationally recognised experts in geriatric care, clinical pharmacology and psychopharmacology using a modified Delphi technique to reach consensus. The most recent version of Beers criteria (AGS 2012) comprises three lists. The first list comprises 34 individual medications or classes of medications that should be avoided in older adults and their concerns (Table 6). The second list includes diseases or conditions and drugs that should be avoided in older adults with these conditions (Table 7). The third list provides medications to be used with caution in older adults (Table 8). The statements in each list are rated on the basis of quality of evidence and the strength of recommendations using the American College of Physicians' Guideline Grading System.
2. Updated Beers (2003) criteria for potentially inappropriate medication use in older adults: independent of diagnosis or condition.
| Drug | Concern |
Severity rating (high or low) |
| Propoxyphene (Darvon) and combination products (Darvon with ASA, Darvon‐N and Darvocet‐N) |
Offers few analgesic advantages over paracetamol (acetaminophen), yet is associated with the adverse effects of other narcotic drugs | Low |
| Indomethacin (Indocin and Indocin SR) | Of all available NSAIDs, this drug produces the most CNS adverse effects | High |
| Pentazocine (Talwin) | Narcotic analgesic that causes more CNS adverse effects, including confusion and hallucinations, more commonly than other narcotic drugs. Additionally, it is a mixed agonist and antagonist | High |
| Trimethobenzamide (Tigan) | One of the least effective antiemetic drugs, yet it can cause extrapyramidal adverse effects | High |
| Muscle relaxants and antispasmodics: methocarbamol (Robaxin), carisoprodol (Soma), chlorzoxazone (Paraflex), metaxalone (Skelaxin), cyclobenzaprine (Flexeril) and oxybutynin (Ditropan). Do not consider the extended‐release formulation of Ditropan XL | Most muscle relaxants and antispasmodic drugs are poorly tolerated by elderly patients because they cause anticholinergic adverse effects, sedation and weakness. Additionally, their effectiveness at doses tolerated by elderly patients is questionable | High |
| Flurazepam (Dalmane) | This benzodiazepine hypnotic has an extremely long half‐life in elderly patients (often days), producing prolonged sedation and increasing the incidence of falls and fracture. Medium‐ or short‐acting benzodiazepines are preferable | High |
| Amitriptyline (Elavil), chlordiazepoxide‐amitriptyline (Limbitrol) and perphenazine‐amitriptyline (Triavil) | Because of its strong anticholinergic and sedation properties, amitriptyline is rarely the antidepressant of choice for elderly patients | High |
| Doxepin (Sinequan) | Because of its strong anticholinergic and sedating properties, doxepin is rarely the antidepressant of choice for elderly patients | High |
| Meprobamate (Miltown and Equanil) | This is a highly addictive and sedating anxiolytic. Those using meprobamate for prolonged periods may become addicted and may need to be withdrawn slowly |
High |
| Doses of short‐acting benzodiazepines: doses greater than lorazepam (Ativan) 3 mg; oxazepam (Serax) 60 mg; iprazolam (Xanax) 2 mg; temazepam (Restoril) 15 mg and triazolam (Halcion) 0.25 mg | Because of increased sensitivity to benzodiazepines in elderly patients, smaller doses may be effective and safer. Total daily doses should rarely exceed the suggested maximum | High |
| Long‐acting benzodiazepines: chlordiazepoxide (Librium), chlordiazepoxide‐amitriptyline (Limbitrol), clidinium‐chlordiazepoxide (Librax), diazepam (Valium), quazepam (Doral), halazepam (Paxipam) and chlorazepate (Tranxene) | These drugs have a long half‐life in elderly patients (often several days), producing prolonged sedation and increasing the risk of falls and fractures. Short‐ and intermediate‐acting benzodiazepines are preferred if a benzodiazepine is required | High |
| Disopyramide (Norpace and Norpace CR) | Of all antiarrhythmic drugs, this is the most potent negative inotrope and therefore may induce heart failure in elderly patients. It also has strong anticholinergic effects. Other antiarrhythmic drugs should be used as well | High |
| Digoxin (Lanoxin) (should not exceed 0.125 mg/d except when treating atrial arrhythmias) | Decreased renal clearance may lead to increased risk of toxic effects | Low |
| Short‐acting dipyridamole (Persantine). Do not consider the long‐acting dipyridamole (which has better properties than the short‐acting formulation in older adults) except with patients with artificial heart valves | May cause orthostatic hypotension | Low |
| Methyldopa (Aldomet) and methyldopa‐hydrochlorothiazide (Aldoril) | May cause bradycardia and exacerbate depression in elderly patients | High |
| Reserpine at doses > 0.25 mg | May induce depression, impotence, sedation and orthostatic hypotension | Low |
| Chlorpropamide (Diabinese) | It has a prolonged half‐life in elderly patients and could cause prolonged hypoglycaemia. Additionally, it is the only oral hypoglycaemic agent that causes SIADH | High |
| GI antispasmodic drugs: dicyclomine (Bentyl), hyoscyamine (Levsin and Levsinex), propantheline (Pro‐Banthine), belladonna alkaloids (Donnatal and others) and clidinium‐chlordiazepoxide (Librax) | GI antispasmodic drugs have potent anticholinergic effects and have uncertain effectiveness. These drugs should be avoided (especially for long‐term use) | High |
| Anticholinergics and antihistamines: chlorpheniramine (Chlor‐Trimeton), diphenhydramine (Benadryl), hydroxyzine (Vistaril and Atarax), cyproheptadine (Periactin), promethazine (Phenergan), tripelennamine, dexchlorpheniramine (Polaramine) | All non‐prescription and many prescription antihistamines may have potent anticholinergic properties. Non‐anticholinergic antihistamines are preferred in elderly patients for the treatment of allergic reactions | High |
| Diphenhydramine (Benadryl) | May cause confusion and sedation. Should not be used as a hypnotic, and when used to treat emergency allergic reactions, it should be used in the smallest possible dose | High |
| Ergot mesyloids (Hydergine) and cyclandelate (Cyclospasmol) | Have not been shown to be effective in the doses studied | Low |
| Ferrous sulphate > 325 mg/d | Doses > 325 mg/d do not dramatically increase the amount absorbed but greatly increase the incidence of constipation | Low |
| All barbiturates (except phenobarbital) except when used to control seizures | Are highly addictive and cause more adverse effects than most sedative or hypnotic drugs in elderly patients | High |
| Meperidine (Demerol) | Not an effective oral analgesic in doses commonly used. May cause confusion and has many disadvantages compared with other narcotic drugs | High |
| Ticlopidine (Ticlid) | Has been shown to be no better than aspirin in preventing clotting and may be considerably more toxic. Safer, more effective alternatives exist | High |
| Ketorolac (Toradol) | Immediate and long‐term use should be avoided in older people, as a significant number have asymptomatic GI pathological conditions | High |
| Amphetamines and anorexic agents | These drugs have the potential to cause dependence, hypertension, angina and myocardial infarction | High |
| Long‐term use of full‐dosage, longer half‐life, non–COX‐selective NSAIDs: naproxen (Naprosyn, Avaprox and Aleve), oxaprozin (Daypro) and piroxicam (Feldene) | Have the potential to produce GI bleeding, renal failure, hypertension and heart failure | High |
| Daily fluoxetine (Prozac) | Long half‐life of drug and risk of producing excessive CNS stimulation, sleep disturbances and increasing agitation. Safer alternatives are available | High |
| Long‐term use of stimulant laxatives: bisacodyl (Dulcolax), cascara sagrada and Neoloid except in the presence of opiate analgesic use | May exacerbate bowel dysfunction | High |
| Amiodarone (Cordarone) | Associated with QT interval problems and risk of provoking torsades de pointes. Lack of efficacy in older adults | High |
| Orphenadrine (Norflex) | Causes greater sedation and anticholinergic adverse effects than safer alternatives | High |
| Guanethidine (Ismelin) | May cause orthostatic hypotension. Safer alternatives are available | High |
| Guanadrel (Hylorel) | May cause orthostatic hypotension | High |
| Cyclandelate (Cyclospasmol) | Lack of efficacy | Low |
| Isoxsurpine (Vasodilan) | Lack of efficacy | Low |
| Nitrofurantoin (Macrodantin) | Potential for renal impairment. Safer alternatives are available | High |
| Doxazosin (Cardura) | Potential for hypotension, dry mouth and urinary problems | Low |
| Methyltestosterone (Android, Virilon and Testrad) | Potential for prostatic hyperplasia and cardiac problems | High |
| Thioridazine (Mellaril) | Greater potential for CNS and extrapyramidal adverse effects | High |
| Mesoridazine (Serentil) | CNS and extrapyramidal adverse effects | High |
| Short‐acting nifedipine (Procardia and Adalat) | Potential for hypotension and constipation | High |
| Clonidine (Catapres) | Potential for orthostatic hypotension and CNS adverse effects | Low |
| Mineral oil | Potential for aspiration and adverse effects. Safer alternatives are available | High |
| Cimetidine (Tagamet) | CNS adverse effects including confusion | Low |
| Ethacrynic acid (Edecrin) | Potential for hypertension and fluid imbalances. Safer alternatives are available | Low |
| Desiccated thyroid | Concerns about cardiac effects. Safer alternatives are available | High |
| Amphetamines (excluding methylphenidate hydrochloride and anorexic agents) | CNS stimulant adverse effects | High |
| Oestrogens only (oral) | Evidence of the carcinogenic (breast and endometrial cancer) potential of these agents and lack of cardioprotective effects in older women | Low |
Source: Fick 2003. CNS: central nervous system; COX: cyclo‐oxygenase; CR: controlled release; GI: gastrointestinal; NSAID: non‐steroidal anti‐inflammatory drug; SIADH: syndrome of inappropriate antidiuretic hormone hypersecretion; SR: slow release.
3. Updated Beers (2003) criteria for potentially inappropriate medication use in older adults: considering diagnoses or conditions.
| Disease or condition | Drug | Concern |
Severity rating (high or low) |
| Heart failure | Disopyramide (Norpace) and high‐sodium‐content drugs (sodium and sodium salts (alginate bicarbonate, biphosphate, citrate, phosphate, salicylate and sulphate)) | Negative inotropic effect. Potential to promote fluid retention and exacerbation of heart failure | High |
| Hypertension | Phenylpropanolamine hydrochloride (removed from the market in 2001), pseudoephedrine; diet pills and amphetamines | May produce elevation of blood pressure secondary to sympathomimetic activity | High |
| Gastric or duodenal ulcers |
NSAIDs and aspirin (> 325 mg) (COXIBs excluded) | May exacerbate existing ulcers or produce new/additional ulcers | High |
| Seizures or epilepsy | Clozapine (Clozaril), chlorpromazine (Thorazine), thioridazine (Mellaril) and thiothixene (Navane) | May lower seizure thresholds | High |
| Blood clotting disorders or receiving anticoagulant therapy | Aspirin, NSAIDs, dipyridamole (Persantin), ticlopidine (Ticlid) and clopidogrel (Plavix) | May prolong clotting time and elevate INR values or inhibit platelet aggregation, resulting in increased potential for bleeding | High |
| Bladder outflow obstruction |
Anticholinergics and antihistamines, gastrointestinal antispasmodics, muscle relaxants, oxybutynin (Ditropan), flavoxate (Urispas), anticholinergics, antidepressants, decongestants and tolterodine (Detrol) | May decrease urinary flow, leading to urinary retention |
High |
| Stress incontinence | α‐Blockers (doxazosin, prazosin and terazosin), anticholinergics, tricyclic antidepressants (imipramine hydrochloride, doxepin hydrochloride and amitriptyline hydrochloride) and long‐acting benzodiazepines | May produce polyuria and worsening of incontinence | High |
| Arrhythmias | Tricyclic antidepressants (imipramine hydrochloride, doxepin hydrochloride and amitriptyline hydrochloride) | Concern due to proarrhythmic effects and ability to produce QT interval changes | High |
| Insomnia | Decongestants, theophylline (Theodur), methylphenidate (Ritalin), MAOIs and amphetamines | Concern due to CNS stimulant effects | High |
| Parkinson's disease | Metoclopramide (Reglan), conventional antipsychotics and tacrine (Cognex) | Concern due to their antidopaminergic/cholinergic effects | High |
| Cognitive impairment | Barbiturates, anticholinergics, antispasmodics and muscle relaxants. CNS stimulants: dextroamphetamine (Adderall), methylphenidate (Ritalin), methamphetamine (Desoxyn) and pemolin | Concern due to CNS‐altering effects | High |
| Depression | Long‐term benzodiazepine use. Sympatholytic agents: methyldopa (Aldomet), reserpine and guanethidine (Ismelin) | May produce or exacerbate depression | High |
| Anorexia and malnutrition |
CNS stimulants: dextroamphetamine (Adderall), methylphenidate (Ritalin), methamphetamine (Desoxyn), pemolin and fluoxetine (Prozac) | Concern due to appetite‐suppressing effects | High |
| Syncope or falls | Short‐ to intermediate‐acting benzodiazepine and tricyclic antidepressants (imipramine hydrochloride, doxepin hydrochloride and amitriptyline hydrochloride) | May produce ataxia, impaired psychomotor function, syncope and additional falls | High |
| SIADH/hyponatraemia | SSRIs: fluoxetine (Prozac), citalopram (Celexa), fluvoxamine (Luvox), paroxetine (Paxil) and sertraline (Zoloft) | May exacerbate or cause SIADH | Low |
| Seizure disorder | Bupropion (Wellbutrin) | May lower seizure threshold | High |
| Obesity | Olanzapine (Zyprexa) | May stimulate appetite and increase weight gain | Low |
| COPD | Long‐acting benzodiazepines: chlordiazepoxide (Librium), chlordiazepoxide‐amitriptyline (Limbitrol), clidinium‐chlordiazepoxide (Librax), diazepam (Valium), quazepam (Doral), halazepam (Paxipam) and chlorazepate (Tranxene). β‐Blockers: propranolol | CNS adverse effects. May induce respiratory depression. May exacerbate or cause respiratory depression | High |
| Chronic constipation | Calcium channel blockers, anticholinergics and tricyclic antidepressants (imipramine hydrochloride, doxepin hydrochloride and amitriptyline hydrochloride) | May exacerbate constipation | Low |
Source: Fick 2003. COPD: chronic obstructive pulmonary disease; COXIB: cyclo‐oxygenase inhibitor; INR: international normalised ratio; MAOI: monoamine oxidase inhibitor; NSAID: non‐steroidal anti‐inflammatory drug; SIADH: syndrome of inappropriate antidiuretic hormone secretion; SSRIs: selective serotonin reuptake inhibitors.
4. Updated Beers (2012) criteria for potentially inappropriate medication usage in older adults: independent of diagnosis or condition.
| Organ System or Therapeutic Category or Drug | Rationale | Recommendation | Quality of Evidence | Strength of Recommendation |
| Anticholinergics (excludes TCAs) | ||||
| First‐generation antihistamines (as single agent or as part of combination products) Brompheniramine Carbinoxamine Chlorpheniramine Clemastine Cyproheptadine Dexbrompheniramine Dexchlorpheniramine Diphenhydramine (oral) Doxylamine Hydroxyzine Promethazine Triprolidine |
Highly anticholinergic; clearance reduced with advanced age, and tolerance develops when used as hypnotic; greater risk of confusion, dry mouth, constipation and other anticholinergic effects and toxicity Use of diphenhydramine in special situations such as short‐term treatment of severe allergic reaction may be appropriate |
Avoid | Hydroxyzine and promethazine: high; all others: moderate | Strong |
| Antiparkinson agents Benztropine (oral) Trihexyphenidyl |
Not recommended for prevention of extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson's disease | Avoid | Moderate | Strong |
| Antispasmodics Belladonna alkaloids Clidinium‐chlordiazepoxide Dicyclomine Hyoscyamine Propantheline Scopolamine |
Highly anticholinergic, uncertain effectiveness | Avoid except in short‐term palliative care to decrease oral secretions | Moderate | Strong |
| Antithrombotics | ||||
| Dipyridamole, oral short‐acting* (does not apply to extended‐release combination with aspirin) | May cause orthostatic hypotension; more effective alternatives available; intravenous form acceptable for use in cardiac stress testing | Avoid | Moderate | Strong |
| Ticlopidine* | Safer effective alternatives available | Avoid | Moderate | Strong |
| Anti‐infective | ||||
| Nitrofurantoin | Potential for pulmonary toxicity; safer alternatives available; lack of efficacy in patients with CrCl < 60 mL/min due to inadequate drug concentration in the urine | Avoid for long‐term suppression; avoid in patients with CrCl < 60 mL/min | Moderate | Strong |
| Cardiovascular | ||||
| Alpha1‐blockers Doxazosin Prazosin Terazosin |
High risk of orthostatic hypotension; not recommended as routine treatment for hypertension; alternative agents have superior risk/benefit profile | Avoid use as an antihypertensive | Moderate | Strong |
| Alpha‐agonists, central Clonidine Guanabenz* Guanfacine* Methyldopa* Reserpine (> 0.1 mg/d)* |
High risk of adverse CNS effects; may cause bradycardia and orthostatic hypotension; not recommended as routine treatment for hypertension | Avoid clonidine as a first‐line antihypertensive Avoid others as listed |
Low | Strong |
| Antiarrhythmic drugs (Class Ia, Ic, III) Amiodarone Dofetilide Dronedarone Flecainide Ibutilide Procainamide Propafenone Quinidine Sotalol |
Data suggest that rate control yields better balance of benefits and harms than rhythm control for most older adults Amiodarone is associated with multiple toxicities, including thyroid disease, pulmonary disorders and QT interval prolongation |
Avoid antiarrhythmic drugs as first‐line treatment of atrial fibrillation | High | Strong |
| Disopyramide* | Disopyramide is a potent negative inotrope and therefore may induce heart failure in older adults; strongly anticholinergic; other antiarrhythmic drugs preferred | Avoid | Low | Strong |
| Dronedarone | Worse outcomes have been reported in patients taking dronedarone who have permanent atrial fibrillation or heart failure. In general, rate control is preferred over rhythm control for atrial fibrillation | Avoid in patients with permanent atrial fibrillation or heart failure | Moderate | Strong |
| Digoxin > 0.125 mg/d | In heart failure, higher dosages are associated with no additional benefit and may increase risk of toxicity; slow renal clearance may lead to risk of toxic effects | Avoid | Moderate | Strong |
| Nifedipine, immediate release* | Potential for hypotension; risk of precipitating myocardial ischaemia | Avoid | High | Strong |
| Spironolactone > 25 mg/d | In heart failure, the risk of hyperkalaemia is higher in older adults, especially if taking > 25 mg/d or taking concomitant NSAID, angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker or potassium supplement | Avoid in patients with heart failure or with a CrCl < 30 mL/min | Moderate | Strong |
| Central nervous system | ||||
| Tertiary TCAs, alone or in combination: Amitriptyline Chlordiazepoxide‐amitriptyline Clomipramine Doxepin > 6 mg/d Imipramine Perphenazine‐amitriptyline Trimipramine |
Highly anticholinergic, sedating and causing orthostatic hypotension; safety profile of low‐dose doxepin (≤ 6 mg/d) is comparable with that of placebo | Avoid | High | Strong |
| Antipsychotics, first (conventional) and second (atypical) generation (see AGS 2012 for full list) | Increased risk of cerebrovascular accident (stroke) and mortality in persons with dementia | Avoid use for behavioural problems of dementia unless non‐pharmacological options have failed and patient is threat to self or others | Moderate | Strong |
| Thioridazine Mesoridazine |
Highly anticholinergic and risk of QT interval prolongation | Avoid | Moderate | Strong |
| Barbiturates Amobarbital* Butabarbital* Butalbital Mephobarbital* Pentobarbital* Phenobarbital Secobarbital* |
High rate of physical dependence; tolerance to sleep benefits; risk of overdose at low dosages | Avoid | High | Strong |
| Benzodiazepines Short‐ and intermediate‐acting: Alprazolam Estazolam Lorazepam Oxazepam Temazepam Triazolam Long‐acting: Clorazepate Chlordiazepoxide Chlordiazepoxide‐amitriptyline Clidinium‐chlordiazepoxide Clonazepam Diazepam Flurazepam Quazepam |
Older adults have increased sensitivity to benzodiazepines and slower metabolism of long‐acting agents. In general, all benzodiazepines increase risk of cognitive impairment, delirium, falls, fractures and motor vehicle accidents in older adults May be appropriate for seizure disorders, rapid eye movement sleep disorders, benzodiazepine withdrawal, ethanol withdrawal, severe generalised anxiety disorder, periprocedural anaesthesia and end‐of‐life care |
Avoid benzodiazepines (any type) for treatment of insomnia, agitation or delirium | High | Strong |
| Chloral hydrate* | Tolerance occurs within 10 days, and risks outweigh benefits in light of overdose with doses only 3 times the recommended dose | Avoid | Low | Strong |
| Meprobamate | High rate of physical dependence; very sedating | Avoid | Moderate | Strong |
| Non‐benzodiazepine hypnotics Eszopiclone Zolpidem Zaleplon |
Benzodiazepine‐receptor agonists that have adverse events similar to those of benzodiazepines in older adults (e.g. delirium, falls, fractures); minimal improvement in sleep latency and duration | Avoid long‐term use (> 90 days) | Moderate | Strong |
| Ergot mesylates* Isoxsuprine* |
Lack of efficacy | Avoid | High | Strong |
| Endocrine | ||||
| Androgens Methyltestosterone* Testosterone |
Potential for cardiac problems and contraindicated in men with prostate cancer | Avoid unless indicated for moderate to severe hypogonadism | Moderate | Weak |
| Desiccated thyroid | Concerns about cardiac effects; safer alternatives available | Avoid | Low | Strong |
| Oestrogens with or without progestins | Evidence of carcinogenic potential (breast and endometrium); lack of cardioprotective effect and cognitive protection in older women Evidence that vaginal oestrogens for treatment of vaginal dryness are safe and effective in women with breast cancer, especially at dosages of estradiol < 25 μg twice weekly |
Avoid oral and topical patch Topical vaginal cream: acceptable to use low‐dose intravaginal oestrogen for the management of dyspareunia, lower urinary tract infection and other vaginal symptoms |
Oral and patch: high Topical: moderate |
Oral and patch: strong Topical: weak |
| Growth hormone | Effect on body composition is small and is associated with oedema, arthralgia, carpal tunnel syndrome, gynaecomastia, impaired fasting glucose | Avoid, except as hormone replacement after pituitary gland removal | High | Strong |
| Insulin, sliding scale | Higher risk of hypoglycaemia without improvement in hyperglycaemia management regardless of care setting | Avoid | Moderate | Strong |
| Megestrol | Minimal effect on weight; increases risk of thrombotic events and possibly death in older adults | Avoid | Moderate | Strong |
| Sulphonylureas, long duration Chlorpropamide Glyburide |
Chlorpropamide: prolonged half‐life in older adults; can cause prolonged hypoglycaemia; causes syndrome of inappropriate antidiuretic hormone secretion. Glyburide: greater risk of severe prolonged hypoglycaemia in older adults |
Avoid | High | Strong |
| Gastrointestinal | ||||
| Metoclopramide | Can cause extrapyramidal effects including tardive dyskinesia; risk may be even greater in frail older adults | Avoid, unless for gastroparesis | Moderate | Strong |
| Mineral oil, oral | Potential for aspiration and adverse effects; safer alternatives available | Avoid | Moderate | Strong |
| Trimethobenzamide | One of the least effective antiemetic drugs; can cause extrapyramidal adverse effects | Avoid | Moderate | Strong |
| Pain | ||||
| Meperidine | Not an effective oral analgesic in dosages commonly used; may cause neurotoxicity; safer alternatives available | Avoid | High | Strong |
| Non–COX‐selective NSAIDs, oral Aspirin > 325 mg/d Diclofenac Diflunisal Etodolac Fenoprofen Ibuprofen Ketoprofen Meclofenamate Mefenamic acid Meloxicam Nabumetone Naproxen Oxaprozin Piroxicam Sulindac Tolmetin |
Increase risk of GI bleeding and peptic ulcer disease in high‐risk groups, including those aged > 75 or taking oral or parenteral corticosteroids, anticoagulants or antiplatelet agents. Use of proton pump inhibitor or misoprostol reduces but does not eliminate risk. Upper GI ulcers, gross bleeding or perforation caused by NSAIDs occurs in approximately 1% of patients treated for 3 to 6 months and in approximately 2% to 4% of patients treated for 1 year. These trends continue with longer duration of use | Avoid long‐term use unless other alternatives are not effective and patient can take gastroprotective agent (proton pump inhibitor or misoprostol) | Moderate | Strong |
| Indomethacin Ketorolac, includes parenteral |
Increase risk of GI bleeding and peptic ulcer disease in high‐risk groups (see above Non–COX‐selective NSAIDs) Of all the NSAIDs, indomethacin has the most adverse effects |
Avoid | Indomethacin: moderate Ketorolac: high |
Strong |
| Pentazocine* | Opioid analgesic that causes CNS adverse effects, including confusion and hallucinations, more commonly than other narcotic drugs; also a mixed agonist and antagonist; safer alternatives available | Avoid | Low | Strong |
| Skeletal muscle relaxants Carisoprodol Chlorzoxazone Cyclobenzaprine Metaxalone Methocarbamol Orphenadrine |
Most muscle relaxants are poorly tolerated by older adults because of anticholinergic adverse effects, sedation, risk of fracture; effectiveness at dosages tolerated by older adults is questionable | Avoid | Moderate | Strong |
| Source: AGS 2012.
CNS: central nervous system; COX: cyclo‐oxygenase; CrCl: creatinine clearance; GI: gastrointestinal; NSAID: non‐steroidal anti‐inflammatory drug; TCA: tricyclic antidepressant. *Infrequently used drugs. | ||||
5. Updated Beers (2012) criteria for potentially inappropriate medication usage in older adults due to drug–disease or drug–syndrome interactions that may exacerbate the disease or syndrome.
| Disease or syndrome | Drug | Rationale | Recommendation | Quality of evidence | Strength of recommendation |
| Cardiovascular | |||||
| Heart failure | NSAIDs and COX‐2 inhibitors Non‐dihydropyridine CCBs (avoid only for systolic heart failure) Diltiazem Verapamil Pioglitazone, rosiglitazone Cilostazol Dronedarone |
Potential to promote fluid retention and exacerbate heart failure | Avoid | NSAIDs: moderate CCBs: moderate Thiazolidinediones (glitazones): high Cilostazol: low Dronedarone: moderate |
Strong |
| Syncope | AChEIs Peripheral alpha‐blockers Doxazosin Prazosin Terazosin Tertiary TCAs Chlorpromazine, thioridazine and olanzapine |
Increase risk of orthostatic hypotension or bradycardia | Avoid | Alpha‐blockers: high TCAs, AChEIs and antipsychotics: moderate |
AChEIs and TCAs: strong Alpha‐blockers and antipsychotics: weak |
| Central nervous system | |||||
| Chronic seizures or epilepsy | Bupropion Chlorpromazine Clozapine Maprotiline Olanzapine Thioridazine Thiothixene Tramadol |
Lower seizure threshold; may be acceptable in patients with well‐controlled seizures in whom alternative agents have not been effective | Avoid | Moderate | Strong |
| Delirium | All TCAs Anticholinergics (see AGS 2012 for full list) Benzodiazepines Chlorpromazine Corticosteroids H2‐receptor antagonist Meperidine Sedative‐hypnotics Thioridazine |
Avoid in older adults with or at high risk of delirium because of inducing or worsening delirium in older adults; if discontinued drugs used long‐term, taper to avoid withdrawal symptoms | Avoid | Moderate | Strong |
| Dementia and cognitive impairment | Anticholinergics (see AGS 2012 for full list) Benzodiazepines H2‐receptor antagonists Zolpidem Antipsychotics, long‐term and as‐needed use |
Avoid because of adverse CNS effects Avoid antipsychotics for behavioural problems of dementia unless non‐pharmacological options have failed and patient is a threat to himself or others. Antipsychotics are associated with increased risk of cerebrovascular accident (stroke) and mortality in persons with dementia |
Avoid | High | Strong |
| History of falls or fractures | Anticonvulsants Antipsychotics Benzodiazepines Non‐benzodiazepine hypnotics Eszopiclone Zaleplon Zolpidem TCAs and selective serotonin reuptake inhibitors |
Ability to produce ataxia, impaired psychomotor function, syncope and additional falls; shorter‐acting benzodiazepines are not safer than long‐acting ones | Avoid unless safer alternatives are not available; avoid anticonvulsants except for seizure disorders | High | Strong |
| Insomnia | Oral decongestants Pseudoephedrine Phenylephrine Stimulants Amphetamine Methylphenidate Pemoline Theobromines Theophylline Caffeine |
CNS stimulant effects | Avoid | Moderate | Strong |
| Parkinson's disease | All antipsychotics (see AGS 2012 for full list, except for quetiapine and clozapine) Antiemetics Metoclopramide Prochlorperazine Promethazine |
Dopamine receptor antagonists with potential to worsen parkinsonian symptoms Quetiapine and clozapine appear to be less likely to precipitate worsening of Parkinson's disease |
Avoid | Moderate | Strong |
| Gastrointestinal | |||||
| Chronic constipation | Oral antimuscarinics for urinary incontinence Darifenacin Fesoterodine Oxybutynin (oral) Solifenacin Tolterodine Trospium Non‐dihydropyridine CCB Diltiazem Verapamil First‐generation antihistamines as single agent or part of combination products Brompheniramine (various) Carbinoxamine Chlorpheniramine Clemastine (various) Cyproheptadine Dexbrompheniramine Dexchlorpheniramine (various) Diphenhydramine Doxylamine Hydroxyzine Promethazine Triprolidine Anticholinergics and antispasmodics (see AGS 2012 for full list of drugs with strong anticholinergic properties) Antipsychotics Belladonna alkaloids Clidinium‐chlordiazepoxide Dicyclomine Hyoscyamine Propantheline Scopolamine Tertiary TCAs (amitriptyline, clomipramine, doxepin, imipramine and trimipramine) |
Can worsen constipation; agents for urinary incontinence: Antimuscarinics overall differ in incidence of constipation; response variable; consider alternative agent if constipation develops | Avoid unless no other alternatives | For urinary incontinence: high All others: moderate to low |
Weak |
| History of gastric or duodenal ulcers | Aspirin (> 325 mg/d) Non–COX‐2–selective NSAIDs |
May exacerbate existing ulcers or cause new or additional ulcers | Avoid unless other alternatives are not effective and patient can take gastroprotective agent (proton pump inhibitor or misoprostol) | Moderate | Strong |
| Kidney and urinary tract | |||||
| Chronic kidney disease Stages IV and V | NSAIDs Triamterene (alone or in combination) |
May increase risk of kidney injury | Avoid | NSAIDs: moderate Triamterene: low |
NSAIDs: strong Triamterene: weak |
| Urinary incontinence (all types) in women | Oestrogen oral and transdermal (excludes intravaginal oestrogen) | Aggravate incontinence | Avoid in women | High | Strong |
| Lower urinary tract symptoms, benign prostatic hyperplasia | Inhaled anticholinergic agents Strongly anticholinergic drugs, except antimuscarinics for urinary incontinence (see AGS 2012 for complete list) |
May decrease urinary flow and cause urinary retention | Avoid in men | Moderate | Inhaled agents: strong All others: weak |
| Stress or mixed urinary incontinence | Alpha‐blockers Doxazosin Prazosin Terazosin |
Aggravate incontinence | Avoid in women | Moderate | Strong |
| Source: AGS 2012. CCB: calcium channel blocker; AChEI: acetylcholinesterase inhibitor; CNS: central nervous system; COX: cyclo‐oxygenase; NSAID: non‐steroidal anti‐inflammatory drug; TCA: tricyclic antidepressant. | |||||
6. Updated Beers (2012) criteria for potentially inappropriate medications to be used with caution in older adults.
| Drug | Rationale | Recommendation | Quality of evidence | Strength of recommendation |
| Aspirin for primary prevention of cardiac events | Lack of evidence of benefit versus risk in individuals aged ≥ 80 | Use with caution in adults aged ≥ 80 | Low | Weak |
| Dabigatran | Greater risk of bleeding than with warfarin in adults aged ≥ 75; lack of evidence of efficacy and safety in individuals with CrCl < 30 mL/min | Use with caution in adults aged ≥ 75 or if CrCl < 30 mL/min | Moderate | Weak |
| Prasugrel | Greater risk of bleeding in older adults; risk may be offset by benefit in highest‐risk older adults (e.g. with prior myocardial infarction or diabetes mellitus) | Use with caution in adults aged ≥ 75 | Moderate | Weak |
| Antipsychotics Carbamazepine Carboplatin Cisplatin Mirtazapine Serotonin–norepinephrine reuptake inhibitor Selective serotonin reuptake inhibitor Tricyclic antidepressants Vincristine |
May exacerbate or cause syndrome of inappropriate antidiuretic hormone secretion or hyponatraemia; need to monitor sodium level closely when starting or changing dosages in older adults because of increased risk | Use with caution | Moderate | Strong |
| Vasodilators | May exacerbate episodes of syncope in individuals with history of syncope | |||
| Source: AGS 2012. CrCl = creatinine clearance. | ||||
STOPP/START criteria: this is a comprehensive tool that enables a doctor to review an older patient's prescription medications in the context of his or her diagnoses (Gallagher 2008). A panel of 18 experts completed two rounds of a Delphi consensus technique to form the content validity of the criteria. STOPP consists of 65 clinically important criteria for potentially inappropriate prescribing, while START is made up of 22 evidence‐based prescribing indicators for common diseases in older people.
SFINX/PHARAO databases: measure two classes of drug‐drug interactions (SFINX database). These are that interactions can be handled and interactions should be avoided. Pharao concerns medication‐related risk load and has two classes – moderate risk of adverse events and high risk.
RENBASE: focuses on the risk of drug‐induced impairment of renal function, and includes two classes: that modification is needed and should be avoided (RENBASE).
Meds75+ database: focuses on potentially inappropriate medications (Meds75+). Its purpose is to support pharmacotherapy decision‐making in people aged over 75 years and to improve medication safety. The database has recommendations and recommendations for almost 500 drugs or their combinations.
The PRISCUS List: a list of potentially inappropriate medications developed for specific use in Germany (The Priscus List).
Appendix 3. Search strategies 2021
MEDLINE (Ovid)
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE 1946 to 13 January, 2021
Search date: 13 January 2021
| 1 | polypharmacy/ | 5187 |
| 2 | inappropriate prescribing/ | 3512 |
| 3 | potentially inappropriate medication list/ | 543 |
| 4 | deprescriptions/ | 525 |
| 5 | medication errors/ | 13403 |
| 6 | polypharma*.ti,ab. | 8732 |
| 7 | ((beer* or shan? or mcleod?) adj3 criter*).ti,ab. | 768 |
| 8 | ("fit for the aged" adj3 (criter* or list? or instrument or classif*)).ti,ab. | 27 |
| 9 | ((forta or rasp or priscus) adj3 (criter* or list? or instrument)).ti,ab. | 95 |
| 10 | (stopp criter* or stopp list?).ti,ab. | 184 |
| 11 | ((concomitant* or concurrent* or inappropriat* or appropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or excess* or multip* or inadvert* or discontinu*) adj1 (medicine? or medicat* or prescrib* or prescription* or drug*)).ti,ab. | 31087 |
| 12 | ((over adj1 (prescrib* or prescript*)) or (over‐prescrib* or overprescrib*) or ("or more" adj (medication* or prescrib* or prescript*))).ti,ab. | 3008 |
| 13 | ((under adj1 prescrib*) or underprescrib* or under‐prescrib*).ti,ab. | 602 |
| 14 | (deprescrib* or deprescript*).ti,ab. | 828 |
| 15 | "medication appropriateness index*".ti,ab. | 140 |
| 16 | (quality adj2 (prescribing or prescription* or medication*)).ti,ab. | 1561 |
| 17 | (improv* adj2 (prescrib* or pharmaco* or prescription*)).ti,ab. | 7970 |
| 18 | (prescrib* adj cascade*).ti,ab. | 62 |
| 19 | ("assessing care of vulnerable elders" or acove).ti,ab. | 92 |
| 20 | ((multi‐drug* or multidrug*) adj2 (prescrib* or prescription* or regimen? or therap* or treatment?)).ti,ab. | 5265 |
| 21 | or/1‐20 | 71228 |
| 22 | exp aged/ | 3187162 |
| 23 | geriatrics/ | 30233 |
| 24 | (elder* or geriatric*).ti,ab. | 297175 |
| 25 | ((old* or aged) adj (person* or adult* or people or patient* or inpatient* or outpatient*)).ti,ab. | 208333 |
| 26 | aged care.ti,ab. | 2884 |
| 27 | veterans/ | 17451 |
| 28 | veteran*.ti,ab. | 37184 |
| 29 | or/22‐28 | 3390148 |
| 30 | 21 and 29 | 19771 |
| 31 | exp *polypharmacy/ | 2671 |
| 32 | 31 and 29 | 1877 |
| 33 | exp randomized controlled trial/ | 521501 |
| 34 | controlled clinical trial.pt. | 94008 |
| 35 | randomi#ed.ti,ab. | 654064 |
| 36 | placebo.ab. | 214533 |
| 37 | drug therapy.fs. | 2267421 |
| 38 | randomly.ti,ab. | 350242 |
| 39 | trial.ab. | 536963 |
| 40 | groups.ab. | 2143873 |
| 41 | or/33‐40 | 4921187 |
| 42 | Clinical Trials as topic.sh. | 194197 |
| 43 | trial.ti. | 233231 |
| 44 | or/33‐36,38,42‐43 | 1388249 |
| 45 | exp animals/ not humans/ | 4775258 |
| 46 | 44 not 45 | 1280754 |
| 47 | 32 or (30 and 46) | 4270 |
| 48 | (2018* or 2019* or 202*).dc,dp,ed,ep,yr. | 4862752 |
| 49 | 47 and 48 | 1271 |
Embase (Ovid)
Embase 1974 to 2021 January 13
Search date: 13 January 2021
| 1 | polypharmacy/ | 17649 |
| 2 | inappropriate prescribing/ | 4549 |
| 3 | medication error/ | 18546 |
| 4 | polypharma*.ti,ab. | 14559 |
| 5 | ((beer* or shan? or mcleod?) adj3 criter*).ti,ab. | 1393 |
| 6 | ("fit for the aged" adj3 (criter* or list? or instrument or classif*)).ti,ab. | 38 |
| 7 | ((forta or rasp or priscus) adj3 (criter* or list? or instrument)).ti,ab. | 145 |
| 8 | (stopp criter* or stopp list?).ti,ab. | 435 |
| 9 | ((concomitant* or concurrent* or inappropriat* or appropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or excess* or multip* or inadvert* or discontinu*) adj1 (medicine? or medicat* or prescrib* or prescription* or drug*)).ti,ab. | 50843 |
| 10 | ((over adj1 (prescrib* or prescript*)) or (over‐prescrib* or overprescrib*) or ("or more" adj (medication* or prescrib* or prescript*))).ti,ab. | 4942 |
| 11 | ((under adj1 prescrib*) or underprescrib* or under‐prescrib*).ti,ab. | 925 |
| 12 | (deprescrib* or deprescript*).ti,ab. | 1310 |
| 13 | "medication appropriateness index*".ti,ab. | 225 |
| 14 | (quality adj2 (prescribing or prescription* or medication*)).ti,ab. | 2598 |
| 15 | (improv* adj2 (prescrib* or pharmaco* or prescription*)).ti,ab. | 11789 |
| 16 | (prescrib* adj cascade*).ti,ab. | 102 |
| 17 | ("assessing care of vulnerable elders" or acove).ti,ab. | 152 |
| 18 | ((multi‐drug* or multidrug*) adj2 (prescrib* or prescription* or regimen? or therap* or treatment?)).ti,ab. | 6875 |
| 19 | or/1‐18 | 112374 |
| 20 | aged/ | 3080471 |
| 21 | frail elderly/ | 10469 |
| 22 | very elderly/ | 216042 |
| 23 | aged hospital patient/ | 974 |
| 24 | veteran/ | 26984 |
| 25 | exp geriatrics/ | 38070 |
| 26 | (elder* or geriatric*).ti,ab. | 424162 |
| 27 | ((old* or aged) adj (person* or adult* or people or patient* or inpatient* or outpatient*)).ti,ab. | 281330 |
| 28 | aged care.ti,ab. | 3257 |
| 29 | veteran*.ti,ab. | 48874 |
| 30 | or/20‐29 | 3355755 |
| 31 | *polypharmacy/ | 4415 |
| 32 | 30 and 31 | 2499 |
| 33 | 19 and 30 | 28021 |
| 34 | random*.ti,ab. | 1625467 |
| 35 | factorial*.ti,ab. | 40193 |
| 36 | (crossover* or cross over*).ti,ab. | 111054 |
| 37 | ((doubl* or singl*) adj blind*).ti,ab. | 241736 |
| 38 | (assign* or allocat* or volunteer* or placebo*).ti,ab. | 1084483 |
| 39 | crossover procedure/ | 65901 |
| 40 | single blind procedure/ | 41553 |
| 41 | randomized controlled trial/ | 641556 |
| 42 | double blind procedure/ | 180691 |
| 43 | or/34‐42 | 2448047 |
| 44 | exp animal/ not human/ | 4906061 |
| 45 | 43 not 44 | 2205514 |
| 46 | 32 or (33 and 45) | 5979 |
| 47 | limit 46 to yr="2018 ‐Current" | 1764 |
| 48 | limit 47 to embase | 1059 |
The Cochrane Library (Wiley)
Search date: 13 January 2021
| #1 | [mh polypharmacy] | 222 |
| #2 | [mh "inappropriate prescribing"] | 149 |
| #3 | [mh "potentially inappropriate medication list"] | 20 |
| #4 | [mh deprescriptions] | 28 |
| #5 | [mh "medication errors"] | 431 |
| #6 | polypharma*:ti,ab | 801 |
| #7 | ((beer* or shan* or mcleod*) near/3 criter*):ti,ab | 71 |
| #8 | ("fit for the aged" near/3 (criter* or list* or instrument or classif*)):ti,ab | 3 |
| #9 | ((forta or rasp or priscus) near/3 (criter* or list* or instrument)):ti,ab | 26 |
| #10 | (stopp criter* or stopp list*):ti,ab | 106 |
| #11 | ((concomitant* or concurrent* or inappropriat* or appropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or excess* or multip* or inadvert* or discontinu*) near/1 (medicine* or medicat* or prescrib* or prescription* or drug*)):ti,ab | 6256 |
| #12 | ((over near/1 (prescrib* or prescript*)) or (over‐prescrib* or overprescrib*) or ("or more" near/1 (medication* or prescrib* or prescript*))):ti,ab | 387 |
| #13 | ((under near/1 prescrib*) or underprescrib* or under‐prescrib*):ti,ab | 68 |
| #14 | (deprescrib* or deprescript*):ti,ab | 153 |
| #15 | (quality near/2 (prescribing or prescription* or medication*)):ti,ab | 406 |
| #16 | (improv* near/2 (prescrib* or pharmaco* or prescription*)):ti,ab | 967 |
| #17 | (prescri* near/1 cascade*):ti,ab | 2 |
| #18 | ("assessing care of vulnerable elders" or acove):ti,ab | 13 |
| #19 | ((multi‐drug* or multidrug*) near/2 (prescrib* or prescription* or regimen* or therap* or treatment*)):ti,ab | 567 |
| #20 | {or #1‐#19} | 9361 |
| #21 | [mh aged] | 207497 |
| #22 | [mh geriatrics] | 204 |
| #23 | (elder* or geriatric*):ti,ab | 49463 |
| #24 | ((old* or aged) near/1 (person* or adult* or people or patient* or inpatient* or outpatient*)):ti,ab | 51125 |
| #25 | aged next care:ti,ab | 307 |
| #26 | [mh veterans] | 970 |
| #27 | veteran*:ti,ab | 5735 |
| #28 | {or #21‐#27} | 281805 |
| #29 | #20 and #28 | 2609 |
| #30 | #20 and #28 with Cochrane Library publication date Between Jan 2018 and Dec 2021 | 1137 |
CINAHL (EBSCO)
Search date: 13 January 2021
| S1 | (MH "Polypharmacy") | 4,661 |
| S2 | (MH "Inappropriate Prescribing") | 2,837 |
| S3 | (MH "Medication Errors") | 14,070 |
| S4 | polypharma* | 6,451 |
| S5 | (beer* or shan* or mcleod*) N3 criter* | 500 |
| S6 | "fit for the aged" N3 (criter* or list* or instrument or classif*) | 17 |
| S7 | (forta or rasp or priscus) N3 (criter* or list* or instrument) | 37 |
| S8 | stopp criter* or stopp list* | 189 |
| S9 | (concomitant* or concurrent* or inappropriat* or appropriat* or suboptim* or sub‐optim* or unnecessary or incorrect* or excess* or multip* or inadvert* or discontinu*) N1 (medicine* or medicat* or prescrib* or prescription* or drug*) | 22,536 |
| S10 | ((over N1 (prescrib* or prescript*)) or (over‐prescrib* or overprescrib*) or ("or more" N0 (medication* or prescrib* or prescript*))) | 4,388 |
| S11 | (under N1 prescrib*) or underprescrib* or under‐prescrib* | 279 |
| S12 | deprescrib* or deprescript* | 597 |
| S13 | "medication appropriateness index*" | 82 |
| S14 | quality N2 (prescribing or prescription* or medication*) | 1,265 |
| S15 | prescrib* N0 cascade* | 42 |
| S16 | "assessing care of vulnerable elders" or acove | 61 |
| S17 | (multi‐drug* or multidrug*) N2 (prescrib* or prescription* or regimen* or therap* or treatment*) | 1,850 |
| S18 | improv* N2 (prescrib* or pharmaco* or prescription*) | 2,901 |
| S19 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 | 49,512 |
| S20 | (MH "Aged+") | 851,285 |
| S21 | (MH "Geriatrics") | 5,571 |
| S22 | (MH "Veterans") | 17,512 |
| S23 | elder* or geriatric* | 144,994 |
| S24 | (old* or aged) N0 (person* or adult* or people or patient* or inpatient* or outpatient*) | 143,981 |
| S25 | "aged care" | 4,745 |
| S26 | veteran* | 32,518 |
| S27 | S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 | 960,691 |
| S28 | S19 AND S27 | 13,257 |
| S29 | (MM "Polypharmacy") | 2,029 |
| S30 | S27 AND S29 | 1,396 |
| S31 | (MH "Random Assignment") | 65,642 |
| S32 | (MH "Clinical Trials+") | 310,857 |
| S33 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 317,673 |
| S34 | PT clinical trial | 107,048 |
| S35 | PT randomized controlled trial | 125,495 |
| S36 | S31 OR S32 OR S33 OR S34 OR S35 | 488,488 |
| S37 | S28 AND S36 | 9,553 |
| S38 | S30 OR S37 | 10,826 |
| S39 | S38 Limiters ‐ Published Date: 20180101‐20211231; Exclude MEDLINE records | 678 |
ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov/)
Search date: 13 January 2021
| polypharmacy | senior | 69 | |
| "inappropriate prescribing" | senior | 26 | |
| appropriate prescribing | senior | 5 | |
| "inappropriate medication" | senior | 30 | |
| "appropriate medication" | senior | 16 | |
| deprescribing | senior | 1 | |
| Total= | 147 | |
| Rerun Feb 2018 | polypharmacy OR "inappropriate prescribing" OR "appropriate prescribing" OR "inappropriate medication" OR "appropriate medication" OR deprescribing | Senior | 209 |
| Rerun Jan 2021 | INTERVENTION: polypharmacy OR "inappropriate prescribing" OR "appropriate prescribing" OR "inappropriate medication" OR "appropriate medication" OR deprescribing & Older adults (65+) | 213 |
WHO International Clinical Trials Registry Platform (ICTRP)
Search date: 13 January 2021
| Search terms | Rerun Jan 2021 |
| polypharmacy | 192 |
| inappropriate prescribing | 33 |
| appropriate prescribing | 9 |
| inappropriate medication | 22 |
| appropriate medication | 12 |
| deprescribing | 57 |
| Total= | 325 |
Appendix 4. Reviews screened for included studies
(1) Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. Journal of the American Academy of Nurse Practitioners 2005 Apr;17(4):123‐32. (2) Garcia RM. Five ways you can reduce inappropriate prescribing in the elderly: a systematic review. Journal of Family Practice 2006 Apr;55(4):305‐12. (3) George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs & Aging 2008;25(4):307‐24. (4) Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. American Journal of Geriatric Pharmacotherapy 2007;5(4):345‐51. (5) Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008;2(CD000011). (6) Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. British Journal of Clinical Pharmacology 2008 Mar;65(3):303‐16. (7) Huss A, Stuck AE, Rubenstein LZ, Egger M, Clough‐Gorr KM. Multidimensional preventive home visit programs for community‐dwelling older adults: a systematic review and meta‐analysis of randomized controlled trials. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences 2008;63(3):298‐307. (8) Jano E, Aparasu RR. Healthcare outcomes associated with Beers' criteria: a systematic review. The Annals of Pharmacotherapy 2007 Mar;41(3):438‐47. (9) Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs & Aging 2009;26(12):1013‐28. (10) Maeda K. Systematic review of the effects of improvement of prescription to reduce the number of medications in the elderly with polypharmacy. Yakugaku Zasshi 2009 May;129(5):631‐45. (11) Milton JC, Hill‐Smith I, Jackson SH. Prescribing for older people. BMJ 2008 Mar 15;336(7644):606‐9. (12) Rollason V, Vogt N. Reduction of polypharmacy in the elderly: a systematic review of the role of the pharmacist. Drugs & Aging 2003;20(11):817‐32. (13) Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta‐analysis. Quality & Safety in Health Care 2006 Feb;15(1):23‐31. (14) Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 2007;370(9582):173‐84. (15) Wenger NS, Roth CP, Shekelle P, ACOVE I. Introduction to the assessing care of vulnerable elders‐3 quality indicator measurement set. Journal of the American Geriatrics Society 2007 Oct;55(Suppl 2):S247‐s52. (16) Yourman L, Concato J, Agostini JV. Use of computer decision support interventions to improve medication prescribing in older adults: a systematic review. American Journal of Geriatric Pharmacotherapy 2008 Jun;6(2):119‐29.
(17) Alldred DP, Raynor DK, Hughes C, Barber N, Chen TF, Spoor P. Interventions to optimise prescribing for older people in care homes. Cochrane Database of Systematic Reviews 2013; 2:CD009095.
(18) Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2013;2:CD008986.
(19) Clyne B, Bradley MC, Hughes C, Fahey T, Lapane KL. Electronic prescribing and other forms of technology to reduce inappropriate medication use and polypharmacy in older people: a review of current evidence. Clinics in Geriatric Medicine 2012;28(2):301‐22.
(20) Fleming A, Browne J, Byrne S. The effect of interventions to reduce potentially inappropriate antibiotic prescribing in long‐term care facilities: a systematic review of randomised controlled trials. Drugs & Aging 2013;30(6):401‐8.
(20) Forsetlund L, Eike MC, Gjerberg E, Vist GE. Effect of interventions to reduce potentially inappropriate use of drugs in nursing homes: a systematic review of randomised controlled trials. BMC Geriatrics 2011;11:16.
(21) Frazier SC. Health outcomes and polypharmacy in elderly individuals: an integrated literature review. Journal of Gerontological Nursing 2005;31(9):4‐11.
(22) George J, Elliott RA, Stewart DC. A systematic review of interventions to improve medication taking in elderly patients prescribed multiple medications. Drugs & Aging 2008;25(4):307‐24.
(23) Loganathan M, Singh S, Franklin BD, Bottle A, Majeed A. Interventions to optimise prescribing in care homes: systematic review. Age and Ageing 2011;40(2):150‐62.
(24) Maeda K. Systematic review of the effects of improvement of prescription to reduce the number of medications in the elderly with polypharmacy. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan 2009;129(5):631‐45.
(25) Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M. Interventions to reduce antipsychotic polypharmacy: a systematic review. Schizophrenia Research 2013;143(1):215‐20.
(26) Tjia J, Velten SJ, Parsons C, Valluri S, Briesacher BA. Studies to reduce unnecessary medication use in frail older adults: a systematic review. Drugs & Aging 2013;30(5):285‐307.
(27) Alldred DP, Kennedy M, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database of Systematic Reviews 2016;2:CD009095.
(28) Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database of Systematic Reviews 2016;2: CD008986.
(29) Curtain C, Peterson G. Review of computerized clinical decision support in community pharmacy. J Clin Pharm Ther 2014;39(4):343‐348.
(30) Desborough J, Twigg M. Pharmacist‐led medication reviews in primary care. Reviews in Clinical Gerontology 2014;24(01):1‐9.
(31) Fried TR, O'Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health Outcomes Associated with Polypharmacy in Community‐Dwelling Older Adults: A Systematic Review. J Am Geriatr Soc 2014;62(12):2261‐2272.
(32) Hill‐Taylor B, Walsh K, Stewart S, Hayden J, Byrne S, Sketris I. Effectiveness of the STOPP/START (Screening Tool of Older Persons' potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta‐analysis of randomized controlled studies. J Clin Pharm Ther 2016;41(2):158‐169.
(33) Lehnbom EC, Stewart MJ, Manias E, Westbrook JI. Impact of medication reconciliation and review on clinical outcomes. Ann Pharmacother 2014 Oct;48(10):1298‐1312.
(34) Lonsdale DO, Baker EH. Understanding and managing medication in elderly people. Best Practice & Research Clinical Obstetrics & Gynaecology 2013;27(5):767‐788.
(35) Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert opinion on drug safety 2014;13(1):57‐65.
(36) Penge J, Crome P. Appropriate prescribing in older people. Reviews in Clinical Gerontology 2014;24(01):58‐77.
(37) Petrovic M, Somers A, Onder G. Optimization of geriatric pharmacotherapy: role of multifaceted cooperation in the hospital setting. Drugs Aging 2016;33(3):179‐188.
(38) Shade MY, Berger AM, Chaperon C. Potentially inappropriate medications in community‐dwelling older adults. Research in gerontological nursing 2014;7(4):178‐192.
(39) Walsh KA, O'Riordan D, Kearney PM, Timmons S, Byrne S. Improving the appropriateness of prescribing in older patients: a systematic review and meta‐analysis of pharmacists' interventions in secondary care. Age Ageing 2016;45(2):201‐209.
(40) Tasai S, Kumpat N, Dilokthornsakul P, Chaiyakunapruk N, Saini B, Dhippayom T. Impact of Medication Reviews Delivered by Community Pharmacist to Elderly Patients on Polypharmacy: A Meta‐analysis of Randomized Controlled Trials. J Patient Saf 2021; 1;17(4): 290‐298. doi: 10.1097/PTS.0000000000000599. PMID: 30920431.
(41) Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT. Meta‐analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc 2018; 66(2): 282‐288. doi: 10.1111/jgs.15195.
(42) Thillainadesan J, Gnjidic D, Green S, Hilmer SN. Impact of Deprescribing Interventions in Older Hospitalised Patients on Prescribing and Clinical Outcomes: A Systematic Review of Randomised Trials. Drugs Aging 2018;35(4):303‐319. doi: 10.1007/s40266‐018‐0536‐4. PMID: 29541966.
(43) Nurse interventions to improve medication adherence among discharged older adults: a systematic review. Verloo H, Chiolero A, Kiszio B, Kampel T, Santschi V. Age and Ageing 2017; 46: 747‐754 doi: 10.1093/ageing/afx076.
(44) Dills H, Shah K, Messinger‐Rapport B, Bradford K, Syed Q. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomised controlled trials. JAMDA 2018; 19:923‐935. doi: https://doi.org/10.1016/j.jamda.2018.06.021
(45) Baumgartner AD, Clark CM, LaValley SA, Monte SV, Wahler RG Jr, Singh R. Interventions to deprescribe potentially inappropriate medications in the elderly: Lost in translation? J Clin Pharm Ther. 2020; 45(3):453‐461. doi: 10.1111/jcpt.13103. Epub 2019 Dec 24. PMID: 31873955; PMCID: PMC7200270.
(46) Monteiro L, Maricoto T, Solha I, Ribeiro‐Vaz I, Martins C, Monteiro‐Soares M. Reducing Potentially Inappropriate Prescriptions for Older Patients Using Computerized Decision Support Tools: Systematic Review. J Med Internet Res. 2019; 14;21(11):e15385. doi: 10.2196/15385. PMID: 31724956; PMCID: PMC6883366.
(47) Ali MU, Sherifali D, Fitzpatrick‐Lewis D, Kenny M, Liu A, Lamarche L, Mangin D, Raina P. Polypharmacy and mobility outcomes. Mechanisms of Ageing and Development 2020; 192. Doi: https://doi.org/10.1016/j.mad.2020.111356.
(48) Mizokami F, Mizuno T, Kanamori K, Oyama S, Nagamatsu T, Lee JK, Kobayashi T. Clinical medication review type III of polypharmacy reduced unplanned hospitalizations in older adults: A meta‐analysis of randomized clinical trials. Geriatr Gerontol Int. 2019;19(12):1275‐1281. doi: 10.1111/ggi.13796. Epub 2019 Nov 22. PMID: 31758656.
(49) Soler O, Barreto JOM. Community‐Level Pharmaceutical Interventions to Reduce the Risks of Polypharmacy in the Elderly: Overview of Systematic Reviews and Economic Evaluations. Front Pharmacol. 2019;2;10:302. doi: 10.3389/fphar.2019.00302. PMID: 31001117; PMCID: PMC6454558.
(50) Huiskes VJ, Burger DM, van den Ende CH, van den Bemt BJ. Effectiveness of medication review: a systematic review and meta‐analysis of randomized controlled trials. BMC Fam Pract. 2017; 17;18(1):5. doi: 10.1186/s12875‐016‐0577‐x. PMID: 28095780; PMCID: PMC5240219.
(51) Raban MZ, Gasparini C, Baysari MT, Westbrook JI. Effectiveness of interventions targeting antibiotic use in long‐term aged care facilities: a systematic review and meta‐analysis. BMJ Open 2020;10:e028494. doi:10.1136/bmjopen‐2018‐028494.
(52) Hansen CR, O'Mahony D, Kearney PM, Sahm LJ, Cullinan S, Huibers CJA, Thevelin S, Rutjes AWS, Knol W, Streit S, Byrne S. Identification of behaviour change techniques in deprescribing interventions: a systematic review and meta‐analysis. Br J Clin Pharmacol. 2018;84(12):2716‐2728. doi: 10.1111/bcp.13742. Epub 2018 Sep 22. PMID: 30129139; PMCID: PMC6255994.
(53) Earl TR, Katapodis ND, Schneiderman SR, Shoemaker‐Hunt SJ. Using Deprescribing Practices and the Screening Tool of Older Persons' Potentially Inappropriate Prescriptions Criteria to Reduce Harm and Preventable Adverse Drug Events in Older Adults. J Patient Saf. 2020;16(3S Suppl 1):S23‐S35. doi: 10.1097/PTS.0000000000000747. PMID: 32809998; PMCID: PMC7447181.
(54) Beuscart JB, Pont LG, Thevelin S, Boland B, Dalleur O, Rutjes AWS, Westbrook JI, Spinewine A. A systematic review of the outcomes reported in trials of medication review in older patients: the need for a core outcome set. Br J Clin Pharmacol. 2017;83(5):942‐952. doi: 10.1111/bcp.13197. Epub 2017 Jan 18. PMID: 27891666; PMCID: PMC6436185.
(55) Bloomfield HE, Greer N, Linsky AM, Bolduc J, Naidl T, Vardeny O, MacDonald R, McKenzie L, Wilt TJ. Deprescribing for Community‐Dwelling Older Adults: a Systematic Review and Meta‐analysis. J Gen Intern Med. 2020;35(11):3323‐3332. doi: 10.1007/s11606‐020‐06089‐2. Epub 2020 Aug 20. PMID: 32820421; PMCID: PMC7661661.
(56) Nakham A, Myint PK, Bond CM, Newlands R, Loke YK, Cruickshank M. Interventions to Reduce Anticholinergic Burden in Adults Aged 65 and Older: A Systematic Review. J Am Med Dir Assoc. 2020;21(2):172‐180.e5. doi: 10.1016/j.jamda.2019.06.001. Epub 2019 Jul 24. PMID: 31351858.
(57) Skjøt-Arkil H, Lundby C, Kjeldsen LJ,Skovgårds DM, Almarsdóttir AB, Kjølhede T,Duedahl TH, Pottegård A, Graabæk T. Multifaceted pharmacist‐led interventions in the hospital setting: a systematic review. Basic and Clinical Pharmacology and Toxicology 2018; 123(4): 363‐379. https://doi.org/10.1111/bcpt.13030
(58) Almutairi H, Stafford A, Etherton‐Beer C, Flicker L. Optimisation of medications used in residential aged care facilities: a systematic review and meta‐analysis of randomised controlled trials. BMC Geriatr 20, 236 (2020). https://doi.org/10.1186/s12877-020-01634-4.
(59) Santos NS, Marengo LL, Moraes FS, Barberato‐Filho S. Interventions to reduce the prescription of inappropriate medicines in older patients. Rev Saude Publica. 2019;53:7.
(60) Granata N, Traversoni S, Kardas P, Kurczewska‐Michalak M, Costa E, Midao L, Giardini A. Methodological features of quantitative studies on medication adherence in older patients with chronic morbidity: a systematic review. Patient Education and Counseling 2020; 103(10): 2132‐2141.
(61) Tomlinson J, Cheong VL, Fylan B, Silcock J, Smith H, Karban K, Blenkinsopp A. Successful care transitions for older people: a systematic review and meta‐analysis of the effects of interventions that support medication continuity. Age and Ageing 2020; 49(4): 558‐569. Doi: https://doi.org/10.1093/ageing/afaa002.
(62) Anderson LJ, Schnipper JL, Nuckols TK, Shane R, Sarkisian C, Le MM, Pevnick JM; Members of the PHARM‐DC group. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019; 15;76(21):1777‐1787. doi: 10.1093/ajhp/zxz196. PMID: 31612924; PMCID: PMC7170727.
Appendix 5. GRADE evidence profile: Pharmaceutical care compared with usual care for older people receiving polypharmacy
Certainty assessment of evidence for each outcome
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness† | Imprecision | Other* |
Certainty (overall score)§ |
| Outcome: Medication appropriateness (as measured by an implicit tool) | |||||||
| 8 studies | Randomised trials | Downgrade by 1 level of evidence: risk of bias assessments across all studies (particularly allocation concealment and blinding of participants and personnel) are likely to lower confidence in estimate of effect. | Downgrade by 2 levels of evidence: heterogeneity between studies (I2 = 97%, P < 0.00001), not all 95% CIs overlap. | Downgrade by 1 level of evidence: not all studies included a validated assessment of under prescribing and, therefore, the findings are not a direct assessment of appropriate polypharmacy (primary outcome). | Downgrade by 1 level of evidence: 95% CI of pooled effect estimate is wide (‐9.26 to ‐2.06). | None. Too few studies to assess publication bias (< 10). | ⊕⊝⊝⊝ very low |
| Outcome: The number of potentially inappropriate medications | |||||||
| 9 studies | Randomised trials | Downgrade by 2 levels of evidence: risk of bias assessments across all studies are likely to lower confidence in estimate of effect; multiple domains across studies with unclear or high risk. | Downgrade by 2 levels of evidence: heterogeneity between studies (I2 = 67%, P = 0.002), not all 95% CIs overlap. | Downgrade by 1 level of evidence: not all studies included a validated assessment of under‐prescribing and, therefore, the findings are not a direct assessment of appropriate polypharmacy (primary outcome). | Do not downgrade level of evidence: no serious imprecision. | None. Too few studies to assess publication bias (< 10). | ⊕⊝⊝⊝ very low |
| Outcome: The proportion of patients with one or more potentially inappropriate medication | |||||||
| 13 studies | Randomised trials | Downgrade by 2 levels of evidence: risk of bias assessments across all studies are likely to lower confidence in estimate of effect; multiple domains across studies with unclear or high risk. | Downgrade by 2 levels of evidence: heterogeneity between studies (I2 = 84%, P < 0.00001), not all 95% CIs overlap. | Downgrade by 1 level of evidence: not all studies included a validated assessment of under prescribing and, therefore, the findings are not a direct assessment of appropriate polypharmacy (primary outcome). | Do not downgrade level of evidence: no serious imprecision. | None. No evidence of publication bias. | ⊕⊝⊝⊝ very low |
| Outcome: The number of potential prescribing omissions | |||||||
| 3 studies | Randomised trials | Downgrade by 2 levels of evidence: risk of bias assessments across all studies are likely to lower confidence in estimate of effect; multiple domains across studies with unclear or high risk. | Downgrade by 2 levels of evidence: heterogeneity between studies (I2 = 92%, P < 0.00001), not all 95% CIs overlap. | Do not downgrade level of evidence: included validated assessments of under‐prescribing. | Downgrade by 1 level of evidence as 95% CI crosses line of no effect. | None. Too few studies to assess publication bias (< 10 studies). | ⊕⊝⊝⊝ Very low |
| Outcome: The proportion of patients with one or more potential prescribing omission | |||||||
| 7 studies | Randomised trials | Downgrade by 2 levels of evidence: risk of bias assessments across all studies are likely to lower confidence in estimate of effect; multiple domains across studies with unclear or high risk. | Downgrade by 2 levels of evidence: heterogeneity between studies (I2 = 95%, P < 0.00001), not all 95% CIs overlap. | Downgrade by 1 level of evidence: not all studies included a validated assessment of under prescribing and, therefore, the findings are not a direct assessment of appropriate polypharmacy (primary outcome). | No serious imprecision: do not downgrade. | None. Too few studies to assess publication bias (< 10). | ⊕⊝⊝⊝ very low |
| Outcome: Hospital admissions | |||||||
| 14 studies | Randomised trials | Downgrade by 2 levels of evidence: risk of bias assessments across most studies are likely to lower confidence in estimate of effect; multiple domains across studies with unclear or high risk. All studies had at least one domain judged to be high risk of bias. Six studies had two or more domains with high risk of bias, two studies had four domains at high risk of bias and one study had five. | Do not downgrade level of evidence: As hospital admissions was not included in the meta‐analysis, an estimate of heterogeneity was not calculated. However, we can point to some consistency in that this outcome was determined in all studies by examination of hospital records. Conversely, the outcome was reported at varying time points. | Downgrade by 1 level of evidence: we are unable to conclude if all hospital admissions were medication‐related. | Do not downgrade level of evidence: as a meta‐analysis was not done for this outcome, we cannot comment on precision. | None. It is not expected that publication bias is an important factor. | ⊕⊕⊝⊝ low |
| Outcome: Quality of life | |||||||
| 16 studies | Randomised trials | Downgrade by two levels of evidence: 14 out of 16 trials had at least one domain judged to be at high risk of bias, and six trials had at least two domains at high risk of bias. | Do not downgrade level of evidence: As quality of life was not included in the meta‐analysis, an estimate of heterogeneity was not calculated. Some inconsistency is indicated in that seven different measures of quality of life were used and the outcome was assessed at varying time points. |
Downgrade by 1 level of evidence: we are unable to conclude if quality of life was medication‐related. | Do not downgrade level of evidence: as a meta‐analysis was not done for this outcome, we cannot comment on precision. | None. It is not expected that publication bias is an important factor. | ⊕⊕⊝⊝ low |
Footnotes
†Indirectness includes consideration of:
indirect (between‐study) comparisons;
indirect (surrogate) outcomes;
applicability (study populations, interventions or comparisons that are different than those of interest, e.g. whether the study used a validated measure of medication appropriateness).
*Other considerations for downgrading include publication bias. Other considerations for upgrading include a strong association with no plausible confounders, a dose response relationship, and if all plausible confounders or biases would decrease the size of the effect (if there is evidence of an effect), or increase it if there is evidence of no harmful effect (safety).
§4 High = This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
3 Moderate = This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
2 Low = This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
1 Very low = This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high.
** Substantially different = a large enough difference that it might affect a decision.
Data and analyses
Comparison 1. Postintervention analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Medication appropriateness (as measured by an implicit tool) | 8 | 947 | Mean Difference (IV, Random, 95% CI) | ‐5.66 [‐9.26, ‐2.06] |
| 1.2 Medication appropriateness (as measured by an implicit tool) (excluding Crotty 2004a) | 7 | 876 | Mean Difference (IV, Random, 95% CI) | ‐5.97 [‐10.08, ‐1.85] |
| 1.3 Number of potentially inappropriate medications | 9 | 2404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.34, ‐0.05] |
| 1.4 Proportion of patients with one or more potentially inappropriate medication | 13 | 4534 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.98] |
| 1.5 Number of potential prescribing omissions | 3 | 691 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.05, 0.09] |
| 1.6 Proportion of patients with one or more potential prescribing omission | 7 | 2765 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.27, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auvinen 2021.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Setting: home care settings in Finland Unit of allocation/analysis: participant Follow‐up: 6 months Duration: unclear Providers: interprofessional team consisting of a pharmacist, physician and registered nurse |
|
| Participants | 512 randomised (intervention = 258; usual care = 254 patients) Mean age 84 years Women = 72%; men = 28% Race: not given The mean Charlson Comorbidity Index of participants was 2.5 (SD 1.6); 92% of patients had cardiovascular disease; 61% had a disease of the musculoskeletal system; 36% had diabetes; 33% had cerebrovascular disease; 31% had dementia; 81% had at least mild renal insufficiency (glomerular filtration rate 80 mmol/min or less). The mean number of regularly taken drugs was 9.2 (range: 2 to 20) in the intervention and 9.5 (range: 1 to 20) in the usual care group. The number and range of drugs taken as needed was 3.5 (0 to 20) and 3.8 (0 to 13) in the intervention and usual care groups, respectively. |
|
| Interventions | Model of care: structured medication assessment conducted by a pharmacist, physician and registered nurse Timing: one medication assessment in patient's home care setting Baseline measurements consisted of a nurse checking patient prescriptions and over the counter drugs with them at their home, asking the patient about the actual use of drugs and updating the medication lists. Assessments carried out by nurse of patients’ physical functioning and performance in daily activities. Data collected on demographic variables and patient characteristics Physician on the home care team documented patients’ diagnoses from existing medical records. A modified Charlson Co‐Morbidity Index was used to describe the home care patients’ disease burden. The glomerular filtration rate was calculated. Within 2 weeks of the baseline measurements, an interprofessional team consisting of a pharmacist, physician and registered nurse who worked regularly in home care conducted the structured medication assessment. Patients’ updated and verified medication lists; current health measurements and electronic medical records, including medical history, were available during the assessment. Before the team meeting, the pharmacist reviewed the patients’ medication lists using 4 databases, which were available in the Terveysportti.fi health portal. The databases were used to identify DDIs, medication‐induced renal risks, risks of clinically relevant adverse effects at a single drug level and as pharmacodynamic risk loads in the whole medication, and the appropriateness of drugs for older people. The physician gathered information from patients’ medical records and on current clinical status. In the interprofessional team meeting, the professionals discussed patients’ current clinical condition and reviewed their medications, considering the current health status and clinically significant aspects. The pharmacist focused his or her medication review recommendations on clinically relevant issues that came up in the team discussion. The physician made clinical decisions and wrote recommendations into patients’ medical records. After the team meeting, the nurse updated the patient’s medication regimen. If the patient did not participate in the team meeting, the nurse informed the patient about the changes and the changes were implemented if the patient accepted them. The pharmacists involved had a qualification in comprehensive medication review or current continuing professional development in clinical pharmacy. All interprofessional team members received a one‐day training or personal introduction on the FIMA protocol. Control group: usual care |
|
| Outcomes | All drugs (number of drugs regularly taken and number of drugs taken as needed); measured at baseline and 6 months Drug‐drug interactions (measured using SFINX database – 2 classes – drug interactions can be handled and drug interactions should be avoided); measured at baseline and 6 months Risk of drug‐induced impairment of renal function (RENBASE – 2 classes – drug modification needed and the drug should be avoided); measured at baseline and 6 months Medication‐related risk load (PHARAO database – 2 classes – moderate risk of adverse events and high risk); measured at baseline and 6 months PIMs (Meds75+ database). Drugs are placed in 4 categories to indicate how suitable they are for people aged over 75 years. Category A indicates the drug is suitable; category B indicates little research evidence, practical experience or efficacy in older persons; category C highlights that the drug is suitable for older persons but with specific cautions; and category D indicates that the drug should be avoided in older people. The authors used the Meds75+ class D to define the use of PIMs among home care patients. This was measured at baseline and 6 months. |
|
| Notes | Funding: The FIMA Study concept, design and acquisition of data were funded by the Ministry of Social Affairs and Health, Finland. Preparation of the present manuscript was supported by the South Savo Regional Fund of the Finnish Cultural Foundation, The Finnish Medical Foundation and Outpatient Care Research Foundation. Contact with authors: we contacted the authors of this study to ascertain whether two of their medication databases were validated measures of prescribing appropriateness. The authors replied to confirm that they were validated measures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised into the intervention or control group in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | It is not stated who carried out allocation or randomisation or if they knew of the next upcoming assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the participants or personnel implementing the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to make decision. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were completed and presented in the Results section. |
| Selective reporting (reporting bias) | Low risk | Not detected. |
| Protection against contamination | High risk | The same interprofessional teams examined patients from intervention and control groups so there is potential for contamination. |
Basger 2015.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: private hospital and homes of older patients in Sydney, Australia Unit of allocation/analysis: participant Follow‐up: 3 months post discharge Duration: unclear Providers: clinical pharmacist, GPs and registered nurses |
|
| Participants | Quote: “216 older patients (over 65 years old) were randomised into control or intervention groups at discharge from a 50 bed private hospital in Sydney, Australia. Patients were admitted for treatment of chronic medical conditions such as diabetes and heart failure, in addition to rehabilitation after joint replacement surgery performed at other hospitals. Their medical conditions and medications were representative of older Australian community patients. Eligibility criteria consisted of age over 65 years, English speaking, taking five or more medications and living within a 15 km radius of the hospital. Patients with cognitive impairment were excluded” Focus on polypharmacy: included participants taking 5 or more medications (number of regular medications reported as control patients: 10.6 ± 3.2, range 4 to 20; intervention patients 11.3 ± 3.3, range 4 to 20; P value = 0.11) Age (mean): 82.7 ± 7.3 years, range 65 to 97 years intervention, 80.2 ± 6.7 years, range 65 to 93 years control Male: 22.5% intervention, 22.8% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: pharmacists worked on hospital wards as a clinical pharmacy service, the pharmacist(s) conducted an independent medication review together with participants during a face‐to‐face encounter, which was sent to the patient’s own GP Training: unclear if training was provided as part of the intervention Timing of intervention: at hospital discharge Quote: “Intervention patients then received medication counselling and an in‐depth interview from the clinical pharmacist to facilitate completion of a medication review, sent to their GP within 3 days of discharge. Medication review consisted of medication reconciliation, identification of (potential) causes of DRPs and recommendations for their resolution and prevention. Opportunities for self‐management were discussed with the patient. Reviews explained medication changes made in hospital. They were completed by a clinical pharmacist (BJB) with postgraduate qualifications in clinical pharmacy, 15 years’ experience in medication review and accreditation through proof of continuing education and by examination. Recommendations represented an evidence‐based risk–benefit evaluation of the consequences of discontinuing or initiating medication. Intervention patients received a copy of the review. Separately and as per hospital protocol, a registered nurse explained each patients discharge medications to them—both control and intervention—with a copy sent to the patients GP, together with a medical summary written for those patients attended by a specialist Control participants received usual care” |
|
| Outcomes | Change in the number of prescribing appropriateness criteria met (prescribing appropriateness criteria‐set for application in older Australians); measured at baseline and 3 months Change in HRQoL (SF‐36); measured at baseline and 3 months |
|
| Notes | Funding: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The clinical pharmacist (one of the authors) collected all relevant demographic, medical and medication data and intervention patients then received medication counselling and an in‐depth interview from the clinical pharmacist to facilitate completion of a medication review; lack of blinding also acknowledged as a limitation of the study” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 intervention patients and 11 control group patients were lost to follow‐up: analysis was based on patients available at follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Bladh 2011.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: internal medical wards at a university hospital in the city of Gothenburg, Sweden Unit of allocation/analysis: participant Follow‐up: 6 months follow‐up Duration: unclear Providers: pharmacist |
|
| Participants | 400 older patients (199 intervention and 201 control) Focus on polypharmacy: median (IQR) number of drugs at baseline was 7 (4 to 9) intervention, 7 (4 to 10) control Age (median (IQR)): 81 (72 to 87) years intervention, 82 (75 to 86) years control Male: 39% intervention, 40% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: medication reviews by pharmacists with feedback to the physicians, drug treatment discussion with patients at discharge and medication reports Training: no educational intervention was specified Timing of intervention: during inpatient stay Quote: “In the intervention group, patients were treated by the same physicians/nurses and the following additional interventions were performed by one of three pharmacists (LB, EO or JK): ‐ Continuous medication reviews including oral feedback on prescribing to physicians; ‐ Drug treatment discussion with the patient at discharge; ‐ A medication report, given to the patient at discharge and sent to the patient’s GP (the regular discharge summary was sent to the patient’s GP independent of the study). Data on prescribing were obtained from the medical records. No medication history was taken by the pharmacists. Medication reviews were performed with a computer support system (MiniQ), which identified potentially inappropriate prescribings according to the three drug‐specific quality indicators (PIPs) analysed in the present study, established by the Swedish National Board of Health and Welfare for evaluation of drug therapy in the elderly: ‐ Drugs that should be avoided in the elderly: for example long‐acting benzodiazepines and drugs with anticholinergic action. ‐ Three or more psychotropic drugs: that is antipsychotics, anxiolytics, hypnotic‐sedatives and antidepressants. ‐ Potentially serious drug‐drug interactions: category D according to the pharmaceutical specialities in Sweden (FASS), that is, drug combinations that should be avoided” Patients in the control group received normal care |
|
| Outcomes | Drug‐specific quality indicators of potentially inappropriate prescribing (PIPs) ‐ the Swedish National Board of Health and Welfare for evaluation of drug therapy in the elderly; measured at admission to hospital and at discharge Quality of life (EQ‐5D); measured at baseline and 6 months later |
|
| Notes | NCT0106301 Funding: the study was supported by the Swedish National Board of Health and Welfare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: “Sequentially numbered, sealed envelopes were opened after participant details were written and transferred to the assignment card via a carbon paper inside the envelope” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Protection against contamination | High risk | Patients in the intervention and control groups were treated in the same wards by the same physicians. |
Blum 2021.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Setting: inpatient wards within university‐based hospitals in major cities in 4 European countries (Switzerland, Netherlands, Belgium and Republic of Ireland) Unit of allocation/analysis: cluster defined at level of attending hospital doctor Follow‐up: 12 months Duration: participation in study ‐ 12 months Providers: doctor and pharmacist working with hospital doctors |
|
| Participants | 2008 randomised 54 clusters in intervention group, median cluster size (interquartile range) 16.5 (10.0 to 23.8) 56 clusters in control group, median cluster size 16.0 (11.8 to 25.2) Age < 80 years: intervention 521 (54%), control 557 (53%) 80 years or over: intervention 442 (46%), control 488 (47%) Median age 79 years (interquartile range 74 to w84 years) Sex: women: 898 (44.7%) Race: not given Median no. of comorbidities: intervention: 11 (interquartile range 8 to 16). control: 10 (8 to 15) Cluster speciality type: Medical: intervention 764 (79.3%), control 825 (78.9%) Surgical: intervention 199 (20.7), control 220 (21.1) Trial site: Bern, Switzerland: intervention 446 (46%), control 376 (36) Cork: intervention 138 (14), control 208 (20) Louvain, Belgium: intervention 150 (16), control 238 (23) Utrecht, Netherlands: intervention 229 (24), control 223 (21) Median number of drugs: intervention 10 (interquartile range 7 to 13), control 9 (7 to 12) |
|
| Interventions | Model of care: structured drug review using a decision support system Timing: unclear but the first consultation was to complete a questionnaire and the second was to discuss planned medication changes. These were at the beginning of the study and before the first follow‐up point (at 2 months). Follow‐up data were collected through telephone interviews with the participants or their proxies at 2, 6 and 12 months post‐randomisation. The intervention was performed at individual patient level and consisted of a structured drug review using STRIP, a process developed to support pharmacotherapy optimisation in older patients. STRIP combines the STOPP/START criteria to detect drug overuse and underuse with implicit drug appropriateness assessment methods, such as structured questions on drug history, treatment adherence, adverse drug reactions and shared decision‐making with the patient on proposed changes to medication. STRIPA is a decision support system that takes into account clinically relevant interactions, dose adjustment according to renal function and predictable adverse drug effects. The STRIP Assistant (STRIPA) version 2.0 is a stand‐alone, web‐based software tool that was used to perform a pharmaceutical analysis, an important step of the STRIP process. Data on diagnoses and current drug use (collected via SHiM and the actual medical record), recent measurements and laboratory values (e.g. renal function, blood pressure) and possible adverse drug reactions, as listed in the patient’s medical record and according to patient information (SHiM) were entered in STRIPA. The assignment of drugs to diseases has been implemented through a drag and drop mechanism (see Methods appendix Figure). START A1 and START A2 were merged to one and STOPP A2 could not be converted into an algorithm, leaving a total of 79 STOPP and 33 START algorithms implemented into the clinical decision support system. Based on these data, pharmacotherapy optimisation signals were generated by the clinical decision support software and evaluated for appropriateness at the individual patient level by the research physician and pharmacist. Preadmission drug use was assessed with the Structured History taking of Medication (SHiM) questionnaire and entered into STRIPA along with the patient’s current diagnoses and relevant laboratory values. A trained research doctor and pharmacist jointly performed the STRIP drug review and generated patient‐specific prescribing recommendations based on STOPP/START criteria, with possible adaptations after discussion with the attending hospital doctor and the patient to take patient preferences into account. After considering additional in‐hospital clinical information (e.g. new diagnoses, history of adverse drug reactions), a final report was sent to the patient’s GP with further recommendations that could not be implemented during the index hospital admission. Blinded team members collected follow‐up and collected outcome data through telephone interviews with the participants or their proxies at 2, 6 and 12 months post‐randomisation. When a hospital admission (at the index hospital or any other hospital) was identified, a second unblinded team gathered data on the hospital admission and concealed all information identifying the intervention allocation before sending it to the adjudication team. The control group received usual care that could include unstructured drug review by the attending hospital doctors, which was not specifically encouraged or discussed. Usual care was performed according to the site‐specific standards of care that did not include application of STOPP/START criteria or STRIP. To mimic the intervention for blinding purposes of the participants and team members, the intervention team administered a sham intervention to all participants through completion of the Morisky medication adherence measure questionnaire (MMAS‐8). |
|
| Outcomes | Drug misuse (PIM); measured at 2 months Drug overuse (PIM); measured at 2 months Drug underuse (PPO) no. long term prescription drugs; measured at 2 months No. of STOPP/START recommendations by STRIP method made to attending hospital doctors and implemented at 2 months First drug‐related hospital admission after discharge following the index hospital admission within 12 months of enrolment First hospital admission; within 12 months First preventable drug‐related hospital admission; within 12 months First drug‐related hospital admission in patients with 1 or more STOPP recommendation implemented after 2 months All‐cause mortality; within 12 months Cancer mortality; within 12 months First fall; within 12 months In‐hospital death; within 2 months Quality of life, EQ‐5D‐VAS pain/discomfort score, EQ‐5D; measured at 2, 6 and 12 months Activities of daily living, Barthel Index; measured at 2, 6 and 12 months Drug compliance, MMAS‐8; measured at 2 and 12 months Clinically significant drug‐drug interactions, assessed using a validated consensus‐based list of 66 drug‐drug interaction criteria (Anyrs et al 2021); measured at 2 months Recommendations implemented at 2 months |
|
| Notes | Funding: This work is part of the project OPERAM: OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634238, and by the Swiss State Secretariat for Education, Research, and Innovation (SERI) under contract number 15.0137. The opinions expressed herein are those of the authors and do not necessarily reflect the official views of the European Commission and the Swiss government. This project was also partially funded by the Swiss National Scientific Foundation (SNSF 320030_188549). The funder of the study had no role in the study design; data collection, analysis and interpretation; or writing of the report. MR and ST had full access to all the data in the study, and all authors had final responsibility for the decision to submit for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how the sequence was generated. |
| Allocation concealment (selection bias) | Low risk | A person blinded to the allocation of recruiting clusters screened and enrolled patients in order to avoid selection bias. Coded information (gender, age, multimorbidity, degree of polypharmacy and so on) from all screened patients was collected and regularly monitored centrally to assess the risk of selection bias. The blinded person worked separately from the rest of the trial team at that site and all team members signed a non‐disclosure form to limit unblinding of this person. Further, the recruitment team, the teams conducting follow‐up telephone calls and the adjudication teams consisting of pharmacists and doctors were fully blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants, hospital doctors and general practitioners were partially blinded and received only general information on the trial without specific details about the intervention. Control patients received a sham intervention. The intervention team consisted of a doctor and a pharmacist – neither was blinded to enable direct interactions with both the attending hospital doctors and the participants. Each cluster‐defining hospital doctor was instructed to keep trial arm allocations confidential. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded team members collected follow‐up and outcome data. When a hospital admission was identified, a second unblinded team gathered data on hospital admission and concealed all information identifying the intervention allocation before sending it to the adjudication team (pharmacists and doctors). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data complete. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported with relevant data. |
| Protection against contamination | Low risk | Cluster randomisation was at the doctor and not hospital level, and authors recognised that potential for contamination in control clusters could not be completely ruled out. However, doctors were independent in the treatment decisions on their units and were instructed to keep trial arm allocations confidential by not to sharing information with their doctor or pharmacist colleagues. Cluster defining hospital doctors worked on separate hospital units and were autonomous in their treatment decisions, further minimising contamination between clusters. |
Boersma 2019.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial. Setting: outpatient clinic of hospital in Utrecht, the Netherlands Unit of allocation/analysis: cluster defined at level of attending resident doctor Follow‐up: 12 months Duration: participation in study ‐ 12 months Providers: resident doctors working at the geriatric outpatient clinic at the University Medical Centre Utrecht; 3 residents took part as research physicians; supervisors of the residents; nurses |
|
| Participants | 34 resident doctors were randomised and the allocation of doctors determined the allocation of patients. The intervention group had 96 patients and the control group 74 patients. Mean age: intervention group: 77.8 years ± 5.7; control: 79.0 ± 6.0 Sex: intervention: 34 male (52.3%); control: 30 (50.8%) Race: not given CCI (Charlson Comorbidity Index), median (interquartile range): intervention: 3 (0 to 9); control: 3 (0 to 10) Total number of medications used per patient (median) = 9 intervention group; 9 control group |
|
| Interventions | Model of care: prescribing recommendations made by independent physician based on STRIP Assistant, a clinical decision support system. Timing: not clear. The prescribing recommendations were made before the patient’s preoperative assessment and implemented at the resident’s discretion. Secondary outcomes were prescribing appropriateness according to STOPP/START criteria version 2, 3‐month and 1‐year postoperative mortality rates and 3‐month changes in MMSE, Katz‐ADL and Fried criteria. The intervention consisted of written prescribing recommendations prepared by an independent, clinically experienced research physician using the STRIP Assistant. The input data consisted of medication use (as reported by the SHiM use (Structured History taking of Medication use)), age, sex, medical history, current medical problems, blood pressure, pulse and estimated glomerular filtration rate. Prescribing recommendations were based on PPOs, PIMs and suboptimal dosages identified by STRIP Assistant and the research physician. The recommendations were given to the resident before the comprehensive geriatric assessment. Whether these recommendations were implemented, either by direct changes to the medication regimen or by recommendations forwarded to the surgeon or general practitioner, was at the resident's discretion. For patients receiving usual care, a pharmacy assistant took the SHiM prior to the comprehensive geriatric assessment. Findings were recorded in the patient's electronic medical record. The standard comprehensive geriatric assessment, performed by a resident and supported by a nurse, provided information about smoking habits and alcohol use, the Charlson Comorbidity Index, 15‐point Katz Index of Independence in Activities of Daily Living (Katz‐ADL), and Mini‐Mental State Examination (MMSE). The resident also reviewed the patients' medication. Any medication changes made by the resident (direct changes as well as recommendations to the surgeon or general practitioner regarding the medication regimen) were registered in the medical record. |
|
| Outcomes | PPOs (no. of implemented medication changes per patient made by a resident during the comprehensive geriatric assessment) PIMs (no. of implemented medication changes per patient made by a resident during the comprehensive geriatric assessment) Number of implemented medication changes per patient made by a resident during the comprehensive geriatric assessment: suboptimal dosages identified by the research physician using the STRIP Assistant (which uses STOPP/START) Prescribing appropriateness (no. of PPOs and PIMs before and after intervention/usual care identified using STOPP/START) Mortality; measured at 3 months and 1 year Mini‐Mental State Exam; measured at 3 months Katz‐ADL (activities of daily living); measured at 3 months Fried criteria (not analysed due to missing data); measured at 3 months |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator randomly assigned the residents to the intervention group (even numbers) and the control group (odd numbers) in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Unclear who performed/generated the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the intervention, resident doctors and research physicians generating the prescribing recommendations could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Research physicians not blinded. Supervisors of residents and nurses who gathered information about comorbidity, cognitive function and functional status were blinded to the intervention. Residents from the intervention group were asked not to discuss the prescribing recommendations they received with colleagues to prevent contamination of the control group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Owing to missing data, the difference in the secondary outcomes MMSE (62.9% missing), Katz‐ADL (28.2% missing), Fried criteria (24.2% missing) between baseline and 3 months postoperatively, and 1 year postoperative mortality (47.9% missing), could not be analysed. |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported, or mentioned if not fully reported. It does not appear that any were intentionally left out. |
| Protection against contamination | Low risk | Residents from the intervention group were asked not to discuss the prescribing recommendations they received with colleagues to prevent contamination of the control group. |
Bucci 2003.
| Study characteristics | ||
| Methods | Study design: randomised trial (block design, using a computerised randomisation scheme) Setting: outpatient clinic at Toronto General Hospital, Canada Unit of allocation/analysis: participant Follow‐up: 1 month after intervention Duration: unclear Providers: pharmacists |
|
| Participants | 80 participants (39 intervention and 41 control) Focus on polypharmacy: mean number of medications at baseline 7.6 intervention, 6.0 control Age (mean): 56.4 years intervention, 60.2 years control Male: 78.9% intervention, 78% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care pharmacists: worked as part of a multi‐disciplinary team in outpatient clinics, the pharmacist(s) conducted an independent medication review together with participants during a face‐to‐face encounter, which was discussed with the multi‐disciplinary team members Training: education was provided to prescribers and other healthcare professionals included in the multi‐disciplinary team Timing of intervention: at hospital discharge Quote: “The intervention involved receipt of pharmacist services, that is, functioning as part of a healthcare team, meeting participants' drug‐related needs and ensuring continuity of care. Specifically, this involved the pharmacist reviewing the appropriateness of drug therapy, making recommendations for change and providing information about medications, their administration and their adverse effects Those randomly assigned to the non‐intervention group received usual care from other clinic staff” |
|
| Outcomes | Appropriateness of prescribing was determined by preintervention and postintervention mean MAI scores; measured at baseline and 1 month Number of medications; measured at baseline and 1 month |
|
| Notes | Quote: “The participant chart was reviewed by a research assistant pharmacist who was blinded to the intervention, and information required to assess the appropriateness of medications was abstracted. A summated MAI score was determined for each participant at least 1 month after the intervention. Follow‐up took place at a scheduled clinic visit or by telephone.” Funding: the research was supported by a Research and Education Grant from the Canadian Society of Hospital Pharmacists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Using a computerised randomisation scheme” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The research assistant was blinded to the intervention. Patient charts were reviewed by the research assistant, blinded to the intervention, and information to assess the appropriateness of medications was abstracted” Unclear if staff or patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patient outcomes were assessed by the research assistant (blinded to the intervention) at baseline and at follow‐up” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the intervention group had died at follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination | High risk | Quote: “The presence of the pharmacist in the clinic may have contaminated medication appropriateness results of the non‐intervention group” |
Campins 2017.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: participants living in the communities of Mataro and Argentona, large towns in the province of Barcelona, Spain Unit of allocation/analysis: participant Follow‐up: 1 year Duration: unclear Providers: clinical pharmacist |
|
| Participants | 503 older patients (252 intervention and 251 control) Focus on polypharmacy: included participants taking 8 or more medications Age (mean ± SD): 79.16 ± 5.5 years intervention, 78.78 ± 5.5 years control Male: 39.7% intervention, 42.6% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: clinical pharmacist evaluated all drugs prescribed to each patient using the GP–GP algorithm, which were discussed with the patient’s physician Training: no educational intervention was specified Timing of intervention: during a single GP visit Quote: “The intervention consisted of 3 consecutive phases. First, a trained and experienced clinical pharmacist evaluated all drugs prescribed to each patient using the GP–GP algorithm and basing their decision about appropriateness on the STOPP/START criteria. Second, the pharmacist discussed recommendations for each drug with the patient’s physician in order to come up with a final set of recommendations. Drug assessment was conducted in all cases by the same clinical pharmacist (IG). Finally, these recommendations were discussed with the patient, and a final decision was agreed by physicians and their patients in a face‐to‐face visit. All changes in prescribed medication were registered in the electronic clinical notes and in the study’s record form. The goal of the study intervention was to improve current prescription medication in community‐dwelling elderly persons in our setting and so improve routine clinical practice. Control group patients followed the usual treatments and control procedures of their physicians” |
|
| Outcomes | Drug appropriateness (STOPP/START criteria); measured at baseline, 3, 6 and 12 months Hospitalisations; measured at 3, 6 and 12 months Quality of life (EQ‐5D); measured at baseline, 3 months and 6 months Adherence (Morisky‐Green); measured at baseline, 3 months and 6 months |
|
| Notes | Funding: this project was funded by a grant from the Spanish Ministry of Health (Independent Health Research Ref. EC11‐313) and a grant from the Catalan Government Health Service (SLT/682/2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “One‐to‐one assignment was based on a list of random numbers generated by a statistical program” |
| Allocation concealment (selection bias) | Low risk | Quote: “Each family physician received 10 sealed, opaque envelopes with identification numbers (assigned consecutively in strict chronological order of recruitment) on the back. Each envelope contained a card with the same identification number and the intervention group to which the subject was assigned” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Open‐label trial; physicians aware of patients’ allocation to intervention and control groups” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The results were not evaluated blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differences in losses to follow‐up between intervention and control group |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but all outcomes outlined in the methods section are analysed and reported |
| Protection against contamination | High risk | Quote: “A second limitation is possible intervention‐to‐control contagion, given that the prescribing physicians who received recommendations from the pharmacist regarding intervention group patients also had patients in the control group. The control group could thus have indirectly benefited from the intervention, thereby diluting—but not increasing—the effect of the intervention study” |
Clyne 2015.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: GP practices in greater Dublin; most classified as urban and a small number as 'mixed'. Control group practices were in more socioeconomically deprived areas. Unit of allocation: GP practices Unit of analysis: participant Follow‐up: unclear Duration: unclear Providers: GPs and pharmacist |
|
| Participants | 196 patients from 12 GP practices within the greater Dublin area were invited to participate by email with a follow‐up telephone call. Practices were eligible if they had at least 80 patients aged 70 years or older and were based in greater Dublin. Consenting practices were instructed to randomly select 50 patients from this age group with capacity to provide informed consent. Focus on polypharmacy: number of repeat medications, mean (SD), 10.2 (4.5) intervention, 9.5 (4.1) control Age (mean): 77.1 (4.9) years intervention, 76.4 (4.8) years control Male: 55.6% intervention, 51.5 control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: medication review provided by the GP Training: education in the form of academic detailing with the pharmacist was provided to GPs; patients also received information leaflets during the medicine reviews Timing of intervention: during a single GP visit Quote: “Intervention participants received a complex, multifaceted intervention incorporating academic detailing; review of medicines with web‐based pharmaceutical treatment algorithms that provide recommended alternative‐treatment options; and tailored patient information leaflets The multifaceted intervention involved academic detailing with a pharmacist on how GPs can review medicines with participating patients; the medicine reviews were supported by web‐based pharmaceutical treatment algorithms for GPs that provided evidence based alternative treatment options to PIP drugs, and tailored patient information leaflets Control practices delivered usual care and received simple, patient‐level PIP feedback” |
|
| Outcomes | The proportion of patients with potentially inappropriate prescriptions; measured at baseline and intervention completion, which was 4 to 6 months after baseline The mean number of potentially inappropriate prescriptions based on STOPP criteria; measured at baseline and intervention completion, which was 4 to 6 months after baseline |
|
| Notes | Funding: this study is independent research, funded by the Health Research Board (HRB) PhD Scholars Programme in Health Services Research under grant PHD/2007/16 and the HRB Centre for Primary Care Research under grant HRC/2007/1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Practices were allocated to intervention and control groups by an independent researcher using minimisation” |
| Allocation concealment (selection bias) | Low risk | Quote: “Selection bias was minimized by collecting baseline data before minimization, which was carried out by an independent third party” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Patients and GPs were not blinded to allocations” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Outcome assessor was blinded to allocations” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No practices lost to follow‐up and losses of patients within intervention and control arms were equal (6 patients in each arm). Analyses were performed according to ITT. |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. |
| Protection against contamination | Low risk | Quote: “A cluster design was chosen to avoid the possibility of contamination across arms” |
Coronado‐Vazquez 2019.
| Study characteristics | ||
| Methods | Study design: quasi‐randomised, multicentre, controlled trial Setting: health centres in Aragon and Andalusia, Spain (could be rural or urban; not clear) Unit of allocation/analysis: individual physician; patients not randomised Follow‐up: after 6 months Duration: unclear Providers: family physicians and nurses from health centres were recruited for the study and were providers of the decision support tool. |
|
| Participants | 22 physicians were randomised. Those randomised to the intervention group selected 61 patients; those randomised to the control group selected 68 patients. Patients were not randomised. Age: details not given for physicians. Patients: intervention group mean age 78.9 (SD 0.94); control group 79.9 (0.73). Sex: Patients: intervention group: female n = 38 (66.7%), male 19 (33.3%). Control group: female 40 (61.5), male 25 (38.5). Race: not given Co‐morbidities: hypertension: intervention 52 (91.2%), control 58 (89.2); diabetes: intervention 25 (43.9), control 24 (36.9); renal failure: intervention 12 (21.1), control 9 (13.8); liver failure: intervention 2 (3.5), control 0 The mean number of chronically prescribed drugs was 9 (SD 2.9). |
|
| Interventions | Model of care: to determine the effectiveness of a shared decision‐making intervention for medication appropriateness in patients with chronic diseases and polypharmacy. Timing: patients were seen twice – initially and 6 months later. The duration of each consultation is not clear. Physicians who participated in the intervention group received information on the designed DST, as well as a link to a video about the shared decision‐making process, which was available on the web. Once the patients were selected, the family physicians reported the inappropriateness found in their medication according to the Beers and START/STOPP criteria, and the possible alternatives they had, agreeing on the changes to be made in the treatment through a deliberative process. The selected patients were assessed by family physicians to verify that they met the inclusion criteria. They were informed about the study and gave informed consent to participate in it. From the electronic medical record, the personal data and medical record of the patients were collected, including the updated treatment. The nurses performed a functional, mental and social assessment, also determining the level of adherence to the treatment. The family physician analysed the adequacy of the treatment for each patient following the Beers, START and STOPP criteria through the data collected in the electronic medical records, which were verified during the visit. The patients were summoned to the doctor’s office to carry out the medication appropriateness survey using the DST in the intervention group or the usual clinical practice in the control group. After 6 months, the family physician contacted the participants again, either through regular visits to follow up chronic patients or by telephone. In this second meeting with the family physician, the medication was re‐checked, making a new intervention in case of inappropriate medications. In the control group, family physicians discussed with patients the inappropriate medications. No DST was used in this group, and care was not standardised. |
|
| Outcomes | Medications withdrawn at first consultation and 6 months (difference in groups regarding medication appropriateness, in particular the withdrawal of drugs according to STOPP/Beers) Medications started at first consultation (START) Total withdrawn and started drugs (STOPP, Beers and START) Medication appropriateness (proportion of patients whose medication was adapted after 6 months of follow‐up. Subgroups: sex, adherence to treatment, treatment with BDZ, treatment with NSAIDs, and level of education). |
|
| Notes | Funding: this project received the Esteve Grant for Health‐Related Innovation and Health Care for the Chronic Patient in its 6th edition. Contact with authors: we contacted the authors to ascertain the time point of data presented in Table 2. No response was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A block randomisation procedure was carried out to ensure the equal size of the groups, however it is not clear how the randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not clear. Quote: "Physicians who agreed to participate in the study were randomised and assigned to the intervention or the control group. A block‐randomisation procedure was carried out to ensure the equal size of the groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not possible because randomised physicians were delivering the intervention or usual care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Seems to have been done by the patients’ own doctors who also implemented the intervention, therefore blinding was not possible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all outcome data were complete, such as those reported in Table 2 (page 7). The percentages were calculated from the overall number of patients included in the study, not from number in the intervention group or control group. We contacted the authors to ascertain the time point of data presented in Table 2. No response was received. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Protection against contamination | Low risk | The authors stated that because the intervention affects professionals’ way of communicating, to avoid contamination no randomisation of patients was done. |
Crotty 2004a.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: care facilities for the elderly in Adelaide, a major city in Australia Unit of allocation: 10 residential facilities Unit of analysis: participant Follow‐up: 3 months Duration: 2 case conferences 6 to 12 weeks apart Providers: resident's GP, geriatrician, pharmacist, home care staff and Alzheimer's Society representative |
|
| Participants | 154 residents (100 intervention and internal control and 54 external control) Focus on polypharmacy: residents were prescribed more than 5 medications Age (mean): 85.3 years (95% CI 84.0 to 86.6) intervention, 83.6 years (95% CI 81.3 to 85.9) external control Male: 44% intervention, 43% external control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: the pharmacist conducted an independent medication review using participant notes, which were then discussed with a multi‐disciplinary team during case conferences Training: education (provided by the Alzheimer’s Association of South Australia) in the form of a training workshop was provided to all members of the multi‐disciplinary team Timing of intervention: during a single nursing home visit Quote: “A medication review was conducted before a multi‐disciplinary case conference. The resident's GP, a geriatrician, a pharmacist, carers and a representative from the Alzheimer's Association of South Australia attended the case conferences, which were held at the nursing home. At the case conference, care staff expanded on any issues in the case notes that required discussion, and the Alzheimer's representative discussed non‐pharmacological management of dementia‐related behaviour. A problem list was developed by the GP in collaboration with the care staff. A half‐day training workshop examining use of a toolkit in the management of challenging behaviours was provided to all facilities in the study, including control facilities” |
|
| Outcomes | Medication appropriateness was assessed using the MAI. Change in MAI was reported. All residents had their medication charts reviewed before and after the intervention by an independent pharmacist. Measured at baseline and 3 months. The Nursing Home Behaviour Problem Scale (NHBPS) was used to assess the effect of the intervention on residents' behaviour. Measured at baseline and 3 months. Monthly drug costs for all regular medications on the government's pharmaceutical benefits scheme were calculated for all residents in the intervention and control groups. |
|
| Notes | Funding: Quality Use of Medicines Evaluation Program 2000‐2001, Health and Aged Care, General Practice National Innovations Funding Pool 1999‐2000, Health and Aged Care. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer‐generated random numbers were used by a researcher independent of investigators” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomly allocated by the pharmacy department using sequential sealed opaque envelopes to receive the case conferences” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Those lost to follow‐up were described, and an ITT analysis was used. |
| Selective reporting (reporting bias) | Low risk | The impact of case conferences on appropriateness of medication and participant behaviours were stated as the objectives. |
| Protection against contamination | Low risk | No evidence was found of a carry‐over effect to other residents within the facilities. |
Crotty 2004b.
| Study characteristics | ||
| Methods | Study design: single‐blind randomised trial Setting: metropolitan hospitals in Adelaide, Australia Unit of allocation/analysis: participants Follow‐up: at 8 weeks Duration: unclear Providers: transition co‐ordinator pharmacist, nurses |
|
| Participants | 110 (56 intervention and 54 control) eligible patients making first‐time transition from a hospital to 1 of 85 long‐term residential care facilities. Patients were eligible if they or their carer gave consent and they had a life expectancy > 1 month. Focus on polypharmacy: the number of preadmission medicines was 6.6 intervention group and 7.7 control group Age (mean): 82 years (95% CI 80.2 to 83.7) intervention, 83.4 years (95% CI 81.7 to 85.1) control Female: 58.9% intervention, 63% control Ethnicity: non‐English speaking background: 8.9% intervention, 5.6% control |
|
| Interventions | Model of pharmaceutical care: the pharmacist conducted an independent medication review using participant notes, which was then discussed with a multi‐disciplinary team during case conferences Training: education was provided to all members of the multi‐disciplinary team Timing of intervention: during hospital discharge to a nursing home Quote: “The intervention focused on transferring information on medications to care providers in long‐term care facilities (first‐time transition). When discharged from hospital to long‐term care facilities, participants' family physicians and community pharmacists were faxed a medication transfer summary compiled by the transition pharmacist. After transfer, the transition pharmacist co‐ordinated an evidence‐based medication review that was conducted by community pharmacists within 10 to 14 days of transfer. A case conference that involved the transition co‐coordinator, the family physician, the community pharmacist and the nurse was held within 14 to 28 days of transfer. Usual hospital discharge process was received by controls and included a standard hospital discharge summary.” |
|
| Outcomes | MAI score; measured at baseline and 8 weeks Secondary outcome measures were adverse events including unplanned visits to the emergency department or hospital readmissions, ADEs, falls, worsening of mobility, behaviours, pain and increasing confusion; measured at 8 weeks |
|
| Notes | Funding: the project was funded by a grant from the Australian Commonwealth Department of Health and Ageing National Demonstration Hospitals Program, Phase 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer‐generated allocation sequence that used block randomisation” |
| Allocation concealment (selection bias) | Low risk | Quote: “Centralised hospital pharmacy service used for randomisation” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Independent pharmacists who were blinded to study group allocation assessed patients' medication charts and case notes” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants in the intervention group and 10 in the control group died or did not complete the study for other reasons. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Protection against contamination | High risk | Quote: “The transition pharmacist also co‐ordinated a case conference involving himself or herself, the family physician, the community pharmacist and a registered nurse at the facility within 14 to 28 days of the transfer. At this case conference, the transition pharmacist provided information concerning medication usage and appropriateness” |
Curtin 2020.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Setting: hospitals in Cork city, Ireland Unit of allocation/analysis: participant Follow‐up: 3 months Duration: between 27 March 2018 and 3 April 2019 patients were randomised; planned closure date was 30 June Providers: research physician (experienced specialist registrar in geriatrics) communicated with patients’ physicians. Qualifications etc. not clear for attending physicians who implemented intervention. |
|
| Participants | 130 were randomised: 65 to the intervention arm and 65 to the control arm Age: intervention 84.49 (SD 5.60); control 85.68 (SD 5.87) Sex: intervention: female 42 (64.61%); control: female 38 (58.46%) Race: not given Charlson Comorbidity Index score: intervention: 6.8 (SD 2.31); control: 6.33 (SD 1.86) Disease: control n (%); intervention n (%): Dementia: 48 (73.8%); 49 (75.4%) Heart failure: 10 (15.4%); 16 (24.6%) Atrial fibrillation: 27 (41.5%); 24 (36.9%) Chronic kidney disease: 15 (23.1%); 16 (24.6%) Active cancer: 6 (9.2%); 5 (7.7%) Osteoporosis: 18 (27.7%); 19 (29.2%) Drugs: no. of regular medications (mean): intervention: 11.52; control: 10.89 |
|
| Interventions | Model of care: to examine the effect of applying the STOPPFrail, a recently developed deprescribing tool, to the medication regimens of older patients with advanced frailty Timing: the intervention was applied at a single time point during the patients’ hospital admission at the time of trial enrolment. It is not clear how long the intervention/mediation withdrawal plan took. From the protocol: "We will advise intervention group participants’ doctors that their patient has been randomised to the intervention group. The unblinded researcher (an experienced Specialist Registrar in geriatric medicine) will offer medication advice based on the STOPPfrail criteria. Where there is a risk of an adverse drug withdrawal event (table 1, 2), it will be recommended that that particular medication be withdrawn slowly according to an evidence based protocol (table 2). Prior to recommending the dose reduction or cessation of a medication, the unblinded researcher will inform the participant, NOK and nursing staff of potential adverse withdrawal effects associated with that drug. The nurse manager and medical team will have the mobile phone number of the primary researcher and will be encouraged to contact him if there are any concerns about adverse withdrawal effects in the participant. If the participant is discharged to a nursing home during the period of drug withdrawal, a written drug withdrawal plan will be sent to the nursing home director of nursing as well as to the medical officer overseeing the care of patients in that nursing home. The mobile phone number of the researcher will be included in this written plan and the primary researcher will be available to respond to queries or concerns." Intervention: STOPPFrail criteria were recently developed to assist clinicians with deprescribing decisions in older people approaching end of life. The criteria consist of 27 indicators that highlight instances of potentially inappropriate prescribing in this particular population of older patients. STOPPFrail‐guided deprescribing was shown to have substantial interrater reliability among clinicians of different specialities, and it may be a reasonable and potentially efficient alternative to a specialist medication review where this is unavailable. For participants randomised to the intervention arm, a medication withdrawal plan was devised by the research physician. The recommended medication withdrawal plan was communicated directly to one of the participant's attending physicians and also documented in the patient's medical record. Medications associated with an increased risk of an adverse withdrawal reaction were recommended to be withdrawn slowly according to a standardised trial withdrawal protocol (Supplemental Protocol S1). The attending physician judged whether or not to accept the drug withdrawal plan and implement the recommended changes. Because of the nature of the intervention, the research physician, attending physicians and participating patients could not be blinded to the intervention or control group assignment after randomisation. The intervention was applied at a single time point during the patients’ hospital admission at the time of trial enrolment. Attending physicians and nursing staff were encouraged to report any potential adverse consequences of deprescribing (adverse drug withdrawal events or disease relapse) to the research team. Control group patients received usual care |
|
| Outcomes | Measured at baseline and 3 months: No. of long‐term prescribed medications consumed by participants Prescriptions of neuroleptic antipsychotic medications QoL (QUALIDEM instrument) QoL (ICECAP‐O instrument) Measured at 3 months: Unscheduled medication reviews after discharge from acute hospital ED presentation Falls and non‐vertebral fractures Mortality Unplanned hospital admissions 28‐day cost of prescription medications |
|
| Notes | Funding: three of the authors are supported by the European Union's Horizon 2020 research and innovation program (grant number 634238). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to study arms in a 1:1 ratio using block randomisation. Block sizes of 4 and 6 were generated using the website randomization.com by an administrator external to the study. Randomisation was not stratified by hospital site. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed in sequentially numbered opaque envelopes until the research physician had enrolled participants, completed baseline data collection and identified deprescribing targets using the STOPPFrail criteria. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the intervention, the research physician, attending physicians and participating patients could not be blinded to the intervention or control group assignment after randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were collected by 3 research physicians (EJ, RD and MR) who were blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients randomised = 130 (65 in each group). Included in the primary analysis: 51 in intervention group; 47 in control group Intervention group: 12 deaths, 1 receiving end of life care, 1 withdrawal Control group: 18 deaths In the analysis of the primary outcome, only those patients who completed follow‐up were included. Deceased patients were excluded due to difficulties in determining final, valid, verifiable medication lists. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. Some secondary outcomes were reported as supplementary tables. |
| Protection against contamination | Unclear risk | Randomisation was not stratified by hospital site, however it is not clear if attending physicians could have introduced contamination if they were seeing patients in both intervention and control groups. Comment in discussion: “we did not use a cluster randomization design that would diminish the possibility of contamination bias. Physicians may have simultaneously had both intervention and control patients under their care during the trial and, through a “training effect,” they may have applied STOPPFrail criteria during medication reviews of control patients. However, any possible contamination of this kind would increase the chance of actual effects of the intervention not being detected (ie, type II error). In spite of the possible presence of contamination, significantly different effects of the STOPPFrail intervention were still observed between the groups.” |
Dalleur 2014.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: hospital in city of Brussels, Belgium Unit of allocation/analysis: participant Follow‐up: at discharge and 1 year after discharge Duration: unclear Provider: inpatient geriatric consultation team (IGCT) |
|
| Participants | Quote: “146 (74 intervention and 72 control) frail patients ≥ 75 years of age admitted to Cliniques Universitaires Saint‐Luc, a 975‐bed teaching hospital in Brussels, Belgium.” Focus on polypharmacy: mean number of medications at baseline: 7.2 intervention, 7.3 control Age (median (IQR)): 84 years (IQR 81 to 87) intervention, 86 years (IQR 81 to 89) control Female: 58.1% intervention, 68.1% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: participants' medication lists were screened by a geriatrician Training: unclear if training was provided as part of the intervention Timing of intervention: during inpatient stay Quote: "In the intervention group, geriatricians used 64 STOPP criteria (‘Duplicate drug classes’ was not considered) to systematically screen the list of medications being taken by participants on admission for potentially inappropriate medications and provided oral and written recommendations to the ward physician during hospitalisation for discontinuation of potentially inappropriate medications. Participants also received standard IGCT care." "Participants in the control group received standard care from the IGCT. Control participants' medications were routinely reviewed by the IGCT geriatrician, using an implicit approach (i.e. no explicit tool was used).” |
|
| Outcomes | Number of PIMs on admission to hospital and at discharge Clinical significance of STOPP‐related recommendations ‐ patients with 1 PIM at baseline were followed up 1 year later |
|
| Notes | Funding: O Dalleur was funded by the Federal Public Service Health of the Belgian government as part of a national project on the implementation of clinical pharmacy in hospitals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Eligible participants were allocated by the IGCT nurse to control or intervention group by drawing of lots—Insufficient information to permit judgement” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “IGCT nurse assigned each participant to the geriatrician who had been allocated to the intended group after randomisation—insufficient information on nurse’s involvement in IGCT to permit judgement of yes/no” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “The attending ward physician (who was responsible for prescriptions during hospitalisation and at discharge), the evaluator and participants were blinded to group assignment. However, the IGCT nurse was not blinded, and insufficient information was provided on nurses’ involvement in the IGCT to permit judgement” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The IGCT nurse provided the evaluator with a list of the patients included in the study, which did not specify allocation group. The evaluator gathered data on the primary outcome” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants in the intervention group and 9 in the control group were not included in the primary outcome assessment because they did not receive the allocated intervention, or because data were missing from their discharge letters. Subset of participants in each group was assessed at 1‐year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Characteristics associated with discontinuation of potentially inappropriate medications at discharge were listed as a secondary outcome measure but were not clearly reported in the results. |
| Protection against contamination | Unclear risk | Quote: “To avoid contamination bias, 2 of the 4 geriatricians involved in the IGCT during the study period were allocated to the intervention group because they used the STOPP criteria in their current practice; the other 2, who had never worked with the STOPP criteria, were allocated to the control group. However, this was a single‐site study; therefore the possibility of contamination bias cannot be ruled out” |
Franchi 2016.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: hospitals in Italy (unclear if urban and/or rural) Unit of allocation: hospital wards Unit of analysis: participant Follow‐up: 12 months post‐discharge Duration: unclear Providers: physicians |
|
| Participants | 697 patients (347 in the intervention and 350 in the control arms) were enrolled Focus on polypharmacy: mean number of drugs, 6.3 (3.3) intervention, 5.7 (3.1) control, subpopulation of patients on polypharmacy Age (mean): 83.7 (± 5.9) years intervention, 83.8 (± 5.6) years control Male: 40.9% intervention, 43.7% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: pharmacists worked as part of inpatient services on hospital wards as a clinical pharmacy service Training: education in the form of e‐learning was provided to all clinicians Timing of intervention: during inpatient stay Quote: “E‐learning platform. E‐learning was delivered through an interactive web‐based platform Contents of e‐learning for the intervention arm. The program delivered to clinicians on the wards randomly assigned to the intervention arm included notions of CGA and geriatric pharmacology, together with training for the use of a third generation assessment instrument (InterRAI Acute Care). The course on geriatric pharmacology was structured in three main areas and five modules as follows: Area 1: main concepts of CGA (Module A); Area 2: general geriatric pharmacology notions (Module B); Area 3: prescription appropriateness and related issues in older adults: (a) assessment and management of patients exposed to polypharmacy (Module C); (b) criteria and tools for the revision and evaluation of prescription appropriateness in older people, such as Beers Criteria, Screening Tool of Older Person’s Prescriptions (STOPP), Assessing Care of the Vulnerable Elderly (ACOVE), Inappropriate Prescribing in the Elderly Tool (IPET) and the Medication Appropriateness Index (MAI) (Module D); (c) criteria and tools to evaluate potential drug–drug interactions (Module E) The access to and utilization of each teaching module was linked to a self‐evaluation test and to specific centralized controls. Each module was divided in four sub‐modules that each participant completed with specific case reports and questions. The INTERcheck® software, a computerized prescription support system, was made available to clinicians in the intervention arm through the interactive web‐based platform, separately from the electronic clinical report form Contents of e‐learning for the control arm. The e‐learning program for clinicians of the control arm consisted only of a refresher on the basic notions of geriatric pharmacology using Module B as a weapon. The e‐learning program for clinicians of the control arm consisted only of a refresher on the basic notions of geriatric pharmacology” |
|
| Outcomes | Reduction in the prescriptions at hospital discharge of PIMs (Beers criteria) Reduction of prescription of potential DDIs (PDDIs) or potentially severe DDIs Length of hospital stay, mortality and incidence of any re‐hospitalisation during the 12‐month follow‐up period |
|
| Notes | Funding: the ELICADHE Study was approved and financially supported by the Italian Medicines Agency (AIFA) according to the 2008 Italian Program for Independent Research (Project no. FARM87SA2B). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information was provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Single‐blind controlled study: participating clinicians were not blind to study aims and treatment allocation” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All investigators involved in data collection were blinded to arm allocation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up in intervention and control group were described, and both ITT analysis and per protocol analysis were used. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Protection against contamination | Unclear risk | Insufficient information was provided to permit judgement. |
Frankenthal 2014.
| Study characteristics | ||
| Methods | Study design: randomised trial (parallel‐group) Setting: chronic care geriatric facility in central Israel Unit of allocation/analysis: participant Follow‐up: 12 months Duration: 12 months Providers: chief physician and study pharmacist |
|
| Participants | Quote: “A chronic care geriatric facility in central Israel. The facility has 384 beds. 12 wards: five nursing departments for residents dependent in their activities of daily living (ADLs) with and without cognitive impairment (ADL‐dependent group), four departments for elderly adults independent in their ADLs but dependent in instrumental ADLs (e.g., use of telephone, shopping, food preparation, travel, housekeeping, handling finances (ADL‐independent group), and three departments for residents who are primarily cognitively impaired but are able to walk independently and therefore need special care to prevent them from getting lost (primarily cognitively impaired group)." Focus on polypharmacy: baseline number of medications, mean (SD): intervention n = 183, 8.8 (SD 3.4); control n = 176, 8.2 (SD 3) Age (mean): age, n (%): 65 to 74 years n = 29 (15.8); 75 to 84 years n = 63 (34.4); ≥ 85 n = 91 (49.7) intervention, 65 to 74 years n = 36 (20.5); 75 to 84 years n = 63 (35.8); ≥ 85 n = 77 (43.8) control Male: 29.5% intervention, 37.5 control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: medication reviews conducted by the study pharmacists, which were discussed with the chief physician Training: unclear if training was provided as part of the intervention Timing of intervention: during inpatient stay Quote: “The intervention consisted of a medication review by the study pharmacist for all residents at study opening and 6 and 12 months later. The STOPP/START criteria were applied to identify PIMs and PPOs. Interventional recommendations that the study pharmacist made for residents in the intervention group but not in the control group were discussed with the chief physician at study opening and after 6 months. The chief physician decided whether to accept these recommendations and implement prescribing changes. The control group received usual pharmaceutical care” |
|
| Outcomes | Proportion of potentially inappropriate prescriptions identified by STOPP; measured at baseline, 6 months and 12 months Proportion of PPOs identified by START; measured at baseline, 6 months and 12 months Quality of life (SF‐12), falls, hospitalisations; measured at baseline and 12 months |
|
| Notes | Funding: this work was supported partly by a research grant from Keshet Association for the Elderly in Tel‐Aviv‐Yaffo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: “A physician who was not part of the study randomized participants. Fixed stratified randomization was used to allocate residents to groups according to the three types of residents. Group allocation was concealed from the study pharmacist, and participants were assigned to one of the two groups using sealed envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The intervention consisted of a medication review by the study pharmacist for all residents at study opening and 6 and 12 months later. The study pharmacists and the chief physician were not blinded to group assignment” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Nurses who were unaware of participants’ group assignments assessed the outcome measures in the study population” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants lost to follow‐up in intervention and control group were described and similar across both groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Fried 2017.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: patients had upcoming appointments at a veterans affairs healthcare system in the city of New Haven, Connecticut Unit of allocation/analysis: participant Follow‐up: 3 months post discharge Duration: 3 months Providers: clinical pharmacist |
|
| Participants | 128 older patients (64 intervention and 64 control) Focus on polypharmacy: number of drugs on admission (± SD), 13.4 (± 5.2) intervention, 13.8 (± 4.8) control Age: mean age not reported; participants categorised according to age bands Male: 98.4% intervention, 98.4% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: clinician receives recommendations based on the information provided from the TRIM web tool Training: no educational intervention was specified Timing of intervention: during a single GP visit Quote: “The TRIM consists of two web applications. The first extracts information on medications and chronic conditions from the EHR. The second consists of three components. The first is an interface for data chart review and telephonic patient assessment. These data, along with the extracted EHR data, serve as inputs for the second component, a set of automated algorithms evaluating medication appropriateness. TRIM evaluates medication appropriateness based on a range of criteria, including feasibility in the context of the patient’s cognition and social support, potential overtreatment of DM or hypertension, “traditional” PIMs according to Beers and Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria, inappropriate renal dosing, and patient report of adverse medication effects. The algorithms generate the third component, a patient‐specific medication management feedback report for the clinician. This report includes a complete medication reconciliation, recommendations for discontinuation or dosage changes for inappropriate medications, and a recommendation regarding the need to simplify the regimen of patients with problems with adherence and poor social support. The report was e‐mailed to the clinician 24 hours before the primary care appointment and handed to the clinician just before the appointment. The algorithms also generate a simple, short report for the patient consisting of a listing of medication reconciliation discrepancies and reported problems with medications that is given to the patient just before the appointment with brief coaching on using it to discuss medication concerns with the clinician. The telephone assessments occurred within 3 days before their primary care appointment. The control group received usual care.” |
|
| Outcomes | Number of medications; measured at baseline and 90 days Potentially inappropriate medications (PIMs); assessed at baseline and 90 days Number of Tool to Reduce Inappropriate Medication (TRIM) recommendations implemented (TRIM evaluates medication appropriateness based on a range of criteria, including Beers and Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria); assessed at baseline and 90 days |
|
| Notes |
NCT02501967 Funding: this work was supported by Grant DF11–303 from the Donaghue Foundation; by the Claude D. Pepper Older Americans Independence Center, School of Medicine, Yale University, (#P30AG21342 NIH/NIA); and by National Institutes of Health Grant UL1 RR024139. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement: unclear who assessed patients' medications and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | The study trial registry page is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Gallagher 2011.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: Cork University Hospital, a hospital in the city of Cork, which serves an urban and rural population Unit of allocation/analysis: participant Follow‐up: 2 months, 4 months and 6 months post discharge Duration: unclear Provider: attending medical team |
|
| Participants | 382 hospital inpatients (190 intervention, 192 control) aged 65 years and older admitted to hospital via the emergency department under the care of a general medical physician Focus on polypharmacy: mean number of medications at baseline: 7.4 intervention, 8.0 control Age (median (IQR)): 74.5 years (71.0 to 80.0) intervention, 77.0 years (71.0 to 81.75) control Female: 53.2% intervention, 53.1% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: participants' medication lists were screened by the primary research physician; oral and written recommendations outlining appropriate prescribing changes were then provided to the attending physicians Training: unclear if any training was provided as part of the intervention Timing of intervention: during hospital admission Quote: “The primary research physician applied STOPP/START criteria to baseline data of participants in the intervention group on admission to identify potentially inappropriate prescriptions and prescribing omissions. These were immediately discussed with the attending medical team, and discussion was followed up by written communication within 24 hours. Intervention recommendations comprised simple statements highlighting potentially inappropriate prescriptions according to relevant STOPP/START criteria. The attending physician judged whether these recommendations should be accepted and prescribing changes implemented. Medication changes were included in the discharge summary to the intervention participants' general practitioners” |
|
| Outcomes | Prescribing appropriateness measured using the MAI, STOPP/START criteria and the AUM index; measured at admission to and discharge from hospital, and at 2, 4 and 6 months Mortality, hospital readmissions, falls, frequency of general practitioner visits; measured at 2, 4 and 6 months |
|
| Notes | Funding: the study was funded by the Health Research Board of Ireland, Clinical Research Training Fellowship number CRT/2006/029. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomly assigned to the intervention group or the control group using a randomisation sequence that was determined by an independently generated random‐numbers table using StatsDirect software, version 4.5” |
| Allocation concealment (selection bias) | Low risk | Quote: “The random‐numbers table was retained, independent of researchers, by a physician external to the study, who assigned participants to groups using a sealed‐envelope system. Group allocation was concealed from the research physician and from participants until baseline data had been collected and inclusion criteria verified” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The research physician, attending physician, and participating patients could not be blinded to group assignment after randomization because of the nature of the intervention” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “An interrater reliability analysis of outcome measurements was conducted to ensure that there was no bias toward more favourable ratings in the intervention group as compared to the control group. There was good interrater agreement between the primary researcher and the physician carrying out the blinded evaluation” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants (10 intervention, 8 control) died before the first outcome measure was assessed and were excluded from analysis; a further 24 participants (10 intervention, 14 control) died during the follow‐up period. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement; study conducted at a single hospital |
Garcia‐Gollarte 2014.
| Study characteristics | ||
| Methods | Study design: prospective, randomised, multicentre trial/study Setting: nursing homes in Spain, all in urban areas Unit of allocation: nursing home Unit of analysis: participant Follow‐up: 3 months postintervention Duration: 6 months Providers: nursing home physician |
|
| Participants | Quote: “1018 residents in 37 nursing homes owned by a private company in Spain. Persons older than 65 years, who had been living in the nursing home for at least 3 months and expected to stay in it for the length of the study, were clinically stable (no changes in prescription in the last 2 months) and accepted that their clinical data were used for the study were included. Residents receiving palliative care or those usually cared by other primary care providers outside the nursing home were excluded.” Focus on polypharmacy: number of drugs, 8.25 (3.39) intervention, 7.89 (3.27) control Age (mean): 84.5 (10.4) years intervention, 84.24 (14.6) control Male: 27% total population, 27.9% intervention, 26.0% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: participants' medication lists were screened by the primary research physician Training: a structured educational intervention delivered by a nursing home physician, expert in drug use in older people, was provided to the physicians Timing of intervention: during inpatient stay Quote: “A nursing home physician, expert in drug use in older people, delivered a structured educational intervention. The program included general aspects of prescription and drug use in geriatric patients, how to reduce the number of drugs, to perform a regular review of medications, to avoid inappropriate drug use, to discontinue drugs that do not show benefits, and to avoid undertreatment with drugs that have shown benefits. It also discussed in detail some drugs frequently related to adverse drug reactions in older people. Educational material and references were given to participants. Finally, two 1‐hour workshops reviewed practical real life cases and promoted practice changes in participants. The educator offered further on‐demand advice on prescription for the next 6 months. This intervention was reinforced by a single review by the researchers, using standard appropriateness criteria [Screening Tool of Older Persons Prescriptions (STOPP) Screening Tool to Alert Doctors to Right Treatment (START)], of a random sample of 10 residents cared by each physician in the intervention group, with written feedback on the problems found. Physicians in the control group did not receive any intervention or information about an educational intervention been delivered in other centers” |
|
| Outcomes | Appropriateness and quality of drug use (STOPP‐START criteria); measured at the beginning of the study and 9 months later (3 months after the intervention was finished) Hospital admissions (total number of days spent in hospital), falls, physician and nurse visits; recorded for 3 months before the 6‐month intervention started and the 3‐month period after it ended |
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was done using random number tables” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Physicians in both groups were informed that there was a company program aimed to improve drug prescription (to explain why data on prescription were collected in their centers) but were blinded to the fact that the educational intervention was being assessed” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported |
| Protection against contamination | Unclear risk | Quote: “Cluster RCT design used whereby nursing homes in the intervention and control group were separate. However, authors note that some cross contamination may have occurred because of informal contacts between physicians” |
Haag 2016.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: tertiary care academic medical centre in the Midwestern United States ‐ unclear if rural or urban Unit of allocation/analysis: patient Follow‐up: 30‐day follow‐up Duration: unclear Providers: pharmacist |
|
| Participants | 25 older patients (13 intervention and 12 control) Focus on polypharmacy: number of drugs on admission, median (IQR), 17 (12 to 20) intervention, 15.5 (13 to 18.5) control Age (median (IQR)): 81 (79 to 85) intervention, 86 (79.5 to 87) control Male: 69% intervention, 83% control Ethnicity: 96% white |
|
| Interventions | Model of pharmaceutical care: MTM consultation with a pharmacist, which included a comprehensive review of all prescription, non‐prescription and herbal medications taken Training: no educational intervention was specified Timing of intervention: during a single consultation Quote: “The intervention group received an MTM consultation with a pharmacist by telephone, preferably within 3 (and up to 7) business days after hospital discharge. This intervention was developed using successful methods of pharmacist integration during care transitions, while complementing the services of an existing CTP, to assess the impact on the quality of medication use. The pharmacist obtained the necessary information and clinical assessments from each patient’s electronic medical record to complete a comprehensive review of all prescription, nonprescription, and herbal medications taken. This systematic review of medications included the identification, resolution, and prevention of drug‐related problems, including adverse events or the use of potentially inappropriate medications. In addition, the electronic medical record was investigated for potential prescribing omissions. This review was the foundation for the phone consultation with the patient to ensure medication optimization. Decisions were based on the pharmacist’s clinical judgment after considering practice guidelines, 2 clinical support databases (Truven Health Analytics’ Micromedex and Wolters Kluwer Lexi‐Drugs), or the highest‐quality evidence available, as well as patient preferences. Recommendations were communicated by the pharmacist via a secure messaging function within the electronic medical record to the CTP provider for review on completion of the phone consultation. The usual care group was defined as the pre‐existing CTP without pharmacist intervention.” |
|
| Outcomes | Potentially inappropriate medications (STOPP/START); assessed at baseline and 30 days after discharge from hospital Medication utilisation quality (modified MAI); assessed at baseline and 30 days after discharge from hospital Hospital readmissions, emergency department visits; recorded at the 5‐week phone call follow‐up Adherence (Morisky‐Green); measured at baseline and at the 5‐week phone call follow‐up |
|
| Notes | Funding: this study was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), and the US Department of Health and Human Services (DHHS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly assigned to either the intervention group or to the usual care group by a study coordinator. Randomization was completed during the phone call by the study coordinator, who opened a sealed envelope that contained an indication of which group the patient was assigned to” |
| Allocation concealment (selection bias) | Low risk | Quote: “The study statistician used a random number generator to determine the allocation sequence” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The trial was unblinded (i.e., the participants and the investigators were aware of the intervention), and the patients received a telephone call from the pharmacist if they were randomized to the intervention group” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All outcomes were assessed while blinded to the intervention or the usual care group allocations” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but all expected outcomes are reported in the results |
| Protection against contamination | High risk | Single‐centre trial with potential for contamination |
Hanlon 1996.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: a veterans' hospital in the city of Durham, North Carolina ‐ no details of whether patients from urban or rural residences Unit of allocation/analysis: participant Follow‐up: 3 months and 12 months after randomisation Duration: unclear Providers: geriatrician, clinical pharmacist, nurse |
|
| Participants | 208 patients who were 65 years or older Focus on polypharmacy: included participants were prescribed 5 or more regularly scheduled medications by a Veteran Affairs physician and were enrolled at the Veteran Affairs Medical Center, Durham, North Carolina Age (mean ± SD): 69.7 ± 3.5 years intervention, 69.9 ± 4.1 years control Male: 98.1% intervention, 100% control Ethnicity, white: 79% intervention, 74.8% control |
|
| Interventions | Model of pharmaceutical care: pharmacists worked as part of a multi‐disciplinary team in outpatient clinics; the pharmacist(s) conducted an independent medication review together with participants during a face‐to‐face encounter; written recommendations were then presented to the primary physician Training: education was provided to prescribers and other healthcare professionals, participant education was also provided regarding drug‐related problems and compliance Timing of intervention: during a single attendance at outpatient clinics Quote: “The clinical pharmacist monitored drug therapy outcomes by reviewing each participant's medical record and medication list, ascertained current medication use, identified drug‐related problems by meeting with participants and carers and evaluated participants' medications by applying the MAI. The pharmacist then formulated prioritised written recommendations presented orally and in writing to the primary physician. After the physician visit, the clinical pharmacist educated the participant regarding drug‐related problems and encouraged compliance In the control group, the clinic nurse reviewed participants' current medications before the visit” |
|
| Outcomes | Participant MAI scores were determined by summing MAI medication scores across evaluated medications; measured at baseline, and at 3 and 12 months HRQoL (SF‐36); measured at baseline and 12 months Participant medication compliance and knowledge were assessed by participant self‐report at baseline and 12 months Potential ADEs; measured at 12 months Participant satisfaction; measured at 12 months |
|
| Notes | Funding: this work was supported by a grant from the National Institute on Aging (ROl‐AGO8380) and an Academic Award from the National Institute on Aging (AG00526‐Dr. Schmader) and by the Claude D. Pepper Older Americans Independence Center (P60AGl i268‐Drs. Schmader and Cohen, Weinberger and Feussner). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomly assigned to the control group or the intervention group using a computer‐generated scheme” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Assessments of outcome measures were blinded (appropriateness, prescribing appropriateness, HRQOL, adverse drug events, medication compliance)” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 participants were not interviewed. 5 in control and intervention groups were institutionalised. 5 from the intervention group and 1 from the control group were lost to follow‐up. 7 from the intervention group and 10 from the control group died. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Protection against contamination | High risk | Potential for contamination because physicians had patients in both intervention and control groups |
Koberlein‐Neu 2016.
| Study characteristics | ||
| Methods | Study design: cluster stepped‐wedge randomised trial Setting: general practices in the Westphalia‐Lippe region in northern Germany ‐ no details on rural/urban backgrounds of patients Unit of allocation: GP practices Unit of analysis: patients Follow‐up: up to 15 months follow‐up Duration: unclear Providers: home care specialists, pharmacist, physician |
|
| Participants | 142 older patients Focus on polypharmacy: 5 or more long‐term drug treatments Age (mean ± SD): 76.8 ± 6.3 years Male: 46.5% Ethnicity: not reported |
|
| Interventions | Model of pharmaceutical care: medication management conducted by the primary care physicians; the pharmacist then undertook a comprehensive medication review; recommendations were sent to the home care specialists Training: no educational intervention was specified Timing of intervention: unclear Quote: “The complete intervention consisted of two over lapping strands of action that were complementary to standard care: 1. medication management, and 2. care provided by the Pflege‐ und Wohnberatung (PuW, home‐care specialists), using a case management concept according to the German Society for Care and Case Management (Deutsche Gesellschaft für Care und Case Management, DGCC) . For the purpose of medication management, primary care physicians (PCP) started off by sending information from their patient records to the home‐care specialists. The home‐care specialists arranged a home visit, conducted an assessment of the patient situation they found—including, among others: drugs taken, adherence, medication handling and storage, reported problems with medication therapy and communicated this to the pharmacist, along with the information provided by the primary care physician. The pharmacist then undertook a comprehensive medication review (PCNE type 3). This included drugs taken, medication documented by primary care physicians, available laboratory data, diagnostic data, and insights into every patient’s personal situation as elicited in patient interviews. The results of the analysis were summarized in a letter of recommendation and sent to the home‐care specialists, who in turn added information on the patient’s home situation and passed them on to the primary care physicians. Implementing the recommendations was the responsibility of each primary care physician. Details about such patient‐related advice from physician to pharmacist and detailed information on the second strand of action can be obtained from the authors." “In the WestGem study, an independent biometrician randomized the participating general practices (clusters) to three (changing) cohorts. After a control period, the cohorts switched to the intervention phase at intervals of three months each. The cohort allocation was disclosed only at the time of the changeover. During the control phase, patients received standard care; in the intervention phase they additionally participated in medication management. The intervention phase, depending on the timing of the changeover, was six to 12 months, with a subsequent follow‐up period of 3 months” |
|
| Outcomes | Number of PIM prescribed (based on PRISCUS list) Medication Appropriateness Index Assessment points were at baseline, the end of the recruitment period, after the end of the recruitment period, 6 months after the end of the recruitment period, 12 months after the end of the recruitment period and 15 months after the end of the recruitment period |
|
| Notes | Funding: the study received funding in the context of the Ziel‐2‐Förderreihe IuK & Gender med.NRW from the federal state of North Rhine Westphalia and the European Union. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent biometrician randomized the participating general practices (clusters) to three (changing) cohorts The cohort allocation was disclosed only at the time of the changeover” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Protocol states that Quote: “in this trial the patient is blinded to the pharmacist” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The pharmacists had been blinded when calculating scores as to which cohort a patient was allocated to, but they were involved in some cases in conducting the medication reviews. They can therefore not be regarded as completely independent. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Not all of the study’s pre‐specified primary outcomes have been reported. |
| Protection against contamination | High risk | Cluster‐randomised trial; unclear if/how contamination protected against with stepped wedge design |
Michalek 2014.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: tertiary medical centre in the city of Essen, Germany, which serves "an urban population" Unit of allocation/analysis: participant Follow‐up: unclear Duration: unclear Providers: physicians |
|
| Participants | 114 patients admitted to a 700‐bed tertiary medical centre. Patients were eligible if aged > 70 years, in a stable health condition (defined as no need for intermediate or intensive care unit treatment), had at least 3 diseases in need for drug treatment, and had at least 3 medical prescriptions. Focus on polypharmacy: polypharmacy at admission Age (mean): see notes Male: 21% intervention, 25% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: participants' medication lists were screened by the physician and recommendations were discussed with the study physicians Training: physicians received training throughout the study period Timing of intervention: during inpatient stay Quote: “On the intervention ward (FORTA group), physician education was structured and continuously provided during the study. The physicians were formally instructed about the FORTA‐principle and provided with the relating documents (publications, current FORTA‐list) by 2 lectures before the study commenced. They convened with the FORTA intervention team (study physicians) on a weekly basis (PharmaBoard) to review information, to collect data on patients included in the study and to discuss medication plans with respect to the FORTA system. Though individual recommendations may have been issued ward physicians were free to adopt them or not. The FORTA intervention team had no power and legal sanction to modify medication plans. The ward physicians’ own judgement was leading over FORTA‐based suggestions in the process of finding the appropriate medication. On the control ward all patients were treated based on established medical standards and on the principles of good medical practice. In the intervention group, the drugs were evaluated according to the FORTA list and changed as guided by FORTA within the first week in the hospital. Weekly meetings for intervention were performed that encompassed a thorough evaluation of patient diseases, functional status, prognosis, and need for drugs. Decisions were based on the FORTA suggestions. Drugs were continued despite unfavourable FORTA labelling if patients insisted. Since FORTA is an implicit tool, physicians are not obliged to strictly follow the proposals. Furthermore, overprescription (drugs not matching a diagnosis or FORTA label C/D drugs despite availability of A/B drugs or not indicated) and under‐prescription (no drugs despite treatable disease) were identified and corrected according to FORTA recommendations Patients of the control group were treated according to current medical standards as good clinical practice by geriatricians” |
|
| Outcomes | Impact of the application of the FORTA list on the number and the quality of drugs; number and quality of drugs; measured at admission to hospital and at discharge Number of patients who fell, the frequency of in‐hospital falls and the change in functional status during hospital stay |
|
| Notes | Age median (IQR): 84 (81 to 87) years intervention, 83 (79 to 87) years control Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Patients were assigned randomly by number of entrance to one of two wards. In addition, patients could only be included in the study during the first 3 days of the week due to staff availability” |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were assigned randomly by number of entrance to one of two wards. The assignment was performed by a manager not involved in patient care and blinded to the aim of the study” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Two physicians familiar with the FORTA classification were responsible for the intervention process. They were not involved in the treatment of the patients of the control area. All other staff of both wards were blinded to the aim of the study” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the study protocol. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Protection against contamination | Low risk | Quote: “One ward served as the intervention area and the other ward as the control area. The wards rather than individual subjects were chosen to minimize contamination of results caused by staff” |
Milos 2013.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: Skane County, an area in southern Sweden with approximately 1,150,000 inhabitants. Patients were invited via healthcare centres. Unit of allocation/analysis: participant Follow‐up: 2 months follow‐up Duration: unclear Providers: pharmacist |
|
| Participants | 369 patients (182 intervention, 187 control) Focus on polypharmacy: mean (SD) number of drugs at baseline was 11.4 (4.2), intervention, 12.1 (4.7), control Age (mean ± SD): 87.0 ± 5.8 intervention, 87.7 ± 5.5 years control Male: 24.2% intervention, 24.1% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: pharmacists performed systematic medication reviews without personal patient contact, which were sent to the physician Training: no educational intervention was specified Timing of intervention: unclear Quote: “For patients in the intervention group the pharmacists performed a systematic medication review without personal patient contact. The medication review included assessment of relevant parts of the EMR and collection of data on the patient’s blood sample results for creatinine, estimated glomerular filtration rate (eGFR), cystatin C, haemoglobin, sodium and potassium plasma levels. To identify DRPs the clinical pharmacist initiated medication reviews based on the background information (symptom assessment form and the MDD cards). The working process was carried out in a structured way with formularies compiled from the LIMM model. The following predetermined risk categories for identifying DRPs were taken into account by the pharmacist and documented by the student: • Drugs that required therapeutic monitoring • Inappropriate drugs for elderly according to The National Board of Health and Welfare (PIMs) • Drugs that are not recommended according to the regional drug and therapeutics committee • Problems with administration/handling of the drugs (crush, cut, inhalation technique) • C/D drug–drug interactions (C interactions are those involving a drug combination that could require dose adjustment; D interactions are those involving a drug combination that ought to be avoided) • Drug type or drug dosage not adjusted for the patient (renal function, liver function) • Unclear indication for drug treatment • Suboptimal treatment • Drugs causing potential adverse drug reaction. The check list including the nine risk categories was an instrument to facilitate the medication review. PIMs were identified according to the national guidelines of the Swedish National Board of Health and Welfare regarding drug therapy in the elderly. The pharmacists’ recommendations were documented in patients’ EMRs. The feedback to the physician varied depending on the PHCC’s routines and organisation and consisted of team rounds, written contact, personal contact and telephone contact. To ensure that the pharmacists worked similarly, they were formally instructed in one tutorial by the head pharmacist (E.R.) about the method of medication review, had monthly meetings with the data collector (S.W.) and had one meeting with the head researcher (V.M.). In addition, the head pharmacist was available for consultation throughout the entire study” "Usual care consisted of the health care centre's "normal" routine” |
|
| Outcomes | Number of PIMs at baseline and 2 months Percentage of patients taking 10 or more medications and percentage of patients taking 3 or more psychotropic drugs (from specified groups); measured at baseline and 2 months |
|
| Notes | Funding: the study was conducted with government funding for projects involving improvement of drug therapy in the elderly. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation was performed using a random number generator” |
| Allocation concealment (selection bias) | Low risk | Quote: “The pharmacist used closed, nontransparent envelopes to randomise the patient to one of two groups: control or intervention” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Muth 2016.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: GP practices in the state of Hesse, Germany. Of 20 practices involved in the study, 6 were in urban areas, 9 in suburban and 5 rural. Unit of allocation: GP practices Unit of analysis: patients Follow‐up: 12‐weeks follow‐up Duration: unclear Providers: GPs |
|
| Participants | 100 older patients (50 intervention and 50 control) Focus on polypharmacy: included participants taking 5 or more long‐term prescriptions Age (mean ± SD): 75.8 ± 6.70 years intervention, 72.5 ± 5.88 years control Male: 44% intervention, 52% control Ethnicity: not reported |
|
| Interventions | Model of pharmaceutical care: a brown bag review and a checklist‐based preconsultation interview with the patient conducted by the HCA, a computer‐assisted medication review carried out by the GP and a GP‐patient consultation Training: no educational intervention was specified Timing of intervention: on a single occasion Quote: “The elements of the complex intervention consist of a brown bag review and a checklist‐based preconsultation interview with the patient that is conducted by the HCA, a computer‐assisted medication review carried out by the GP and a GP‐patient consultation. GPs in the intervention group received practice guidelines for older patients and the complex intervention was implemented at their practice on a single occasion. Control group: GPs in the control group also received the practice guidelines for older patients,35 but continued with usual care” |
|
| Outcomes | Medication Appropriateness Index (MAI); measured at baseline, 6 weeks and 12 weeks Health‐related quality of life (EQ‐5D index); measured at baseline, 6 weeks and 12 weeks Self‐reported adherence (Morisky the Medication Adherence Rating Scale ‐ MARS); measured at baseline, 6 weeks and 12 weeks |
|
| Notes | ISRCTN99691973 Funding: provided by the German Federal Ministry of Education and Research, BMBF (grant number 01GK0702) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “An experienced clinical pharmacologist (SH) coded the MAI following a blinded chart review” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were small and similar across both groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Protection against contamination | Unclear risk | Quote: “Reduction in inappropriate prescriptions was observed in both groups, indicating a likely contamination effect in the control group” |
Muth 2018.
| Study characteristics | ||
| Methods | Study design: randomised trial (cluster) Setting: GP practices in the state of Hesse, Germany. Of 72 practices in the study, 22 were located in a city, 16 in a mid‐sized town, 25 in a small town and 9 in a rural area Unit of allocation: GP practices Unit of analysis: patients Follow‐up: 9 months follow‐up Duration: unclear Providers: GPs |
|
| Participants | 505 older patients (252 intervention and 253 control) Focus on polypharmacy: included participants taking 5 or more long‐term prescriptions Age (mean ± SD): 72.5 ± 6.5 years intervention, 71.7 ± 7.4 years control Male: 47% intervention, 48% control Ethnicity: not reported |
|
| Interventions | Model of pharmaceutical care: a brown bag review and a checklist‐based preconsultation interview with the patient conducted by the HCA, a computer‐assisted medication review carried out by the GP and a GP‐patient consultation Training: no educational intervention was specified Timing of intervention: on a single occasion Quote: “There are four elements of the complex intervention. It consists of (1) a brown bag review and (2) a checklist‐based preconsultation interview with the patient that is conducted by the healthcare assistant (HCA), (3) a computerised decision support system (CDSS)‐assisted medication review carried out by the GP, and (4) a GP–patient consultation to optimise and prioritise medication. GPs had the option to use the CDSS to help prepare the medication review with the patient, and during the consultation itself. Trained HCAs and GPs implemented the intervention on a single occasion, which took the GP and the HCA a per‐patient average of 35 and 45 min, respectively.35 The practice team for the intervention group received the GP guidelines for ambulatory geriatric care prepared by the Hesse Guideline Group. Recommendations in the guideline focus on primary and secondary prevention (e.g. physical exercise, fall assessment and prevention). The control group continued to receive usual care but the practice team also received the GP guidelines for ambulatory geriatric care to harmonise usual care in both groups” |
|
| Outcomes | MAI score; measured at baseline, 6 months and 9 months Health‐related quality of life (EQ‐5D); measured at baseline, 6 months and 9 months Functional status; measured at baseline, 6 months and 9 months Pain; measured at baseline, 6 months and 9 months All‐cause hospitalisation; measured at baseline, 6 months and 9 months Adherence (Morisky‐Green); measured at baseline, 6 months and 9 months |
|
| Notes | ISRCTN99691973; NCT01171339 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Practice allocation to treatment groups will be performed by central randomisation by a study‐independent researcher at the IGP after registration of the first patient per practice. Once a practice has been randomised, all the patients recruited for the practice will be deemed intervention or control depending on which arm of the study each practice was allocated. After completion of the baseline documentation of all study patients per practice, the study‐independent researcher at the IGP will inform the study team at the IGP about the practice status as either intervention or control. The study team will send a fax with the randomisation result to the practice” |
| Allocation concealment (selection bias) | High risk | Quote: “Practice allocation to treatment groups will be performed by central randomisation by a study‐independent researcher at the IGP after registration of the first patient per practice. Once a practice has been randomised, all the patients recruited for the practice will be deemed intervention or control depending on which arm of the study each practice was allocated. After completion of the baseline documentation of all study patients per practice, the study‐independent researcher at the IGP will inform the study team at the IGP about the practice status as either intervention or control. The study team will send a fax with the randomisation result to the practice” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Owing to the nature of the intervention, it was not possible to blind GPs, HCAs, patients and the study team” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Treatment allocation was blinded to the clinical pharmacologist conducting medication reviews for the primary outcome (MAI) and to the statistician” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way. |
| Protection against contamination | Low risk | Cluster‐RCT – allocation was by practice |
O'Mahony 2020.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial (pragmatic, multi‐national, parallel‐arm, prospective, randomised, open‐label, blinded endpoint controlled trial) Setting: large academic teaching hospitals in cities in Ireland, Scotland, Spain, Italy, Belgium and Iceland Unit of allocation/analysis: participant Follow‐up: 12 weeks Duration: patients are followed up for 12 weeks but unclear how long patients' duration of participation was Providers: "The Trial Coordinating Committee was staffed by a dedicated Trial Manager, an Endpoint Liaison Officer (both full time) and a Data Manager, Biostatistician, and Trial Monitor (part‐time) who were supervised by the Trial Coordinating Investigator. Each of the six sites was led by a site Principal Investigator (PI), a senior physician specialising in geriatric medicine and with extensive experience in geriatric pharmacotherapy. These PIs oversaw the recruitment, training and conduct of the local trial staff. All site staff were ICH GCP certified researchers with medical, nursing or health science backgrounds. Training consisted of live interactive tutorials, online ICD‐10 training, case‐based ADR adjudications, remote testing of the electronic case report form (eCRF) at each clinical site, a central two day and subsequent one day meeting of all local site staff and web‐based site initiation visits prior to recruitment initiation. Audits were performed by the Clinical Research Facility in Cork (CRF‐C) monitoring staff who were otherwise independent of the running of the trial." |
|
| Participants | 1537 patients randomised: 772 to intervention group and 765 to control Age: overall, years (IQR): 78 (72, 84); intervention: 78 (72, 84); control: 78 (72, 84) Sex: overall, female: n = 725 (47.2%); intervention: 367 (47.5%); control: 358 (46.8%) Race: not given Top 100 most prevalent chronic medical conditions were listed in supplement page 7. Top 10 were essential (primary) hypertension (n = 1121), hyperlipidaemia (330), heart failure (315), COPD (297), chronic ischaemic heart disease (293), non‐insulin‐dependent diabetes mellitus (249), hypothyroidism (232), atrial fibrillation and flutter (219), chronic kidney disease stage 3 (207), pure hypercholesterolaemia (184) The total number of daily medications (median (IQR)) = 10 (8 to 13). The number of daily medications in intervention group = 10 (8 to 13). The number of daily medications in control group = 10 (8 to 13). |
|
| Interventions | Model of care: clinical decision support system‐generated medication advice reports based predominantly on PIP criteria to clinicians attending hospitalised acutely ill older people living with multi‐morbidity. The aim was to test whether this significantly reduced adverse drug reaction incidence. Timing: patients randomised, allocated to either control or intervention arm; outcomes assessed within 14 days of enrolment, follow‐up at 12 weeks post‐discharge. Standard pharmaceutical care plus single time point SENATOR software intervention within 60 hours of admission. Quote: "The central hypothesis in the SENATOR trial is that attending medical staff prescribers working in specialist departments other than geriatric medicine will—when offered advice points relating to potentially inappropriate medication in individual patients under their care—adjust the prescriptions of these patients according to the SENATOR software‐generated advice reports (the intervention). These adjustments will, in turn, significantly reduce ADR incidence in intervention arm patients compared to matched patients receiving standard pharmaceutical care in the same medical centre. The SENATOR software was designed by the project consortium and implemented by the Clanwilliam Group. It produces a report, in the clinician’s native language, that identifies potential risks, and opportunities for improvement, in the participants’ current medication list. The report has 5 components: [1] recommendations for modifying or discontinuing a current medication; [2] recommendations to initiate a new medication (both based on the published STOPP/START guidelines); [3] identification of major drug‐drug and [4] drug‐disease interactions (both based on SafeScript software and other local drug‐drug and drug‐disease interaction databases) [5] and nonpharmacological recommendations considered complementary to patients’ drug therapy. STOPP/START is a widely used series of heuristic rules aimed at optimizing drug prescribing in older patients, which has been validated in a range of settings. SafeScript is a validated software system which uses the Summary of Product Characteristics (SPCs) of ATC coded medications in conjunction with ICD‐10 coded conditions that was developed in the UK. The non‐pharmacological recommendations in the SENATOR report were based on the ‘Optimal evidence‐based Non‐drug Therapies in Older People’ (ONTOP) programme; these evidence‐based recommendations were developed independent of the trial as part of the same FP7 funded project. Only the ONTOP recommendations aimed at reducing the occurrence of incident delirium were completed and available for inclusion in the SENATOR report version used within the current trial. Since all older subjects who are hospitalised are at risk for developing delirium, the recommendations are provided to all subjects randomized to the SENATOR intervention, except those who already have delirium at the time of recruitment. The ONTOP recommendations are included by way of a demonstration of the potential utility of SENATOR as a potential mechanism for promoting non‐pharmacological therapies i.e. as a proof of concept. However, the study was not powered with the aim of estimating the benefits of the ONTOP component. In the intervention arm the participant’s clinical team receive an individualised SENATOR report, at a single time point within 60 hours of hospital admission. Control arm subjects and their doctors receive no additional study specified intervention. All subjects have usual clinical management whereby as part of routine care clinicians routinely review and adjust medications according to their local practice. In addition, in one site the majority of hospitalised patients undergo a routine review of their medications by a dedicated internal liaison team or by a hospital pharmacist. As SENATOR is a decision support tool, it efficiently provides in a single report a range of evidence‐based recommendations, derived from general considerations. Given the multiple complexities of clinical care, none of these recommendations is mandated. Rather clinicians are requested to review them in the context of the patient’s unique clinical circumstances and to make any alterations in accordance with the clinician’s best judgement." |
|
| Outcomes | The proportion of patients with at least one adjudicated probable or certain, non‐trivial incident in‐hospital ADR occurring within 14 days of enrolment during the index hospitalisation The proportion of patients with at least one adjudicated possible, probable or certain, non‐trivial incident in‐hospital ADR occurring within 14 days of enrolment during index hospitalisation The proportion of patients with at least one adjudicated probable or certain, non‐trivial hospital‐acquired, pre‐specified (as listed in protocol, table 2) ADR occurring within 14 days of enrolment during index hospitalisation The number of adjudicated probable or certain, non‐trivial hospital‐acquired ADRs occurring within 14 days of enrolment during the index hospitalisation (i.e. the count of primary endpoint events) The number of adjudicated possible, probable or certain, non‐trivial, incident, in‐hospital ADR occurring within 14 days of enrolment during index hospitalisation The number of adjudicated probable or certain, non‐trivial hospital‐acquired, pre‐specified (as in protocol table 2), non‐trivial, incident, in‐hospital ADR occurring within 14 days of enrolment during index hospitalisation All‐cause mortality within 30 days of randomisation Re‐hospitalisation at 12 weeks post‐discharge QoL (EQ‐5D‐3L); measured at baseline and discharge Healthcare utilisation (outcome not reported) |
|
| Notes | Funding: this work was supported by the European Commission’s Seventh Framework Programme (FP7/2007–2013) (grant number 305930) as part of the SENATOR project. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients are randomised into one of the 2 trial arms with a 1:1 allocation ratio. Randomisation is stratified by study site and by admitting service type (i.e. medical vs surgical). The stratum‐specific randomisation lists are generated using random block sizes by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Trial allocation was delivered using an interactive Web‐Response System developed in conjunction with the main trial database by Clininfo, the data management partner company within the SENATOR project. Participant allocation was released once all necessary baseline information data had been entered into the trial database and a decision made to randomise. In participants randomised to the active intervention, trial data were automatically transferred to the cloud‐based SENATOR software engine maintained by Clanwilliam Health, and the resulting SENATOR report was automatically emailed to the local trial research staff at a designated email. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Given the nature of the intervention it is not possible to effectively mask the intervention from the clinical team or from the on‐site researchers. The randomisation lists were integrated into the eCRF so that researchers could not access them and any given allocations were only revealed once patients were unambiguously enrolled into the trial and their screening information was irreversibly entered onto the eCRF. Blinded co‐principal investigators adjudicated Trigger List event forms relating to patients from other clinical sites |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Potential adverse drug reactions (ADR) were assessed by a blinded adjudication committee. The recording of evidence supporting an ADR was done in a blinded manner using the relevant Potential Endpoint Form. Quote: "Each event is reviewed by up to five Potential Endpoint Adjudication Committee members, excluding members from the same site where the event occurred. Each reviewer independently assesses the likelihood of the event being medication‐related and the severity of the event. If the local site Principal Investigator, upon unblinded review of the patient’s case records and the Primary Endpoint Form, grades the event as either ‘unlikely’ or ‘certain’ and the Endpoint Liaison Officer concurs, then the Evidence Form is reviewed by a single blinded Endpoint Committee member, marking Stage 1. If the stage 1 Reviewer agrees with the Site Principal Investigator, this decision is accepted otherwise the form is reviewed by a second blinded Endpoint Committee member, marking Stage 2. All Potential Endpoints judged by the unblinded Site Principal Investigator to be possible, probable, or indeterminate ADRs are directly reviewed independently by two committee members who are blinded to the initial assessment. The adjudicated conclusion is determined by the Stage 2 Agreement Matrix; where reviewers agree, or any disagreement is minor, an adjudicated result is assigned. For more substantive levels of disagreement, the review progresses to a 3rd blinded Endpoint Committee member (Stage 3) and a majority consensus prevails provided all 3 reviewers judge that the event is at least a possible ADR. Otherwise the event is adjudicated by consensus at a full committee meeting with the Site Principal Investigator being recused." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All analyses were based on the intention‐to‐treat principle. The intention‐to‐treat population consisted of all patients randomised. Results show: Intervention: 772 patients; followed up at 12 weeks: 660 Control: 765; followed up at 12 weeks: 645 Numbers withdrawn/lost to follow‐up were similar between groups. EQ‐5D‐5L was reported in a supplementary table. It was planned to be part of a cost utility analysis, but other aspects of this analysis were not reported, such as QALYs. |
| Selective reporting (reporting bias) | High risk | Healthcare utilisation post‐discharge was assessed but is not reported. It was not stated why this outcome was not reported. |
| Protection against contamination | High risk | Arguments for not adopting a cluster design and acceptance of some degree of contamination: Quote: "We had initially proposed a cluster randomized trial adjusting for difference in baseline ADR risk using an ADR prediction tool such as the previously validated Gerontonet ADR Risk Scale [6]. This approach had the specific advantage of limiting contamination between the intervention and control arm, especially if it transpired that the same investigator was simultaneously attending a subject in both arms of the trial. However, in our feasibility study we discovered a very large degree of heterogeneity in ADR rates between different sites and between specialities within individual sites. Some of this heterogeneity may have resulted from initial limited standardisation of our ADR reporting and adjudication processes and from sampling variability given the limited sizes of individual samples as well as real substantive differences between sites. Furthermore, we found that within our population the ADR predictions tools were inadequate for correcting for the between‐cluster variability in baseline ADR risk. This made it impossible to exclude the possibility that any observed differences in the proposed trial might not simply be the consequence of an unequal distribution of baseline ADR risks across sites. We therefore adopted an individual level randomisation, accepting that a degree of cross arm contamination might dilute the perceived effect size. However, even in a cluster design this effect is not fully prevented because junior medical staff routinely migrate between various specialities within a hospital and senior clinicians typically cross cover other specialist services at weekends and when working on‐call outside of regular daytime hours." |
Olsson 2012.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: hospital in city of Orebro, Sweden Unit of analysis: patients Follow‐up: 12 months follow‐up Duration: unclear Providers: GPs |
|
| Participants | 150 older patients (50 intervention group B, 50 intervention group C and 50 control) Focus on polypharmacy: included participants taking 5 or more drugs Age (mean ± SD): 83.9 ± 5.1 years intervention, 82.5 ± 4.9 years control Male: 36% intervention, 44% control Ethnicity: not reported |
|
| Interventions | Model of pharmaceutical care: home visit by a nurse and a prescription review conducted by nurses then sent to the physician/primary health care centre Training: no educational intervention was specified Timing of intervention: unclear Quote: “Group A (control): home visit by study nurse within one month after discharge, QoL survey by post at six months, and second home visit by study nurse at 12 months. Group B (intervention): as group A and a letter with a prescription review (according to points 1 – 4 below) sent to the physician/primary health care centre. Group C (intervention): as group B combined with a current and comprehensive medication record consisting of the patient's written drug regimen and indications sent to the patient to enable participation in his/her drug treatment. This was accompanied by an instruction to utilize the record throughout the health care system, make notes, and discuss their drug treatment with their physicians. During the home visit patients in all three groups were asked about their drug regimen and compliance to capture their “true” medication record. The study physician completed a prescription review assessing the following as indicators of prescription quality: 1. number of drugs; total, on regular basis and on demand; 2. number of drug‐risk indicators (long‐ and short‐acting benzodiazepines, sleeping pills, NSAIDs, digitalis, diuretics, SSRI, PPI, neuroleptics, and drugs with anticholinergic effects); 3. drug interactions by using a computer program that warns for interactions of C‐type (adjustment of dose recommended) and D‐type (avoidance of drug recommended); 4. number of medication errors and/or discrepancies between medication list (prescriptions) and the patient’s own regime (drugs noted but not taken, drugs taken but not noted, and wrong dosages)” |
|
| Outcomes | Quality of prescriptions (The National Board of Health and Welfare. Indicators for evaluation of quality of drug treatment for elderly); measured at baseline and 12 months Quality of life (EQ‐5D index, EQ VAS); measured at baseline, 6 months and 12 months |
|
| Notes | For the purpose of this review we focused on intervention group C versus control. Funding: this study was supported by grants from Orebro County Council. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “All home visits throughout the study were done by the same study nurse who was blinded to the groups” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “No significant differences between the groups were observed in respect of mortality or dropouts” |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified. |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Pitkala 2014.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: assisted living facility in Helsinki, Finland Unit of allocation/analysis: wards Unit of analysis: participant Follow‐up: unclear, states that repeated assessments were performed at 6 and 12 months Duration: unclear, states that repeated assessments were performed at 6 and 12 months Providers: nurses and consulting physician |
|
| Participants | 227 residents (118 intervention, 109 control) in 20 wards. Inclusion criteria: age 65 years or older; living permanently in an assisted living facility; Finnish speaking; using at least 1 medication; having an estimated life expectancy > 6 months; and being able to provide written informed consent (or have a proxy who is able to provide written informed consent in the case of cognitive impairment) Focus on polypharmacy: mean number of regular medications (SD), 7.5 (2.8) intervention, 7.8 (3.1) control Age (mean): 82.9 (7.5) intervention, 83.5 (6.9) control Male: 34.7% intervention, 22.9% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: nurses identified potential medication‐related problems and discussed these with the consulting physician Training: 2 x 4‐hour training sessions for nursing staff based on the principles of constructive learning theory Timing of intervention: unclear Quote: “The intervention comprised two 4‐hour training sessions for nursing staff based on the principles of constructive learning theory. The training sessions were developed to be activating and interactive. The sessions were designed to enable nurses to better recognize harmful medications and corresponding ADEs. The first 4‐hour afternoon session was primarily lecture‐based, but participants were encouraged to present and openly discuss medication‐related problems experienced by their own residents. The session involved introducing the list of harmful medications and suitable alternatives. This session also involved discussion about medication use for residents with renal impairment and drug‐drug interactions. The second 4‐hour afternoon session was case study based. Using the principles of problem‐based learning, the nurses participated in facilitated discussions about medication‐related problems. To demonstrate the relevance and importance of the topic, nurses were encouraged to present and discuss actual resident cases from their own wards. Throughout the training sessions, the nurses responsible for medication management were invited to reflect on their own procedures and opportunities for improvement. We also invited physicians to participate in the 2 education sessions. Two out of 3 physicians working in the intervention wards attended 1 of the training sessions. The list of harmful medications was provided to all nurses working in the intervention wards. Following the training, the nurses were asked to identify potential medication‐related problems and bring these to the attention of the consulting physician. When this occurred, it was the physician’s responsibility to change or continue a specific medication Control staff received no additional training and continued to provide routine care” |
|
| Outcomes | Use of potentially harmful medications (Beers criteria); measured at baseline, 6 and 12 months HRQoL assessed using the 15 dimensional instrument (15D) of health‐related quality of life; measured at baseline, 6 and 12 months Health service utilisation; measured at baseline, 6 and 12 months Mortality; measured at baseline, 6 and 12 months |
|
| Notes | Funding: This study is supported by Sohlberg Foundation and Helsinki University Hospital development grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The 36 wards were assessed for possible participation, and 20 wards were paired into 10 dyads. The wards in each dyad shared similar resident characteristics. A computerized random number generator was then used to randomize 1 ward in each dyad to the intervention arm and the other to the control arm” |
| Allocation concealment (selection bias) | Low risk | Quote: “A person independent of assessment procedure telephoned another person not familiar with the wards or residents to receive the randomization number (intervention or control) for each ward” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “The study nurses who recruited the residents were not aware which wards had been randomized to the intervention or control groups” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The research nurses performed their assessments at 0, 6 and 12 months. These nurses were independent of the study intervention and unaware of the randomization procedures” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “High attrition rate: 41 residents (18.1%) lost to follow‐up at 6 months and 63 residents (27.8%) lost to follow‐up at 12 months. All residents assessed at baseline and at least 1 of the 2 follow‐ups were included when analyzing changes in the use of medications and HRQoL (modified intention‐to‐treat analyses). All randomized residents were included when analyzing health service utilization and mortality (intention‐to‐treat analyses)” |
| Selective reporting (reporting bias) | Low risk | The study protocol is available; however, there are some discrepancies between the outcome reported in the trial registry document and the paper. 6‐month outcome data for all outcomes are not clearly reported. Cost data are not reported |
| Protection against contamination | Low risk | Quote: “A cluster randomised design was used that involved randomizing wards rather than individual residents. This was necessary to avoid potential contamination of the intervention that may have arisen if nurses had provided care to both residents in the intervention and control arms” |
Romskaug 2020.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Setting: home‐dwelling patients recruited via their family doctors in the counties of Akershus and Oslo, Norway Unit of allocation/analysis: cluster‐randomisation at the family physician level was performed to avoid between‐group contamination. To avoid large variations in cluster sizes, each family physician participated with a maximum of 5 patients, and stratification was performed based on the number of contributing patients (1 to 2 vs 3 to 5). Follow‐up: 24 months Duration: 2 years Providers: physician trained in geriatric medicine, supervised by senior consultant. Patients’ own family physicians involved in relaying medication changes to them. |
|
| Participants | 70 family physicians underwent randomisation, each representing a cluster. Total 174 patients. 36 clusters in intervention group (one cluster per family physician), 34 in control. 87 patients in each group. Does not state how many patients per cluster, but methods section stated maximum of 5. Mean age (SD): intervention 82.2 (7.6); control 84.4 (6.9). Overall, among 174 patients (mean (SD) age, 83.3 (7.3) years). Sex: females, number (%) 118 (67.8%); intervention 52 (59.8); control 66 (75.9) Race: not given Co‐morbidities: not given Cumulative Illness Rating Scale summary score, mean (SD): intervention 16.8 (4.4); control 16.6 (4.1) Regularly used drugs mean (SD): intervention 10.1 (2.7); control 9.5 (2.6) |
|
| Interventions | Model of care: clinical geriatric assessments and collaborative medication reviews by geriatrician and family physician (FP) ‐ aim was to investigate their effect on health‐related quality of life and other patient‐relevant outcomes in home‐dwelling older patients receiving polypharmacy Timing: 1‐hour consultation and follow‐up meeting for intervention. Assessments at baseline, 16 and 24 weeks (also for control group). "The intervention consisted of 3 main parts. 1. Geriatric assessment consisting of a medical history, systematic screening for current problems, clinical examination of the patient, and relevant supplementary tests as well as a detailed review of each medication in use, with emphasis on indication, dosage, possible adverse effects, and interactions. Assessments were done by a physician trained in geriatric medicine, supervised by a senior consultant. On average, 1 hour was spent on each clinical consultation. As soon as possible after randomization, the patients were seen by the geriatric physician. In advance, the geriatrician obtained necessary information on the patient’s medical history and actual medication from hospital records, the FP’s electronic patient record, the home nursing service and other relevant sources. The geriatrician carried out a medical history from the patient (if necessary supplemented by a close relative) and a physical examination, both with focus on conditions most relevant for the patient’s total medication use. Relevant blood analyses and other supplementary tests were ordered if not already available. The geriatric work‐up was aimed at evaluating whether current medications were indicated, whether the relevant conditions were satisfactorily compensated, whether the dosages were appropriate, whether the patient had symptoms of adverse drug reactions, and whether drug‐drug interactions or drug‐disease interactions were present or likely to occur. A drug interaction database, lists of anticholinergic drugs, the STOPP/START criteria and the NORGEP criteria were also used. PATIENT ASSESSMENTS carried out by the geriatrician: Medical history: Go through medical history obtained from family physician. Is the information accurate? Any indistinctness? Systematic screening for current problems Cognition: Known/suspected dementia? (IQCODE, CDR, relatives, impression of patient)? NPS? Depression/anxiety: Screening by ICD‐10 criteria. Nutrition: Weight loss, reduced appetite, nausea, dyspepsia? BMI, MNA‐SF. Pain: Previous/current problem? Is the cause identified? In need of better analgesia? Breathing: Dyspnea? Hydration: Signs of dehydration? Overhydration/edema? Natural functions: Urinary incontinence? Voiding problems? Diarrhea/constipation? Mobility: Gait problems? Dizziness? Walking aids? History of falling? Sleep: Any problems related to sleep? Sort out current main problem(s) concerning the patient's health. Medications: Are all drugs used as prescribed? Any problems with administration? Has the patient any suspicions regarding side effects? Is the patient aware of the indication for different drugs? If symptomatic medications – what is the current situation regarding the target symptom? If unclear indication, explore the patient’s willingness to reconsider dosages or to discontinue the drug in order to assess effectiveness. For prophylactic medications, identify thoughts on the balance between current drug use and reducing future risks. Key elements of the medication review ‐ Is there a clear indication for the drug? ‐ Are treatment effects evaluated and/or reconsidered? ‐ Are dosages appropriate? ‐ Are there any suspected adverse drug reactions? (Also considering whether symptoms considered as related to disease may rather constitute subtle adverse drug reactions, perhaps as the combined effect of several drugs.) ‐ Are drug‐drug interactions or drug‐disease conditions present or likely to occur? ‐ Are all relevant conditions satisfactorily compensated? ‐ Is the patient using drugs associated with particular high risk (e.g. anticholinergic drugs, drugs listed in STOPP/NorGepc)? Clinical examination: with emphasis on relevant conditions and current symptoms. Supplementary tests: Blood pressure (including orthostatic), Pulse rate, respiratory rate, ECG, Relevant blood analyses, Serum concentration of relevant drugs, Pharmacogenetic tests: ‐ CYP2C19, CYP2C9, CYP2D6 (all patients) ‐ CYP3A5 and SLCO1B1 if using statins ‐ SLC6A4 if using SSRI’s ‐ VKORC1 if using warfarin. 2. Second part of the intervention was a meeting between the geriatrician and the FP, with discussion of each medication, establishing a collaborative plan for adjustments and follow‐up. Approximately 15 minutes were spent discussing each patient. The main purpose of this meeting was to combine the competence and knowledge of the geriatrician with that of the FP. The geriatrician summarized the findings from the geriatric assessment and medication review, and the two physicians discussed the patient’s drug list systematically. The geriatrician could suggest changes in the drug regimen, but the FP retained the medical responsibility for the patient and oversaw all ordinations and medication changes. 3. Third was clinical follow‐up by the geriatrician or FP, as agreed on. Follow‐up was in general done by the FP. Depending on medication changes that had been done, the two physicians arranged the necessary follow‐up within the project period. The follow‐up could consist of a clinical evaluation, further drug adjustments, blood tests etc., and could be carried out by the FP, the geriatrician or through telephone contact with the patient, the relative or the home nursing service, depending on the circumstances." The control group received usual care from their family physician. |
|
| Outcomes | QoL, measured by 15D instrument; measured at baseline, 16 weeks and 24 weeks Medication appropriateness, MAI and assessment of underutilisation; measured at baseline, 16 weeks and 24 weeks Short Physical Performance Battery score; measured at baseline, 16 weeks and 24 weeks Gait speed; measured at baseline, 16 weeks and 24 weeks Grip strength; measured at baseline, 16 weeks and 24 weeks Digit span forward; measured at baseline, 16 weeks and 24 weeks Digit span backward; measured at baseline, 16 weeks and 24 weeks Trail making test A; measured at baseline, 16 weeks and 24 weeks Trail making test B; measured at baseline, 16 weeks and 24 weeks Five Digits Test 1; measured at baseline, 16 weeks and 24 weeks Five Digits Test 2; measured at baseline, 16 weeks and 24 weeks Five Digits Test 3; measured at baseline, 16 weeks and 24 weeks Five Digits Test 4; measured at baseline, 16 weeks and 24 weeks Functional Independence Measure; measured at baseline, 16 weeks and 24 weeks Relative Stress Scale score; measured at baseline, 16 weeks and 24 weeks Change in SBP; measured at baseline, 16 weeks and 24 weeks Falls; measured 16 weeks and 24 weeks Weight; measured at baseline, 16 weeks and 24 weeks Hospital admissions; measured at 16 weeks and 24 weeks No. days patient spent in own home during follow‐up; measured at 16 weeks and 24 weeks Use of home nursing service; measured at baseline, 16 weeks and 24 weeks Admission to permanent institutional care; measured at 16 weeks and 24 weeks Mortality; measured at 16 weeks and 24 weeks |
|
| Notes | Funding: this study was funded by the Research Council of Norway (Dr Wyller). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated and carried out in blocks of unknown and variable size. A statistician not otherwise involved in trial procedures prepared the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | The research assistant, who provided all assessments, was blinded with respect to allocation. To avoid selection bias, the clusters (at family physician level) were randomised after all patients had been included in each cluster. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Cluster randomisation was done at the family physician level to avoid between‐group contamination. Due to the nature of the intervention, the geriatrician implementing the intervention and family physicians in intervention clusters could not be blinded. Although patients were repeatedly instructed not to reveal their allocation group to the research assistant, such revelations may have occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research assistant who carried out all outcome assessments was blinded as to allocation. The RA conducted 3 outcome assessments: baseline, 16 weeks and 24 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some of the secondary outcomes are only reported in Supplement 2. Some only have the statistical result reported and not the results of the test. These include falls, weight, hospital admissions, time spent in own home, use of home nursing service, admission to permanent institutional care and mortality. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported. Some are reported in a supplement to the main paper. |
| Protection against contamination | Low risk | Cluster‐randomisation was performed at the family physician level to avoid between‐group contamination. |
Schmader 2004.
| Study characteristics | ||
| Methods | Study design: randomised trial (2 × 2 factorial design) Setting: 11 Veterans Affairs hospitals, in the USA ‐ unclear if urban and/or rural Unit of allocation/analysis: participant Follow‐up: telephone interviews 12 months after randomisation Duration: participants were followed for 12 months Provider: pharmacist/nurse/geriatrician/social worker |
|
| Participants | 834 (430 intervention (inpatient), 404 control (inpatient)) participants who were 65 years of age or older, were hospitalised on a medical ward or surgical ward, had an expected stay of 3 or more days and met criteria for frailty Focus on polypharmacy: at baseline, the mean number of prescription drugs per participant in the geriatric inpatient unit was 7.7; number was 7.6 in the usual inpatient care group Age (ranges): 65 to 73 years (196 people in intervention group, 191 people in control group), 74 years or older (234 people in intervention group, 213 people in control group) Male: 97% intervention, 98% control Ethnicity, white: 71% intervention, 75% control |
|
| Interventions | Model of pharmaceutical care: pharmacists worked as part of a multi‐disciplinary team in outpatient clinics; the pharmacist(s) conducted an independent medication review together with participants during a face‐to‐face encounter Training: no education intervention was specified Duration: during inpatient period Quote: “All 11 inpatient and outpatient geriatric evaluation management programmes had a core team that included a geriatrician, a social worker and a nurse. Pharmacists performed regular assessments and recommendations regarding medications in 7 inpatient and 6 outpatient teams. For participants assigned to the GEM unit or clinic, team members implemented evaluation and management protocols Usual inpatient care was the customary medical or surgical treatment provided by attending physicians Usual outpatient care was the customary care delivered by ambulatory care attending physicians or house staff under their direction” |
|
| Outcomes | Adverse drug reactions and serious adverse drug reactions; measured at baseline, hospital discharge and 12 months Inappropriate prescribing was assessed using the MAI and the Beers list at baseline, hospital discharge and 12 months Polypharmacy and under‐use were also measured using AUM; measured at baseline, hospital discharge and 12 months |
|
| Notes | Funding: financial support was provided by grant AG‐15432 and the Veterans Affairs Cooperative Study Program 006. Additional support was provided by grant AG‐14158 from the National Institute on Aging, Washington, D.C.; grant AI‐51324 from the National Institute of Allergy and Infectious Diseases, Washington, D.C.; the VFW Endowed Chair in Pharmacotherapy for the Elderly, College of Pharmacy, University of Minnesota; and the Veterans Affairs Cooperative HSR&D Service. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Computer‐generated random allocation” |
| Allocation concealment (selection bias) | High risk | Quote: “The centre notified site research assistants of each participant's inpatient assignment by telephone. Outpatient assignment was revealed at hospital discharge” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “All assessments were performed blind to treatment status” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement |
Shim 2018.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial Setting: Duchess of Kent Hospital in the city of Sandakan, Sabah, Malaysia Unit of allocation/analysis: participant Follow‐up: 6 months Duration: end date of study unclear. Patients were followed up for 6 months from recruitment date, and recruitment was from February 2014 to February 2015 Providers: interventions were delivered by a researcher who had been practising as a pharmacist |
|
| Participants | 160 patients were randomised (intervention 80, control 80) Age: overall 65.0 to 87.0, median 71. Intervention: 65.0 to 87.0, median 72.0. Control 65.0 to 84.0, median 71.0. Sex: overall: male n = 87 (57.2%), female 65 (42.8%). Intervention: male 42 (57.5%), female 31 (32.5%). Control: male 45 (57%), female 34 (43%). Race: overall: Chinese n = 97 (63.8%), other 55 (36.2%). Intervention: Chinese 42 (57.5%), other 31 (42.5%). Control: Chinese 55 (69.6%), other 24 (30.4%). Co‐morbidities: circulatory system (n = 156, 23.5%), endocrine, nutritional and metabolic disorders (n = 132, 19.9%), genitourinary system (n = 62, 9.3%) No. of co‐morbidities: overall: 1.0 to 11.0 (median 4.5); intervention group 1.0 to 9.0 (median 4.0); control 2.0 to 11.0 (median 5.0) The most frequently prescribed medications were those for the cardiovascular system (n = 155, 28.2%), followed by those for the gastrointestinal tract and metabolism (n = 136, 24.8%), and for blood and blood‐forming organs (n = 113, 20.6%). |
|
| Interventions | Model of care: the aim of the study was to investigate the effects of collaborative interventions between pharmacists and physicians on health‐related outcomes of elderly patients, and medication adherence and medication appropriateness. This study is a collaboration between pharmacists and physicians and is an example of a complex health care intervention, which is “made up of several components, which may act both independently and inter‐dependently to achieve their desired outcomes”. Elderly patients visit pharmacies to refill their medications, therefore this is an opportunity for pharmacists to review medications. Timing: intervention participants reviewed by pharmacist; it is unclear if patients met with physician but pharmacists collaborated with physicians. Patients were followed up every 2 months after initial meeting for 6 months. Not clear how long each meeting lasted. This study was a RCT, conducted in a single centre, single‐blinded, 2 parallel groups, equal randomisation ratio of 1:1. Any elderly patients who sought treatment in the Medical Outpatient Department (MOPD) of the Duchess of Kent Hospital in Sandakan, Sabah, Malaysia, from February 2014 to February 2015, were invited to participate. Inclusion criteria: 65 years old or over, taking at least 5 medications, who could communicate in English, Bahasa Malaysia or Mandarin Exclusion criteria: medical conditions that could prevent effective communication such as deaf, mute, dementia, psychiatric. Those whose medications were supervised by caregivers/health care personnel. Those taking part in other studies or services. Informed written consent taken and patients followed up for 6 months. Sample size calculated as follows: "if the pharmacist intervention could improve medication adherence of elderly patients by 10%, with a 20% standard deviation (SD) and by using the sample calculator, OpenEpi (www.OpenEpi.com), with 95% confidence interval and 80% power of detection, at least 160 participants would be required for the study, assuming a 20% dropout rate." "Researcher enrolled participants and assigned to control or intervention groups using computerised random number generator, Research Randomizer (www.randomizer.org)." Intervention group participants were provided pharmaceutical care, which included medication reviews and reconciliation, counselling on their medications and how to use them. Importance of medication adherence was emphasised; reasons for non‐adherence noted and resolved. Pharmaceutical care issues (PCIs) were identified by pharmacist and discussed with physician if required to resolve the issue. "Medications of participants in intervention group were reviewed by pharmacist before seeing the physician so that any inappropriateness or DRPs could be identified and resolved in discussion with physician. Another researcher in the team, who is a pharmacist, or an ambulatory care physician, confirmed PCIs." Participants were followed up every 2 months for 6 months. Every 2 months, pharmaceutical care was given and phone calls were made to participants who did not arrive at the appointment. Participants were considered dropouts if they did not attend follow‐ups after 3 reminder calls. All participants were given RM20 as a token of appreciation at the end of the study. A research assistant, a pharmacist who was blinded to allocation of participants, conducted assessment of baseline and endpoint (6 months) outcomes. Medical and medication history were recorded at face‐to‐face interviews and confirmed by checking medical records. Medication appropriateness was assessed based on MAI score. The MAI is comprised of 10 items, which assess 10 elements of the prescribed chronic medications. Each item is weighted according to its importance in determining medication appropriateness. Medication adherence was measured using the Malaysian Medication Adherence Scale (MALMAS), a validated instrument. The MALMAS is comprised of 8 items, with scores less than 6 representing non‐adherence and 6 to 8 representing adherence. The participants’ knowledge of the use of their medications was assessed based on their understanding of correct doses, frequencies, indications and time of administration of their medicines. All data were analysed using SPSS version 20. Descriptive analysis was conducted on all data; numeric data were analysed for mean values, SDs and medians. The Pearson Chi2 test was used to analyse associations between categorical data. Differences in numeric outcomes between intervention and control groups were analysed using the Mann‐Whitney U test for independent samples. Any P value < 0.05 was considered statistically significant. Effect size, which measures the magnitude of difference between intervention and control groups, was reported using the formula recommended by Jin et al (Jin H, Kim Y, Rhie SJ. Factors affecting medication adherence in elderly people. Patient Prefer Adherence. 2016;10:2117‐2125). Effect size was defined as: small = 0.1, medium = 0.3 and large = 0.5.31 A generalised estimating equation (GEE) analysis was performed to confirm the effects of the collaborative interventions on participants’ medication adherence and medication appropriateness based on MAI scores. Patients in the control group received usual outpatient care from the pharmacy, which consisted of dispensing medications with brief instructions on the method of administration. These participants were asked to return to the pharmacy for further assessment only after 6 months. |
|
| Outcomes | Medication adherence scored by MALMAS – Malaysian Medication Adherence Scale; assessed at baseline and 6 months Medication appropriateness, scored by MAI; assessed at baseline and 6 months Medication knowledge (unvalidated questionnaire); assessed at baseline |
|
| Notes | Funding: the University of Malaya provided a research grant for this study (PG017‐2013A). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A researcher enrolled the participants and assigned them to control or intervention groups, according to the random allocation sequence generated using a computerised random number generator, Research Randomizer. |
| Allocation concealment (selection bias) | Unclear risk | Not enough information to make a decision. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was single‐blinded. Participants could not be blinded due to the nature of the intervention. Pharmacists and physicians could not be blinded as they were delivering the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The baseline and end point (at 6 months) outcomes were assessed by a research assistant who was blinded to the allocation of participants to the control and intervention groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete/presented. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Protection against contamination | Unclear risk | Not enough information to make a decision. |
Spinewine 2007.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: Mont‐Godinne teaching hospital near the town of Yvoir, Belgium Unit of allocation/analysis: participant Follow‐up: 1 month, 3 months and 1 year Duration: from admission to discharge Provider: pharmacists |
|
| Participants | 186 hospital inpatients (96 intervention, 90 controls) aged 70 years and older with acute geriatric problems Focus on polypharmacy: at baseline, mean (± SD) number of prescribed drugs was 7.9 (± 3.5) for participants in the intervention group and 7.3 (± 3.3) for those in the control group Age (mean ± SD): 82.4 ± 6.9 years intervention, 81.9 ± 6.2 years control Female: 71.9% intervention, 66.7% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: pharmacists worked as part of inpatient services on hospital wards as a clinical pharmacy service; the pharmacist(s) conducted an independent medication review together with participants during a face‐to‐face encounter, which was discussed with the prescriber Training: education was provided to prescribers Timing of intervention: during the hospital inpatient stay Quote: “The intervention consisted of the provision of pharmaceutical care from admission to discharge by a clinical pharmacist. A pharmacist was present 4 days per week and participated in medical and multi‐disciplinary rounds, had direct contact with participants and carers and had access to participant medical records. For every participant, the pharmacist performed a medication history on admission and prepared a participant record with clinical and pharmaceutical data. Appropriateness of treatment was analysed, and a pharmaceutical care plan was prepared. Whenever an opportunity to optimise prescribing arose, the pharmacist discussed this with the prescriber, who could accept or reject the advice. The pharmacist answered all questions received from healthcare professionals about medications. At discharge the pharmacist provided written and oral information on treatment changes to the participant or carer, as well as written information to the GP” |
|
| Outcomes | Prescribing appropriateness measured using MAI, Beers list, ACOVE; measured at baseline and discharge from hospital Mortality, readmission (hospital admissions) or visit to an emergency department, medications taken, unnecessary drug use and satisfaction with information provided at admission and at discharge; measured at 1 month, 3 months and 1 year after discharge |
|
| Notes | Funding: one of the authors received research support from the National Institutes of Health, Grants RO1 AI 5535901 and K23 AI068582‐01. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was alternate and was stratified for age, number of prescribed medicines and identity of the resident in charge of the participant. A pharmacist external to the main study checked the inclusion criteria and assigned participants to their groups” |
| Allocation concealment (selection bias) | High risk | Quote: “A pharmacist external to the main study checked inclusion criteria and assigned participants to their groups” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The physicians were not blinded to group assignment because of the nature of the project” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not double‐blinded, and MAI evaluations at discharge were unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants in both control and intervention groups were transferred to another unit. 5 participants in each of the groups (10 people in total) died. |
| Selective reporting (reporting bias) | High risk | A secondary outcome, 'medications taken', was not reported. |
| Protection against contamination | High risk | Some physicians cared for control and intervention participants. |
Strauven 2019.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised controlled trial Setting: 54 nursing homes in Belgium Unit of allocation/analysis: cluster at nursing home level Follow‐up: 15 months Duration: e‐learning: 4 modules at 60 mins each. Workshop: 2 hours. 2 consultation meetings at 2 hours each. Face‐to‐face medication reviews with nursing home residents (unclear how long these lasted or how often they were held). Case conferences – 3 per resident over 12 months. Providers: Component 1 – educational material developed by research team. The research team moderated the face‐to‐face workshops. Component 2 ‐ material provided by the research team. The co‐ordinating physician, the pharmacist and the head nurse were invited to take the lead in preparing, organising and implementing the meetings. Component 3 – face‐to‐face medication reviews conducted by an interdisciplinary team consisting of 3 HCPs: the GP, the pharmacist and a nurse (head nurse, or other nurse involved in the care of the resident). The interdisciplinary case conferences were facilitated by the use of a web application. Nurse and GP were supposed to represent the interest of the residents by sharing information on the perception and preferences of the NHRs regarding their current medication regimen. 701 healthcare professionals (55 co‐ordinating physicians, 378 GPs, 85 pharmacists and 183 nurses) |
|
| Participants | 54 nursing homes were randomised, with 1804 participants: 24 in the intervention group with 847 residents; 30 in the control group with 957 residents Age: median (IQR): intervention: 87 (82 to 92); control: 88 (83 to 92) Sex: intervention: 590 females (69.7%); control: 682 (71.3%) Race: not given Median comorbidity score (CIRS‐G) (IQR): intervention 25 (21 to 29), control 24 (21 to 28) The 3 most prevalent comorbidities: hypertension, n (%): intervention 443 (56.0%), control 402 (56.1%); dementia/dementia syndrome/cognitive impairment, n (%): intervention 471 (59.5%), control 388 (54.2%); osteoarthritis, n (%): intervention 501 (63.3%), control 474 (66.2%) Median number of medications at baseline (IQR): intervention group = 9 (6 to 12); control group = 9 (6 to 11) |
|
| Interventions | Model of care: the aim of the study was to investigate the impact of a complex multifaceted intervention on the appropriateness of prescribing for Belgian nursing home residents Timing: e‐learning: 4 modules at 60 mins each. Workshop: 2 hours. 2 consultation meetings at 2 hours each. Face‐to‐face medication reviews with nursing home residents (unclear how long these lasted or how often they were held). Case conferences – 3 per resident over 12 months. The key element of this complex multifaceted intervention was the structured and repeated interdisciplinary resident’s medication review (or ‘interdisciplinary case conferences’) supported by training and local consultation. The intervention consisted of 3 interacting components: Component 1: education and training. A blended learning programme, combining e‐learning with face‐to‐face workshops, was developed. Training needs and desired formats were discussed during focus groups with HCPs. The structure of the e‐learning platform, the format and content of the blended learning programme were developed in collaboration with a team of HCPs and experts in e‐learning and geriatric pharmacotherapy. The e‐learning consisted of four modules on the following topics: drugs and ageing; (in)appropriate prescribing; medication review; and teamwork. Each module took approximately 60 min to complete and was built up from a variety of learning formats including narrative PowerPoints, videos, serious games, assignments, summary tools on specific topics and tests. All participating HCPs from NHs allocated to the intervention group had access to the e‐learning platform that was available during the whole study duration. Educational material developed by the research team was also available through the e‐learning platform. It included a medication review flowchart, a shortlist of STOPP/ START criteria and summary sheets on different topics (e.g. renal function, anticholinergic drugs). The research team moderated two types of face‐to‐face interactive workshops (i.e. on‐site training), 2 hours each, one specific for pharmacists and one with all participating HCPs of one or more NHs. The aim was to apply the theoretical concepts, addressed during the e‐learning, to clinical cases, and to get familiar with research instruments (DRP classification; web application). Specific on‐site training for nurses could be given by the co‐ordinating physician and/or the pharmacist. To encourage this type of training, preparatory material on administration of medications and on detection of ADEs was provided by the research team. As an incentive, the e‐learning modules and on‐site workshops were accredited for GPs as well as for pharmacists. As there was no similar accreditation programme for nurses, they received a certificate of attendance. Component 2: local consultation. At the level of each participating NH, physicians (GPs and co‐ordinating physician), pharmacists and nurses were asked to participate in 2 local consultation meetings. The objectives of this component were (a) to reach consensus on the appropriate use of one specific class of medication within each NH, with the intent that this work could then be used during the interdisciplinary case conferences and (b) to initiate teamwork and communication between HCPs of the same NH. The overall output was expected to be (i) a ‘vision’ or a ‘management plan’ for the treatment of certain condition(s), (ii) a list of (in)valid indications for the use of the discussed medications; (iii) a list of molecules to be preferred or avoided, and underlying reasons and (iv) modalities of the use of the preferred drugs (e.g. dosage, duration, follow‐up). Two medication classes ‐ antidepressants and lipid‐lowering drugs ‐ were selected by the research team. Material to support the preparation of the meetings and the discussion was provided by the research team. Each meeting was expected to last approximately 2 hours. The co‐ordinating physician, the pharmacist and the head nurse were invited to take the lead in preparing, organising and implementing the meetings (i.e. invitations, content, discussion, guidelines, overview of figures and numbers of NHRs taking these medications, summary, report). Depending on the opportunities and willingness, additional HCPs could be invited to participate (e.g. geriatrician, psychiatrist, physiotherapist, occupational therapist). The first meeting had to be held at the beginning of the study, ideally before the first interdisciplinary case conferences. A second meeting took place several months later. The objective of the latter was to review the implementation of the consensus reached after the first meeting, to re‐discuss or amend this consensus if necessary, and/or to start the discussion about the second medication class. The HCPs involved in the study were paid for their attendance at the meetings. Component 3: interdisciplinary case conferences. Face‐to‐face medication reviews had to be conducted for each NHR included in the study by an interdisciplinary team consisting of three HCPs: the GP, the pharmacist, and a nurse (head nurse, or other nurse involved in the care of the resident). The medication review focused on the appropriate and cost‐effective use of all medications taken by the resident. During the discussion, the team determined whether drugs must be additionally prescribed, tapered, discontinued, dose‐adjusted or replaced, and whether other actions are needed. They were also requested to prioritise and time schedule the treatment modifications to be made. Drug‐related problems and interventions had to be recorded using a DRP classification tool adapted from Basger et al and from the PCNE classification V6.2. The interdisciplinary case conferences were facilitated by the use of a web application. This web application was primarily developed to allow electronic data collection (clinical, medical, economic and medication data). It also enabled (i) sharing data about NHRs between HCPs in the intervention group, allowing preparation of the medication review by each HCP and (ii) generating a standardised report of every case conference, including details on DRPs and interventions, with the possibility to re‐discuss or amend these interventions at the next meeting. A total of three case conferences per resident over a 12‐month period were aimed for. Each case conference was estimated to last about 20 mins. For residents with a hospital admission during the study period, HCPs were encouraged to perform an additional medication review in the fortnight after hospital discharge. For residents entering end‐of‐life care, an additional medication review could be conducted with a focus on stopping unnecessary medications and optimising comfort. NHRs did not participate in the case conferences. However, the nurse and the GP were supposed to represent the interest of the residents by sharing information on the perception and preferences of the NHRs regarding their current medication regimen. NHRs and/or their family were given the opportunity to receive feedback from the nurse or the GP on the issues discussed during the case conferences, and to get information on the treatment and the proposed changes. HCPs could agree to postpone the implementation of certain interventions until discussion with and agreement of the NHR and/or family. The HCPs were paid for each interdisciplinary case conference. Data were collected at 3 points in time: at month 1 (baseline), month 8 and month 15 (end of study). Data collection was performed by the HCPs involved in the care of each resident through a web application. Administrative and clinical data (e.g. age, functional status) were collected by the nurse, comorbidities (past and current medical history) and laboratory values by the GP, and medication data by the pharmacist. In the middle (month 8) and at the end of the study (month 15), data on health care use (hospital admissions, emergency visits, GP or specialist visits) were collected by the nurse and the GP. Characteristics of NHs (e.g. number of beds, location) and administrative data about participating HCPs were collected at the beginning of the study (month 1) from the co‐ordinating physician and respective HCPs. HCPs were paid for data collection. In the intervention group, HCPs were also requested to record data on each case conference (participants, time for preparation, duration, DRPs identified, interventions). Nursing homes allocated to the control group received no intervention and continued delivering usual care. After completion of the last data collection, control NHs had access to the e‐learning platform and had the option of attending a symposium that presented a summary of key messages relative to the effect of the intervention. They also received feedback on their own results relative to the appropriateness of prescribing. |
|
| Outcomes | Outcomes were measured at baseline, 8 months and 15 months Appropriateness of prescribing – counts of PIMs or PPOs Mortality No. of medications Visits to specialised physicians Visits to GPs Hospital admissions Visits to emergency department No. of medications, counted in classes Interdisciplinary case conferences: measuring median number of ICC per resident, median number of drug‐related problems per resident, cause and type of identified DRP and interventions, implementation status and drugs involved Cost |
|
| Notes | Funding: The study was funded by the National Institute for Health and Disability Insurance (NIHDI). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Nursing homes were stratified by (a) province/region, (b) experience with multidisciplinary case conferences and (c) type of medication delivery (by a hospital pharmacy or by one or more community pharmacies). Factors (b) and (c) were considered to be possibly significant covariates. Geographical location (factor a) was taken into account because the funding body (NIHDI), requested that (i) at least one NH from each province/region was given the opportunity to implement the intervention and (ii) the number of NHs allocated to each group was balanced per province/region. In 4 of the 10 Belgian regions/provinces, only one NH applied. These 4 NHs were therefore immediately assigned to the intervention group. The characteristics of the other 59 NHs with regard to location (province/region), experience with case conferences and type of delivering pharmacy, were entered into SPSS. This programme generated a series of blocks for each stratum and allocated NHs to control or intervention group randomly within each block. In terms of selection bias, the authors commented that this may have occurred because only NHs that applied freely were included. Further, only NH residents being treated by GPs who were willing to take part in the study were included. From a subsequent process evaluation, it was learned that these GPs were among the most ‘open’. |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by an independent researcher blinded to the identification of the NHs and not involved in the recruitment of NHs or residents. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Because of the nature of the intervention, it was not possible to blind NHs or HCPs to the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collection was performed by the HCPs involved in the care of each resident through a web application. Administrative and clinical data (e.g. age, functional status) were collected by the nurse, comorbidities (past and current medical history) and laboratory values by the GP, and medication data by the pharmacist. In the middle (month 8) and at the end of the study (month 15), data on healthcare use (hospital admissions, emergency visits, GP or specialist visits) were collected by the nurse and the GP. Characteristics of NHs (e.g. number of beds, location) and administrative data about participating HCPs were collected at the beginning of the study (month 1) from the co‐ordinating physician and respective HCPs. In the intervention group, HCPs were also requested to record data on each case conference. HCPs had access only to data collection files of NHRs for which they were responsible. Data export from the web application to the research database was performed with the intervention of a trusted third party (TTP), who was responsible for data coding and small cell analysis. NHRs and HCPs were be known to the research team by study ID number only. It is unclear if this meant that the research team members were blinded to outcomes/data. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The methods section stated that data collection would take place at baseline, middle and end of study. Results seem to be presented only for baseline and end. |
| Selective reporting (reporting bias) | High risk | Results of cost analysis not presented in this paper. Full results of ICCs will be reported in a separate report. |
| Protection against contamination | Low risk | As the intervention was mainly provided at the level of HCPs, a cluster design was chosen to prevent contamination bias. Each co‐ordinating physician, pharmacist and management board could not be involved in more than one application to the national call to nursing homes in Belgium (to maximise variability in the sample and to prevent contamination bias). Each GP only had patients in either the intervention or control group. |
Syafhan 2021.
| Study characteristics | ||
| Methods | Study design: pragmatic, multicentre, randomised controlled trial Setting: GP practices in regions of Northern Ireland and England ‐ likely a mix of rural and urban Unit of allocation/analysis: participant Follow‐up: 6 months Duration: patients involved for 6 months Providers: pharmacists who were based at general practices |
|
| Participants | 356 patients were recruited and randomised: intervention 181; control 175 Age: mean years ± SD 67.9 ± 13.1; median (IQR) 70 (60.0 to 78.0); 18 to 65 years (n (%)) 126 (35.4); > 65 years (n (%)) 230 (64.6); range 25 to 96 Sex: female (n (%)): 192 (53.9); male: 164 (46.1) Race: not given Number of active medical problems (mean ± SD): 7.6 ± 3.4; median (IQR) 7 (5 to 10) The average number of prescribed long‐term medicines: 10.3 |
|
| Interventions | Model of care: the aim of the study was to evaluate the impact of a medicines optimisation intervention, delivered by GP practice‐based pharmacists, to patients at risk of medication‐related problems (MRPs), on patient outcomes and healthcare costs. Recognising pharmacists as part of healthcare teams within primary care, the intervention focused on medication optimisation and how shared decision‐making was an important part of that. Timing: baseline pharmacist intervention and appointment with patient; at 2 months, same as baseline – appointment with pharmacist/patient; 4 months, same as baseline and 2 months; 6 months – end of study questionnaires Prior to the first medicines optimisation intervention by the clinical pharmacist, he/she reviewed the patient’s medical notes, electronic record and laboratory data to identify potential medication‐related issues. When the patient attended the practice, the pharmacist, through discussion with the patient, compiled a complete medication history (including non‐prescription medicine use), reviewed medication adherence and appropriateness of medicines and discussed medication management with each patient. A pharmacist intervention guide, containing forms to complete for each patient at different stages of the research was available to assist in ensuring uniformity of the process within and across practices. Having completed this process, the pharmacist created a list of potential MRPs in order to develop an individualised medicines optimisation intervention plan for each patient. The clinical pharmacist aimed at delivering a holistic, patient‐centred service to each participating patient based on individual needs, expectations and outcomes that mattered to the patient. The interventions included recommending/making changes to medication regimens (in collaboration with GPs), personalised education and counselling on medication management, the correct use of medication administration devices and lifestyle factors, as appropriate for each patient. The pharmacist also, in discussion with the patient, drew up a list of treatment goals. Where necessary, the pharmacist referred the patient to another healthcare professional within the practice for management of other patient matters identified during the appointment, e.g. referred to a diabetic specialist nurse. Having completed the intervention, the pharmacist produced a short report for the patient’s GP outlining actions taken and any further recommendations which required input from the GP. The same procedure was followed at subsequent patient visits at 2 and 4 months, building upon patient progress towards agreed goals. The control group were the normal care group. A bi‐weekly teleconference involving all participating pharmacists and a site visit to each GP practice site were conducted by the university‐based members of the research team as an integral part of project management to ensure that all the pharmacists were delivering a uniform service and completing all paperwork appropriately. The control group continued with usual care. They were asked to complete baseline questionnaires and end of study questionnaires at the 6‐month time point. |
|
| Outcomes | No. of medication‐related problems (MRPs identified using classification devised by AbuRuz et al (ref 36); measured at baseline and 6 months MAI score; measured at baseline and 6 months MARS beliefs about medicines questionnaire; measured at baseline and 6 months HRQoL patient satisfaction with pharmacist service; measured at baseline and 6 months Healthcare resource utilisation; measured at 6 months and the previous 6 months prior to study starting |
|
| Notes | Funding: This study was partially funded by the Association of the British Pharmaceutical Industry (ABPI) (grant number MOIC001). NFS was sponsored by the Indonesia Endowment Fund for Education (LPDP) (20160422046233). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were arranged in random order (random.org) and then randomly assigned to control and intervention groups. The list of patients in each practice who met the inclusion criteria of stratum 1, followed by stratum 2 and who had no exclusion criteria was arranged in a random order (random.org) and then randomly assigned to control and intervention groups. |
| Allocation concealment (selection bias) | Unclear risk | Does not seem to refer to this. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding is not referred to at all. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who implemented the questionnaires or other assessments. Pharmacists undertaking the assessments could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All outcomes listed in the methods section are reported on in the results. Some secondary outcomes have little detail, e.g. no measures of deviation reported. A secondary outcome measure listed in the study’s record detail on clinicaltrials.gov – patient laboratory data – is not mentioned in this report. |
| Selective reporting (reporting bias) | High risk | Based on the difference between the study register and the outcome listed there, which is not mentioned in the report. |
| Protection against contamination | Unclear risk | Contamination is not mentioned. It seems there was potential for contamination as intervention and control patients were interacting with the same health professionals. |
Tamblyn 2003.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: GP practices in Montreal, Canada Unit of allocation: physicians Unit of analysis: participant Follow‐up: terminated after an inappropriate prescription had been initiated or discontinued Duration: 13 months Provider: physician |
|
| Participants | 107 primary care physicians with at least 100 participants, who were 30 years of age or older, had practices in Montreal and spent at least 70% of the week in fee‐for‐service practice, were randomly assigned. Participants were 66 years of age or older, had been seen on 2 or more occasions by the study physician in the past year and were living in the community at the start of the study. Focus on polypharmacy: implied 35.6 intervention/33.8 control prescriptions per elderly patient in the 18 months before the study date Age (mean ± SD): 75.4 ± 6.3 years intervention, 75.3 ± 6.2 years control Female: 61.2% intervention, 64.2% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: physicians delivered the intervention via a computerised support programme, participants' medication lists were screened by the physicians Training: no educational intervention specified Timing of intervention: unclear Quote: “Each physician was given a computer, a printer, health record software and dial‐up access to the Internet. The software documented health problems and medications supplied. For each participant, trained personnel developed a health problem list and documented 26 health problems related to targeted drug‐disease contraindications and other health problems. CDS group physicians downloaded updates of dispensed prescriptions from the Quebec beneficiary, medical‐service and prescription claims database (Regie de l'assurance maladie du Quebec (RAMQ)). Data were integrated into the participant's health record and were categorised as having been prescribed by the study physician or by another physician. Alerts were instituted to identify 159 clinically relevant prescribing problems among the elderly (McLeod 1997). Alerts appeared when the physician accessed the record, when prescription record updates were downloaded from RAMQ and when current health problems and prescriptions were recorded in the chart by the physician. They identified the nature of the problem, possible consequences and suggested alternative therapy in accordance with expert consensus” |
|
| Outcomes | Initiation and discontinuation rates of 159 prescription‐related problems (McLeod criteria); assessed over the 13‐month study period | |
| Notes | Funding: "Funding was provided by the Fonds de recherche en santé du Québec, the Fond d'autoroute à l'information, the Medical Research Council, the National Health Research and Development Program and Clinidata Inc. In addition, Dr. Tamblyn was supported as a health scholar by the National Health Research and Development Program. This study was made possible by support provided by the Régie de l'assurance maladie du Québec, which developed the computerized interface for the drug insurance‐claims database of the seniors drug‐insurance program, and by Clinidata, which developed the software to record disease and drug profiles and to conduct automated surveillance for investigator‐defined prescribing problems." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Physicians were stratified by age, sex, language, location of medical school and number of elderly patients. Half of the physicians within each stratum were randomly assigned to the CDS group” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “Physicians and patients were not told the specific outcomes of the study but were aware of which group they had been assigned to” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of inappropriate scripts started per 1000 visits and number of inappropriate scripts discontinued per 1000 visits were reported. |
| Selective reporting (reporting bias) | Low risk | All results of outcomes specified in the methodology were reported. |
| Protection against contamination | Unclear risk | To minimise the possibility of contamination, only 1 physician per group practice was included. |
Taylor 2003.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: rural Alabama. Patients were recruited via clinics affiliated with the University of Alabama School of Medicine in Tuscaloosa and other towns in Pickens County, Alabama. Pickens County ranks among the 13% poorest counties in the USA. Unit of allocation/analysis: participant Follow‐up: 12 months Duration: baseline until 12 months Provider: pharmacists |
|
| Participants | 33 intervention patients, 36 control who received care at 3 community‐based family medicine clinics Focus on polypharmacy: patients eligible for inclusion were taking 5 or more medications, 12 or more doses per day, or both Age (mean ± SD): 64.4 ± 13.37 years intervention, 66.7 ± 12.3 years control Male: 36.4% intervention, 27.8% control Ethnicity, white: 60.6% intervention, 61.1% control |
|
| Interventions | Model of pharmaceutical care: medication reviews were provided by pharmacists in community‐based family medicine clinics during a face‐to‐face encounter with participants Training: no educational intervention was specified Timing of intervention: during a single attendance at outpatient clinics Quote: “Participants received usual medical care along with pharmacotherapeutic interventions provided by a pharmacist during regularly scheduled clinic visits, based on the principles of pharmaceutical care. A participant typically met with a pharmacist for 20 minutes before seeing a physician. Published therapeutic algorithms and guidelines were used as the basis of the pharmacists' recommendations. Pharmacists were specifically trained to evaluate a therapy's indication, effectiveness and dosage, as well as the correctness and practicality of directions, drug‐drug interactions, drug‐disease interactions, therapeutic duplication and duration of treatment, untreated indications and expense The pharmacist reviewed the medical record for medication‐related problems, conducted a chart review to ensure that information on drug therapy and allergies was accurately documented, examined the medication history to determine compliance with and complications of medications and provided comprehensive individualised participant education, which included a brief review of the disease, important lifestyle modifications and basic drug information. Pharmacists monitored participants' responses to drugs and attempted to improve compliance by consolidating medication regimens, reducing dosage frequency, devising medication reminders and teaching participants techniques for using devices such as inhalers. In addition to this, a system was developed in which the participant, the physician or the nurse reported suspected problems associated with drug therapy. Participants, nurses and physicians were educated about the signs and symptoms of medication misadventures. The control group received standard medical care” |
|
| Outcomes | Number of inappropriate prescriptions at baseline and at 12 months using the MAI Change in number of hospital admissions and emergency department visits at 12 months Medication misadventures, medication compliance (participant self‐report), knowledge and pharmacy‐related satisfaction were recorded at 12 months Quality of life (SF‐36); assessed at baseline and 12 months |
|
| Notes | Funding: This study was supported by the ASHP Research and Education Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly assigned to a control group or an intervention group” Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 participants were not included because they were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described were reported. |
| Protection against contamination | High risk | Although participants were randomly assigned, physicians were not because of the small number of physicians practising in the rural community. |
Thyrian 2017.
| Study characteristics | ||
| Methods | Study design: cluster‐randomised trial Setting: GP practices in the state of Mecklenburg‐Western Pomerania, northern Germany Unit of allocation: GP practices Unit of analysis: patients Follow‐up: 12‐month follow‐up Duration: 12 months Providers: nurses |
|
| Participants | 516 older patients (348 intervention and 168 control) recruited from 95 GP practices in Germany Focus on polypharmacy: number of drugs on admission, 6.4 ± 3.2 Age (mean ± SD): 80.6 ± 5.7 years intervention, 79.8 ± 5.0 years control Male: 38.8% intervention, 39.7% control Ethnicity: not reported |
|
| Interventions | Model of pharmaceutical care: the nurses conducted an in‐depth assessment, computer‐assisted assessment determining a personalised array of intervention modules and subsequent success monitoring Training: no educational intervention was specified Timing of intervention: unclear Quote: “Dementia care management aims to provide optimal care by integrating multi professional and multimodal strategies for improving patient‐ and caregiver‐related outcomes within the framework of the established health care and social service system. It was developed according to current guidelines, targeted at the individual participant level, and delivered at patients’ homes by 6 nurses with dementia‐specific qualifications supported by a computer‐based intervention‐management system(IMS) to improve systematic identification of patients’ and caregivers’ unmet needs. The nurses conducted an in‐depth assessment. Based on these data, the IMS generated an individual preliminary intervention task list, and the nurses discussed and finalized the task list in a weekly interdisciplinary case conference with a nursing scientist, a neurologist/ psychiatrist, a psychologist, and a pharmacist. Afterwards, the list of intervention tasks was summarized in a semi standardized GP information letter. This letter was then discussed between the GP and nurse to establish an individual treatment plan. During the first 6months of the intervention period, the nurse conducted 6 home visits with an average duration of 1 hour, carrying out his or her standard intervention tasks in close cooperation with the caregiver, the GP, and health care and social service professionals. During the subsequent 6 months, the study nurse monitored the completion of all intervention tasks. In line with the Pacala scale for intensive case managements, each study nurse delivered intervention to, on average, 60 patients with dementia Participants cluster‐randomised to the control group received care as usual in a primary care setting” |
|
| Outcomes | Use of potentially inappropriate medication (PRISCUS criteria); measured at baseline and 12 months Quality of life (QoL‐AD); measured at baseline and 12 months |
|
| Notes | Funding: The study was performed in cooperation with and funded by the German Center of Neurodegenerative Diseases and the University Medicine of Greifswald. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The GP was randomized by fair coin tossing to care as usual or intervention group” |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The randomization was done before baseline assessment of the individuals and the intervention cannot be classified as blinded, neither on the level of the GP, nor on the level of the study participant” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: “Because baseline assessment, primary outcome assessment, and delivery of intervention needed to be performed by the same nurses, blinding was not possible” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | High risk | Not all of the study’s pre‐specified secondary outcomes have been reported. |
| Protection against contamination | Low risk | Randomised at practice level |
Wehling 2016.
| Study characteristics | ||
| Methods | Study design: randomised trial Setting: hospitals in the German cities of Mannheim and Essen Unit of allocation/analysis: participant Follow‐up: unclear, admission to discharge i.e. duration of stay Duration: unclear, admission to discharge i.e. duration of stay Providers: ward physicians |
|
| Participants | 409 patients (202 intervention, 207 control) aged > 65 years Focus on polypharmacy: number of patients with 6 to 10 medications (%), 55.0% intervention, 56.5% control Age (mean): 84 intervention, 82 control (see notes) Male: 36.6% intervention, 34.3% control Ethnicity: no information given |
|
| Interventions | Model of pharmaceutical care: medicine reviews were undertaken by the doctor Training: physician education provided during the study Timing of intervention: during inpatient stay Quote: “On the intervention ward (FORTA group), physician education was structured and continuously provided during the study. The physicians were formally instructed about the FORTA‐principle and provided with the relating documents (publications, current FORTA‐list) by 2 lectures before the study commenced. They convened with the FORTA intervention team (study physicians) on a weekly basis (PharmaBoard) to review information, to collect data on patients included in the study and to discuss medication plans with respect to the FORTA system. Though individual recommendations may have been issued ward physicians were free to adopt them or not. The FORTA intervention team had no power and legal sanction to modify medication plans. The ward physicians’ own judgement was leading over FORTA‐based suggestions in the process of finding the appropriate medication On the control ward all patients were treated based on established medical standards and on the principles of good medical practice” |
|
| Outcomes | The quality of medications was assessed by the FORTA score. Secondary endpoints were the impact of FORTA on ADR and clinical outcomes. Outcomes were measured at admission and discharge. |
|
| Notes | Funding: The study was funded by DFG‐German Research Foundation (WE 1184/15‐1 to MW and HB; FR2997/2‐1 to HF). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Randomization had to be guided by random availability of beds on one ward only with the other ward being inaccessible at admission and this may have resulted in observed heterogeneities between the control and intervention groups at baseline” |
| Allocation concealment (selection bias) | Low risk | Quote: “The assignment was performed by a manager blinded to the purpose of the study” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Study described as an “open randomized controlled trial”. No apparent blinding of physicians based on intervention ward: on the intervention ward (FORTA group), physician education was structured and continuously provided during the study. The physicians were formally instructed about the FORTA‐principle and provided with the relating documents (publications, current FORTA‐list) by 2 lectures before the study commenced. They convened with the FORTA intervention team (study physicians) on a weekly basis (PharmaBoard) to review information, to collect data on patients included in the study and to discuss medication plans with respect to the FORTA system” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The assessment of medication quality and the adjudication of adverse drug reactions/clinical endpoints were performed by FORTA‐trained physicians who were not involved in patient recruitment, ward instruction on the study conduct and patient interviewing; thus, this could be done in a blinded manner after discharge of the patient on the base of a note and data review to avoid bias In addition, patients were asked for ADR and clinical records searched for related entries by the study team that was not blinded but did not participate in the endpoint adjudication as described above” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported. |
| Protection against contamination | High risk | Increasing contamination of the control ward by the intervention prevented the study authors from extending the recruitment period. The authors report that during the study, teams on the control ward seemed to have increasingly acquired skills and knowledge from the other ward by migration and/or communication. |
ACOVE: Assessing Care of the Vulnerable Elderly; ADE: adverse drug events; ADR: adverse drug reactions; AUM: under‐utilisation of medication; CDS: computerised decision support; CDSS: computerised decision support; CI: confidence interval; COPD: chronic obstructive pulmonary disease; cRCT: cluster‐randomised controlled trial; DID: difference in difference; DDIs: drug‐drug interaction; DRPs: drug‐related problems; ED: emergency department; eGFR: estimated glomerular filtration rate; EHR: electronic health record; EMR: electronic medical record; FORTA: Fit for The Aged; GP: general practitioner; HCA: healthcare assistant; HCP: healthcare professional; HRQoL: health‐related quality of life; IGCT: inpatient geriatric consultation team; IPET: Inappropriate Prescribing in the Elderly Tool; IQR: interquartile range; ITT: intention‐to‐treat; MAI: Medication Appropriateness Index; MARS: Morisky the Medication Adherence Rating Scale; MTM: medication therapy management; NH: nursing home; NHBPS: Nursing Home Behavior Problem Scale; OBRA: Omnibus Budget Reconciliation Act; PAL: Prescription Advantage List; PIMs: potentially inappropriate medications; PIP: potentially inappropriate prescribing; PPOs: potential prescribing omissions; QALY: quality‐adjusted life year; RAMQ: Régie de l'assurance maladie du Québec; RASP: Rationalisation of home medication by an Adjusted STOPP in older Patients; SBP: systolic blood pressure; SD: standard deviation; SF‐36: Short form 36; START: Screening Tool to Alert doctors to the Right Treatment; STOPP: Screening Tool of Older Person’s Prescriptions; TRIM: Tool to Reduce Inappropriate Medication
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| DRKS00013588 | Setting (nursing homes) |
| Hogg 2009 | Outcome measure: validated appropriateness criteria not applied to control group |
| Hugtenburg 2017 | Not all patients receiving polypharmacy. No measure of appropriateness. |
| Juola 2015 | No appropriate data |
| Rieckert 2020 | The outcome was a composite of hospital admissions and death and we could not disentangle these, and a validated measure of appropriate prescribing was not used. |
| Schmidt‐Mende 2017 | Not all patients receiving polypharmacy |
| Simon 2006 | Outcome measure: appropriateness criteria not validated (expert opinion) |
| Wouters 2017 | Not all patients receiving polypharmacy |
ACOVE: Assessing Care of the Vulnerable Elderly MAI: Medication Appropriateness Index
Characteristics of studies awaiting classification [ordered by study ID]
Aharaz 2021.
| Methods | Aim: to evaluate the feasibility and sustainability of a collaborative deprescribing intervention by a pharmacist and a physician to multimorbid patients in a Danish Subacute Medical Outpatient Clinic (SMOC). A randomised controlled pilot study was conducted, with phone follow‐up at 30 and 365+ days. |
| Participants | 67 patients (mean age 72.5 ± 12.3 years, 57% men) completed the study. Both intervention (n = 34) and control (n = 33) groups were comparable at baseline concerning gender, age, number of medications and number of comorbidities. A total of 38 of the patients (57%) were referred to the SMOC due to either anaemia, dyspnoea, pain, hypertension, oedema or decline in physical function. The 5 most frequent comorbidities in the study group were cardiovascular disease (56 patients, 78%), pain conditions (47 patients, 65%), mental/neurological illness (20 patients, 28%), respiratory disease (18 patients, 25%) and diabetes (16 patients, 22%). Sixty‐six patients (99%) had ≥ 1 diagnoses within the 5 most frequent comorbidities. In total, the five most frequent comorbidities accounted for 198 out of 295 (67%) of all comorbidities. There was no difference in the incidence of acute admissions between the control and intervention groups at 30, 90 and 180 days post inclusion (P ≥ 0.052). |
| Interventions | A senior pharmacist performed a systematic deprescribing intervention using the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) criteria, the Danish deprescribing list and patient interviews. A senior physician received the proposed recommendations and decided which should be implemented. |
| Outcomes | The main outcome was the number of patients having ≥ 1 medication where deprescribing status was sustained 30 days after inclusion. |
| Notes | — |
Del Cura‐Gonzalez 2022.
| Methods | Aim: to investigate a complex MULTIPAP intervention that implements the Ariadne principles in a primary care population of young‐elderly patients with multimorbidity and polypharmacy and to evaluate its effectiveness for improving the appropriateness of prescriptions |
| Participants | Patients aged 65 to 74 years with multimorbidity and polypharmacy FPs were cluster‐randomised to the intervention group (59 FPs, 298 patients) and control group (58 FPs, 295 patients). Participating patients had similar characteristics to non‐participants. Mean age (SD) control group: 69.9 (2.7) years; intervention group 69.6 (2.7) years. |
| Interventions | Family physicians (FPs) were randomly allocated to continue usual care or to provide the MULTIPAP intervention based on the Ariadne principles with 2 components: FP training (eMULTIPAP) and FP patient interviews. The training comprised a 4‐week course, which included multimorbidity, polypharmacy, appropriateness of prescribing, treatment adherence, the Ariadne principles, therapeutic cascade, deprescription and physician‐patient shared decision‐making basic concepts. |
| Outcomes | The primary outcome was the appropriateness of prescribing, measured as the between‐group difference in the mean MAI score change from the baseline to 6‐month follow‐up. A secondary outcome was the appropriateness of prescribing measured as the between‐group difference in the mean MAI score change from the baseline to the 12‐month point. Another secondary outcome was quality of life, using the EuroQol 5D‐5 L questionnaire. Patient perceptions of shared decision‐making were assessed using the collaboRATE measure and question number 33 from the National Health System (NHS) inpatient survey. Medication safety was measured as the incidence (number of events per patient year) of adverse drug reactions reported by the FP and potentially hazardous interactions using the taxonomy proposed by Otero‐López. Medication adherence was measured with the Morisky Medication Adherence score. Use of health services was measured as unplanned and/or number of hospitalisations, number of visits to emergency services, and number of FP and primary care nurse visits. |
| Notes | — |
Grischott 2022.
| Methods | Aim: to study whether a hospital discharge intervention combining medication review with enhanced information transfer between hospital and primary care physicians can delay hospital readmission and impact health care utilisation or other health‐related outcomes of older inpatients with polypharmacy. |
| Participants | 68 senior physicians and their blinded junior physicians included 609 patients ≥ 60 years taking ≥ 5 drugs. |
| Interventions | Participating hospitals were randomised to either integrate a checklist‐guided medication review and communication stimulus into their discharge processes, or follow usual discharge routines. |
| Outcomes | Primary outcome was time‐to‐first‐readmission to any hospital within 6 months, analysed using a shared frailty model. Secondary outcomes included readmission rates, emergency department visits, other medical consultations, mortality, drug numbers, proportions of patients with potentially inappropriate medication and the patients’ quality of life. |
| Notes | — |
Kirwan 2022.
| Methods | Aim: to assess the feasibility of a definitive trial of the MyComrade intervention across 2 healthcare systems (Republic of Ireland (ROI) and Northern Ireland (NI)) |
| Participants | Eligible practices were those in defined geographical areas who had GPs and practice‐based pharmacists (PBPs) (in NI) willing to conduct medication reviews. Eligible patients were those aged 18 years and over, with multimorbidity and taking 10 or more medications. Mean age (SD) of control group: 73 (± 12) years; and intervention group: 73 (± 10) years |
| Interventions | The MyComrade intervention is an evidence‐based, theoretically informed novel intervention which aims to support the conduct of medication reviews for patients with multimorbidity in primary care, using a planned collaborative approach guided by an agreed checklist, within a specified time frame. |
| Outcomes | Feasibility outcomes, using pre‐determined progression criteria, assessed practice and patient recruitment and retention and intervention acceptability and fidelity. Anonymised patient‐related quantitative data, from practice medical records and patient questionnaires were collected at baseline, 4 and 8 months, to inform potential outcome measures for a definitive trial. These included (i) practice outcomes ‐ completion of medication reviews; (ii) patient outcomes ‐ treatment burden and quality of life; (iii) prescribing outcomes ‐ number and changes of prescribed medications and incidents of potentially inappropriate prescribing; and (iv) economic cost analysis. The framework Decision‐making after Pilot and feasibility Trials (ADePT) in conjunction with a priori progression criteria and process evaluation was used to guide the collection and analysis of quantitative and qualitative data. |
| Notes | — |
Kornholt 2022.
| Methods | Aim: to investigate the effects of a comprehensive medication review intervention on health‐related quality of life (HRQoL) and clinical outcomes in geriatric outpatients exposed to polypharmacy |
| Participants | A total of 408 patients were included, with 196 in the medication‐consultation group and 212 in the usual care group. They were all taking 9 or more medications (median for whole study population = 12). Mean age (SD) of control group: 80.8 (7.3) years and intervention group: 80.5 (7.2) years |
| Interventions | The intervention was an additional consultation with a physician focusing on reviewing medication, informing patients about their medicines and increasing cross‐sectoral communication as a supplement to and compared with usual care. |
| Outcomes | The primary outcome was change in HRQoL after 4 months measured with the EuroQoL 5‐dimension 5‐level (EQ‐5D‐5L) questionnaire. Secondary outcomes were HRQoL after 13 months, mortality, admissions, falls and number of medicines after 4 and 13 months. |
| Notes | — |
Mahlknecht 2021.
| Methods | Aim: to achieve clinical benefits for older patients (aged 75+) by means of evidence‐based reduction of polypharmacy (defined as ≥ 8 prescribed drugs) and inappropriate prescribing in general practice |
| Participants | The trial involved 22 GPs and 307 patients in the intervention group; 21 GPs and 272 patients in the control group (northern Italy). |
| Interventions | The intervention consisted of a review of patient’s medication regimens by 3 experts who gave specific recommendations for drug discontinuation. |
| Outcomes | The main outcome measures were non‐elective hospital admissions or death within 24 months (composite primary endpoint). Secondary outcomes were drug numbers, hospital admissions, mortality, falls, fractures, quality of life, affective status, cognitive function. |
| Notes | — |
McCarthy 2022.
| Methods | Aim: to investigate the effect of a general practitioner (GP)‐delivered, individualised medication review in reducing polypharmacy and potentially inappropriate prescriptions (PIPs) in community‐dwelling older adults with multimorbidity in primary care. |
| Participants | Eligible patients were aged 65 years or over and prescribed 15 or more repeat medicines. Intervention group: 26 practices and 217 patients; control group: 25 practices and 205 patients Mean age of whole patient population in study: 76.5 years (SD 6.83); mean number of medicines: 17.37 (SD 3.50) |
| Interventions | Intervention GP practices had access to the SPPiRE website, where they completed an educational module and used a template for an individualised patient medication review that identified PIP, opportunities for deprescribing and patient priorities for care. |
| Outcomes | The 2 primary outcomes were the number of repeat medicines and the proportion of patients with any PIP, from a list of 34 prespecified indicators. Secondary outcomes relating to prescribing included the number of medicines stopped and started, the proportion of patients with a reduction in significant polypharmacy, the number of PIPs, the proportion of patients with a high‐risk PIP, the proportion of patients with any reduction in PIP. Secondary patient‐reported outcomes measures included health‐related quality of life (EuroQoL 5‐dimension 5‐level, EQ‐5D‐5L), revised Patients’ Attitudes Towards Deprescribing (rPATD) and the Multimorbidity Treatment Burden Questionnaire (MTBQ). Healthcare utilisation data were also collected. |
| Notes | — |
McDonald 2022.
| Methods | Aim: to evaluate the effect of an electronic deprescribing decision support tool on ADEs after hospital discharge among older adults with polypharmacy |
| Participants | A total of 5698 participants were enrolled in 3 clusters. Median (range) of whole patient population: 78 (72 to 85) years. There were 3204 patients in the control group and 2494 in the intervention group. |
| Interventions | Personalised reports of deprescribing opportunities generated by MedSafer software to address usual home medications and measures of prognosis and frailty. Deprescribing reports provided to the treating team were compared with usual care (medication reconciliation). |
| Outcomes | The primary outcome was a reduction of ADEs within the first 30 days postdischarge (including adverse drug withdrawal events) captured through structured telephone surveys and adjudicated blinded to intervention status. Secondary outcomes were the proportion of patients with 1 or more PIM deprescribed at discharge and the proportion of patients with an adverse drug withdrawal event (ADWE). |
| Notes | — |
Rankin 2022.
| Methods | Aim: to assess the feasibility of the PolyPrime intervention in primary care in Northern Ireland (NI) and the Republic of Ireland (RoI) |
| Participants | 12 GP practices were recruited and randomised; 68 patients with a mean age of 76.4 (SD 4.4) years for the whole study patient population. |
| Interventions | Practices allocated to the intervention arm watched an online video and scheduled medication reviews with patients on 2 occasions. |
| Outcomes | The feasibility of collecting GP record (medication appropriateness, health service use) and patient self‐reported data (health‐related quality of life (HRQoL), health service use)) were assessed at baseline, 6 and 9 months. HRQoL was measured using the EuroQol‐5 dimension‐5 level questionnaire (EQ‐5D‐5L) and medication‐related burden quality‐of‐life (MRB‐QoL) tool. An embedded process evaluation and health economics analysis were also undertaken. |
| Notes | — |
Rudolf 2021.
| Methods | Aim: to investigate whether special training and the PRISCUS card could lessen PIM and undesired drug–drug interactions (DDI) among elderly patients in primary care |
| Participants | A total of 1138 patients were recruited in 3 clusters (control group with 68 practices and 593 patients; intervention group 1 with 34 practices and 304 patients; and intervention group 2 with 35 practices and 316 patients. Mean age (SD) for the whole patient population in the study was 77.5 (4.92) years. |
| Interventions | In the intervention groups either the primary care physicians alone or the entire practice team received special training; the control group received general instructions about medication. |
| Outcomes | The primary endpoint was the difference in the percentages of patients at the practices with at least one PIM/DDI at baseline (T0) and 12 months later (T1). Secondary endpoints were overall mortality, the percentage of patients with at least one hospital admission and mean quality of life as assessed using the EQ‐5D health‐related quality of life questionnaire. |
| Notes | — |
ADE: adverse drug events AMO: admission medication order BPMH: best possible medication history CAS: computerised alert systems CDS: Clinical Decision Support EMR: electronic medical record FP: family physician GP: general practitioner HRQoL: health‐related quality of life KT: knowledge translation MAI: Medication Appropriateness Index PAPA: medication prescription adapted to elderly PIMs: potentially inappropriate medications PIP: potentially inappropriate prescribing SD: standard deviation START: Screening Tool to Alert doctors to the Right Treatment STOPP: Screening Tool of Older Person’s Prescriptions
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617000665336.
| Study name | Impact of clinical pharmacist medication review on appropriate prescribing in elderly patients: a randomized, trial |
| Methods | Randomised trial |
| Participants | Quote: “Patients are eligible for the study if they 1) attend medical follow up in Specialized Out‐patient Clinic (SOPC) of the Department of Medicine, 2) are 65 years or older, 3) have hyper‐polypharmacy (defined as 10 or more regular drugs and 4) agree to provide oral informed consent” |
| Interventions | Quote: “For the intervention group, clinical pharmacist with 5 years of clinical experience will perform medication chart review prior to the next SOPC follow‐up, The review includes assessing the appropriateness of each of the regular medications based on laboratory findings, medication lists, consultation and discharge notes, procedures and test results. Face‐to‐face interview (lasts for around 30‐45 mins) will then be conducted with patients on the day prior to the SOPC follow‐up. Clinical pharmacists will assess drug use history, identify drug‐related problems and provide drug therapy interventions through written pharmacist note to physicians during the SOPC follow‐up, based on the medication chart review and the above pharmaceutical assessments. Immediately after the SOPC follow up, clinical pharmacist will provide education (which lasts about 15 minutes) on drug‐related problem identified before the visit, reinforce physician’s instruction, and encourage drug compliance using written patient educational leaflets. Phone follow follow‐up will be conducted 1 month after the pharmacist intervention.” “For patient randomized to control group, they will attend the medical follow‐up as usual and receive usual care, in which patients would have visit their physicians during the Specialist Out‐patient Clinic (SOPC) and with their medication dispensed in the Out‐patient pharmacy as usual. No pharmacist medication review will be performed, and no pharmacist interview with patients for the control group” |
| Outcomes | Primary outcome: Medication Appropriateness Index (MAI) Secondary outcomes: Change in number of drugs prescribed to each participant, potentially inappropriate medications (PIMs) identified by Screening Tool of Older Persons’ Prescription (STOPP), potential prescription omission (PPOs) identified by the Screening Tool to Alert Doctors to the Right Treatment (START) Changes in total number of drug‐related problems 30‐day unplanned hospital admission Medication adherence measured by Morisky Score (MMAS‐4) |
| Starting date | July 2017 |
| Contact information | Miss Heidi Chan Pharmacy, Pamela Youde Nethersole Eastern Hospital, 3Lok Man Road, Chai Wan, HK, Hong Kong cyh123@ha.org.hk |
| Notes | Intervention phase complete, no results currently published |
Correard 2020.
| Study name | TEM‐EHPAD |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: aged ≥ 65 years, residing in a nursing home Exclusion (main): life expectancy < 3 months, with severe dementia |
| Interventions | Intervention: medication review done remotely (telemedicine) undertaken by a hospital‐based multidisciplinary team (clinical pharmacist and geriatrically trained internal medicine specialist) Control: care as usual |
| Outcomes | Primary outcome: unplanned hospitalisations (6 months) Main secondary outcomes (3 and 6 months): quality of life; behavioural disturbance; proportion of residents with at least one PIP; fall rates |
| Starting date | May 2019 |
| Contact information | Florian Correard florian.correard@ap‐hm.fr |
| Notes | Trial registry NCT03640845 |
Dauphinot 2017.
| Study name | Optimization of drug prescribing in an elderly population of geriatric consultations (OPTIM) |
| Methods | Multicentre, open‐label, randomised trial |
| Participants | Quote: “Patients aged 65 and over, patients received for the first time in a geriatric or memory consultation, patients living at home, patients with the ability to express themselves orally or in writing in French sufficiently to carry out clinical assessments, patients who led the last drugs prescription from his referring physician, at the geriatric/memory consultation (in current practice, patients should take the last prescription established by the referring physician), and patients accompanied by a caregiver” |
| Interventions | Quote: “The intervention group will participate to the optimization program: clinical medication review performed by a pharmacist in cooperation with the clinician. This aim is to identify actual and potential DRP, to decrease the potential iatrogeny of drug prescription and to improve the drug adherence of the patient. This intervention will be standardized across participating centers through a “Drug prescription optimization” form. The pharmacist will complete this report form including the patient data (medical, social, lab results and medication), their synthesis of medication review, and their PI in order to achieve drug optimization and their counseling/specific strategies in order to improve the drug adherence. In our study, the clinical medication review will be at the inclusion, 6 months and 18 months. The review of current medication performed by the pharmacist, in collaboration with the clinician (specialist physician), will also identify DRP (including pharmacological redundancy, medication overdose, need for a change in dosage form and PIP) taking into account the specificities of drug management in elderly patients. The DRP will be identified through a structured approach for each patient and the pharmacist will perform PI. The medication review will be standardized through various tools, including current national professional guidelines and international recommendations, medications databases, and prescription appropriateness as assessed by a set of validated quality indicators including Screening Tool of Older Persons’ potentially inappropriate prescriptions and Screening Tool to Alert doctors to Right Treatment (STOPP‐START) and Beers criteria. The PI are defined as “any action initiated by a pharmacist directly resulting in a change of the patient’s management or therapy’ to the physician” and including addition of a new drug, discontinuation, switch, dose adjustment, optimization of administration and drug monitoring. In order to optimize drug adherence, the pharmacist will provide comprehensive counseling and perform specific adherence strategies (information about medications and administration)” |
| Outcomes | Proportion of potential inappropriate medication (from clinical trial page) STOPP/START The occurrence and the number of all‐cause hospitalisations and all‐cause emergency department visits Quality of life of the patients measured by the questionnaire EUROQOL‐5D (EQ‐5D) |
| Starting date | May 2016 |
| Contact information | Dr Dauphinot Virginie virginie.dauphinot@chu‐lyon.fr/d_virginie@hotmail.com |
| Notes |
NCT02740764 Intervention phase ongoing |
DRKS00003610.
| Study name | Reduction of potentially inappropriate medication in the elderly |
| Methods | Randomised trial (cluster) |
| Participants | Patient participants: aged 70 years and older, taking at least 6 different drugs on a regular basis, life expectancy of at least 6 months (at the discretion of the treating primary care physician), legal competence, willingness to comply with study arrangements (i.e. assessment in the primary care office, telephone interviews) and to provide written informed consent, accessible by phone |
| Interventions | Quote: “Written information sources (pocket‐sized quick reference guide and comprehensive manual) containing recommendations from the PRISCUS list of potentially inappropriate medications in the elderly will be provided to general practitioners in the intervention arm. General practitioners will also be offered different training opportunities, depending on their needs and requirements, to allow them to get familiar with recommendations and to practice their application” |
| Outcomes | Quote: “Primary: proportion of participants per office with potentially inappropriate medication as defined by PRISCUS list (time frame: after 12 months of follow‐up)” |
| Starting date | May 2020 |
| Contact information | Prof. Hans‐Joachim Trampsich Department of Medical Informatics, Biometry and Epidemiology, University of Bochum, Bochum, Germany hans.j.trampisch@ruhr‐uni‐bochum.de |
| Notes | Intervention phase complete, no results currently published |
Greiver 2019.
| Study name | Supporting Practices in Improving Care for Complex Elderly Patients (SPIDER) |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion (practice): "a) contributes EMR [electronic medical record] data to the repository of a Practice Based Research Network (PBRN) that participates in CPCSSN; and b) includes a primary care provider (PCP) who consents to participate and lead the practice [intervention] team" Inclusion (HCP):" a) practices comprehensive family medicine in an office setting (academic or non‐academic); and b) consents to participate and allow the research staff to provide study information to their eligible patients" Inclusion (patient): "a) 65 years or older; b) has at least one office visit during the past 2 years; and c) has received ten or more different prescription medications (as indicated in the EMR) in the past year" |
| Interventions | Intervention: "The SPIDER intervention will include a family physician‐led inter‐professional practice team participating in 3‐4 Learning Collaboratives over a period of 12 months, reviewing validated and comparable practice EMR data and working with a QI Coach to develop strategies and implement changes to improve care for elderly patients living with complex care needs and taking ten or more unique medications." Control: usual care (received by all participants) |
| Outcomes | Primary outcome: number of PIPs (12 months) Secondary outcomes: patient perception of the intervention; HCP perception of the intervention; cost‐utility |
| Starting date | March 2018 |
| Contact information | Michelle Greiver (michelle.greiver@nygh.on.ca) |
| Notes | Trial registry NCT03689049 |
Husebo 2015.
| Study name | Improving quality of life in nursing home residents: a cluster randomized clinical trial of efficacy (COSMOS) |
| Methods | Pilot study and multicentre, cluster‐randomised effectiveness‐implementation clinical hybrid trial with follow‐up |
| Participants | Patient participants: nursing home patients (n = 571) with and without dementia, ≥ 65 years old, with polypharmacy (≥ 4 drugs) from 67 nursing home units |
| Interventions | Quote: “COmmunication, Systematic assessment and treatment of pain, Medication review, Occupational therapy, Safety (COSMOS): The intervention group will receive a 2‐day education program including written guidelines, repeated theoretical and practical training (credited education of caregivers, physicians and nursing home managers), case discussions and role play. The 1‐day midway evaluation, information and interviews of nursing staff and a telephone hotline all support the implementation process. The control group will receive care as usual, during the trial and follow‐up period” |
| Outcomes | Quote: “Total medication and use of psychotropic drugs in number and dose will be assessed with respect to drug‐related problems and drug–drug interactions using STOPP and START criteria. Other measures include quality of life in late‐stage dementia, hospital admission and mortality” |
| Starting date | Before July 2015 |
| Contact information | Elisabeth Flo Department of Global Public Health and Primary Care, Centre for Elderly – and Nursing Home Medicine, University of Bergen, Kalfarveien 31, N‐5020 Bergen, Norway elisabeth.flo@uib.no |
| Notes |
NCT02238652 Intervention phase complete, no results currently published |
Ie 2020.
| Study name | Medication optimisation protocol efficacy for geriatric inpatients (MPEG) |
| Methods | Randomised, parallel design |
| Participants | Inclusion: medical inpatients aged ≥ 65 years old, taking 5 or more regularly prescribed medications, with a predicted hospital stay after admission of at least 1 week Exclusion: life expectancy < 1 month and inability to take oral medication |
| Interventions | Intervention: medication review, followed by the development of a medication optimisation proposal based on the STOPP/START criteria and a medication optimisation protocol, shared with the GP and the community pharmacist. A multidisciplinary team will be involved (physician, pharmacist, nurse). Control: all patients will receive medication reconciliation by ward‐based pharmacists using data provided by the medical record handbook, patient/family or a referral letter, as well as usual care from the attending physicians |
| Outcomes | Primary outcome: composite of all‐cause death, unscheduled hospital visits and rehospitalisation (48 weeks post‐randomisation) Main secondary outcomes (24 and 48 weeks): regular and PIM; long‐term care required; health‐related quality of life |
| Starting date | 2019 |
| Contact information | Kenya Ie (kenya.ie@marianna‐u.ac.jp) |
| Notes | Trial registry: UMIN000035265 |
ISRCTN18427377.
| Study name | Hospital discharge study |
| Methods | Randomised trial (cluster) |
| Participants | Quote: “Participant inclusion criteria 1. In‐hospital patient at the time of inclusion 2. Male or female of 60 years or older with 5 or more drugs prescribed” |
| Interventions | Quote: “In the intervention group, the senior hospital physicians takes part in a teaching session of two hours duration about how to integrate a structured medication review and specific elements of communication into the daily discharge routine. The senior physicians are responsible for instructing their assistant physicians in patient recruitment and carrying out the correct discharge procedure. The assistant physicians critically review their patients’ medication lists, discuss the results of these reviews and their suggestions with the patients and compile revised medication lists which they then communicate to the patients’ general practitioners with an invitation for discussion. The senior hospital physicians in the control group undergo a two hour instruction addressing multimorbidity, patient in‐ and exclusion and the handling of the different data collection forms. Their assistant physicians will follow the “usual” discharge routine of their clinics” |
| Outcomes | Primary outcome measures: time (in days) without readmission to hospital Secondary outcome measures: Readmission rates Numbers of drugs at discharge and at 1, 3 and 6 months after discharge; proportions of potentially inappropriate medications (PIMs) at discharge and at 1, 3 and 6 months after discharge are (consecutive classification at study centre based on updated Beers criteria, 2012 and PRISCUS list) Patients’ quality of life at discharge and at 1, 3 and 6 months after discharge (EQ‐5D‐3L‐scale) |
| Starting date | January 2017 |
| Contact information | Dr. med. Stefan Neuner‐Jehle MPH (Scientific) stefan.neuner‐jehle@usz.ch |
| Notes | Currently in recruitment phase |
ISRCTN18752158.
| Study name | The general practice‐based pharmacist: supporting medicines management in older adults |
| Methods | Cluster‐randomised trial |
| Participants | Inclusion: aged 65 years, with complex polypharmacy (≥ 10 repeat medications) |
| Interventions | Intervention: "The intervention will involve a pharmacist integrating into the GP practice where they will support prescribing‐related activities. The first component of the intervention will be the medicines optimisation element delivered by targeted patient medication reviews and based on improving safety and addressing national medicines management priorities and guidelines. There will be a focus on high risk prescribing, potentially inappropriate prescribing (PIP) and deprescribing. (...) The second component of the intervention will evaluate the role and impact of a pharmacist on care provision within the general practice when integrated into the practice team. This will involve a pharmacist joining the practice team and engaging in activities to support GPs and other practice staff such as audit, medication review, educational sessions and a medicines information role. Any individual prescribing issues identified will be discussed with the GP. The GP will exercise their own clinical judgement and expertise, and will have the final decision in any medication changes, in consultation with the patient. Data that will be recorded for the purposes of this study will be anonymised practice‐level data on prescribing, a description of the activities that the pharmacist undertakes and the length of time undertaken to complete those activities." Control: care as usual |
| Outcomes | Primary outcome: mean PIPs per patient (baseline, 4 months) Main secondary outcomes: number of repeat medications; proportion of patients with polypharmacy (≥ 10 regular medications); medication changes; health‐related quality of life; patient's beliefs and attitudes; burden of treatment; engagement with other HCP |
| Starting date | March 2020 |
| Contact information | Aisling Croke (aislingcroke@rcsi.ie) |
| Notes | — |
ISRCTN41009897.
| Study name | Pilot testing of a new approach to improving the prescribing of many drugs for older people who live in their own home and are cared for by general practitioners |
| Methods | Cluster‐randomised trial (pilot) |
| Participants | Inclusion: aged ≥ 70 years, receiving ≥ regular medicines |
| Interventions | Intervention: "The existing intervention package consists of two components: (a) an online video demonstrating how GPs can improve appropriate polypharmacy during typical consultations with older patients; (b) a patient recall process (appointment with the GP for a medication review). Rather than introducing new tasks for GPs to perform, the video component seeks to enable GPs to use available time more efficiently by demonstrating how appropriate polypharmacy can be prescribed during routine consultations with older patients (‘Modelling or demonstrating of behaviour’) and emphasising the potentially positive consequences of performing this behaviour (‘Salience of consequences’). The intervention seeks to introduce small, but potentially sustainable changes in GPs’ current clinical practice aimed at improving prescribing for older people." Control: care as usual |
| Outcomes | Primary outcomes: recruitment; medication appropriateness (12 months) Secondary outcomes: fidelity and mechanism of action (assessed at end of study); health economics (assessed at end of study); health‐related quality of life (6 and 9 months post‐intervention); medication‐related burden (6 and 9 months post‐intervention); data to inform sample size calculation |
| Starting date | September 2019 |
| Contact information | Carmel Hughes (c.hughes@qub.ac.uk) |
| Notes | — |
ISRCTN90146150.
| Study name | Improving medicines use in people who take multiple medicines (IMPPP) |
| Methods | Cluster‐randomised trial |
| Participants | Inclusion: persons experiencing potentially problematic polypharmacy in primary care and community settings, anticipated to be aged ≥ 60 years, taking ≥ 10 medications regularly on prescription Main exclusion: receiving end‐of‐life care, chaotic medication use |
| Interventions | Intervention: "The IMPPP intervention will be based in general practice, and will involve GPs and practice pharmacists working together, drawing on the specific skills of each professional sensitive to the context of each practice. This is a complex intervention and will comprise two key elements: 1. A model for conducting a polypharmacy medication review (including pharmacist‐GP collaboration and case finding)
2. Components seeking to enhance professional engagement (education, practice feedback, financial incentives). An informatics tool integrated into GP clinical systems will help support the medication review element as well as the practice feedback component." Control: not described |
| Outcomes | Primary outcome: PIM Main secondary outcomes: quality of life; medication adherence; healthcare service use; patient and medication safety |
| Starting date | February 2019 |
| Contact information | Anna Brroke Anna.Brooke@bristol.ac.uk |
| Notes | — |
Johansen 2018.
| Study name | Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (IMMENSE study) |
| Methods | A non‐blinded randomised trial |
| Participants | Quote: “Inclusion criteria: age ≥70 years, acutely admitted and willing to provide written informed consent (patient or next of kin). Exclusion criteria: admitted to the study ward more than 72 hours before evaluation of eligibility, moved to and discharged from other wards during the index stay, inability to understand Norwegian (patient or next of kin), considered terminally ill or with a short life expectancy, planned discharged on the inclusion day, occupying a bed in a study ward but under the care of physicians from a non‐study ward or if an intervention from a study pharmacist is considered necessary for ethical reasons (before randomisation or in control group)” |
| Interventions | Quote: “Patients randomised to the intervention group receive the IMM‐based intervention including: (1) MedRec at admission, (2) medication review and monitoring during the hospital stay, (3) patient counselling designed to meet the needs of each individual patient, (4) MedRec at discharge together with an updated and structured medication list given to patients and submitted to primary care at discharge and (5) a follow‐up phone call to the patient’s GP and nurses in home care service/nursing home to inform about and discuss current medication therapy and recommendations. Step 5 is in addition to the original IMM model. The study pharmacist is performing all steps in close collaboration with the hospital physician who has the medical responsibility for the patients. Patients assigned to standard care receive treatment from a team consisting of physicians, nurses, nurse assistants, and sometimes occupational therapists and physiotherapists. Standard care may include elements as MedRec, medication review and patient counselling performed by physicians or nurses during the hospital stay” |
| Outcomes | Primary outcome: Rate of ‘acute readmissions and ED visits’ 12 months after discharge Secondary outcomes: Change in self‐reported HRQoL Length of index hospital stay Change in total score of the Medication Appropriateness Index (MAI) from admission to discharge Change in potentially inappropriate medications prescribed identified by The Norwegian General Practice—Nursing Home criteria (NORGEP‐NH), Screening Tool of Older Persons’ Prescriptions (STOPP) V.2 and Screening Tool to Alert doctors to Right treatment (START) V.2 from admission to discharge Change in potentially inappropriate medications prescribed using START V.2, STOPP V.2 and NORGEPNH from discharge to 3 and 12 months |
| Starting date | September 2016 |
| Contact information | Jeanette Schultz Johansen jeajoh@uit.no |
| Notes |
NCT02816086 Intervention phase ongoing |
Jungo 2019.
| Study name | Optimising PharmacoTherapy In the multimorbid elderly in primary CAre (OPTICA) |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: aged ≥ 65 years old, attending an eligible GP practice, multimorbidity, polypharmacy Exclusion: inability to provide informed consent, participation in another interventional study |
| Interventions | Intervention: GPs will perform a STRIPA analysis, which consists of 4 steps: recording diagnosis and medication; structured drug review with integrated STOPP/START criteria; shared decision‐making between GP and patient; and follow‐up through study team Control: treatment in accordance with standard care |
| Outcomes | Primary outcomes (all 6 and 12 months): medication appropriateness; change in medication appropriateness; medication underutilisation; change in medication underutilisation Main secondary outcomes (all 6 and 12 months): polypharmacy; overprescribing; underprescribing; falls and fractures; quality of life; formal and informal care; survival; medical costs |
| Starting date | October 2018 |
| Contact information | — |
| Notes | Trial registry NCT03724539 |
Komagamine 2018.
| Study name | Pharmacist intervention versus usual care for elderly patients hospitalised in orthopaedic wards |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: aged ≥ 70 years, taking ≥ 5 medications or at least one PIP at hospital admission Exclusion: elective hospital admission, expected length of stay < 1 week |
| Interventions | Intervention: pharmacist‐led intervention with several components, including: 1) medication reconciliation; 2) patient education and monitoring; 3) advice to patient's physician regarding unnecessary or inappropriate medications and starting necessary medications; and 4) written summary information on discharge medication, shared with patients, GPs and community pharmacists Control: no intervention |
| Outcomes | Primary outcome: hospital readmission rate (12 months) Secondary outcomes: Emergency Department (ED) visits; all‐cause mortality; new fracture; acute myocardial infarction; ischaemic stroke; number of medications; PIPs; PPOs |
| Starting date | March 2017 |
| Contact information | Junpei Komagamine junpei0919@yahoo.co.jp |
| Notes | Trial registry UMIN000029404 |
Kua 2017.
| Study name | Nursing home team‐care deprescribing study |
| Methods | Cluster‐randomised stepped‐wedge intervention |
| Participants | Nursing home residents at least 65 years old and on 5 or more medications |
| Interventions | Quote: “The intervention will consist of a five‐step multidisciplinary team‐based deprescribing approach using a deprescribing guide adapted from the Beers criteria, Screening Tool of Older People's Prescriptions (STOPP) criteria, as well as a review of medication interactions and side effects. The five‐step team‐care process consists of reviewing, checking, discussion, communication and documentation as described in figure 2, initiated by the pharmacists. Each nursing home in the study is currently served by one to two community‐based pharmacists. They have completed or are currently undertaking their postgraduate studies (Master of Clinical Pharmacy) or Board Certified Geriatric Pharmacist training. All pharmacists (minimum working experience at aged care homes of 1 year) will receive a half‐day face‐to‐face training and familiarisation session on the intervention. Our multidisciplinary teamcare approach involves nurses, pharmacists and doctors and will be implemented during routine doctor and pharmacist nursing home review visits. Pharmacists will initiate deprescribing in medication review, after discussion with ward nurses on the feasibility of deprescribing for each appropriate individual patient. The intervention information filled‐up by the pharmacist will be passed on through the ward nurses to the doctor for review during doctor's visit. Thereafter, the doctor will make the final decision on drugs that will be deprescribed. A copy of the deprescribing reference guide (Beers and STOPP criteria) will be available to all participating healthcare professionals. The Beers and STOPP criteria are intended as a guide for educating pharmacists and doctors regarding the different types of interventions that they could make. For successful deprescribed patients with external institution follow‐up, a copy of the deprescribing details will be pass as memorandum to the external doctor. Additionally, multidisciplinary discussion session may be introduced as part of the nursing home's standard practice at some sites, but implementation depends on case‐by‐case availability and agreement of individual doctor, pharmacist and nurse at each site during routine care. Non‐cognitive impaired residents or next of kins of cognitive‐impaired residents may be contacted in decision making of the intervention where feasible. Control: All participants in the control arm will continue to receive usual care or support that they usually receive from their healthcare professionals. In participants who were randomised to control, there is a possibility that some participants will require a review of their medication. These patients will be documented and analysed separately at the end of study” |
| Outcomes | The number of STOPP criteria and Beers criteria interventions made The type and percentage of drug‐related problems |
| Starting date | November 2016 |
| Contact information | Mr. Chong‐Han Kua chong.kua@monash.edu |
| Notes |
NCT02863341 Intervention phase complete, no results currently published |
Loffler 2014.
| Study name | Optimizing polypharmacy among elderly hospital patients with chronic diseases |
| Methods | Cluster‐randomised trial |
| Participants | Quote: “Patient participants: patients aged 65+ years who take five or more prescribed long‐term drugs and who are likely to spend at least 5 days in the participating hospitals will be recruited and included consecutively” |
| Interventions | Quote: “During in‐patient treatment of chronically ill patients affected by polypharmacy, a pharmacist specially trained in communication skills performs a narrative‐based medication review. Apart from detecting potentially inadequate medication, a major aim is to identify patient preferences and to include them ‐ whenever possible ‐ into a list of evidence‐based medication recommendations. Patients will be motivated to narrate the drugs they currently take and describe their experiences and expectations related to these drugs. Based on this information the pharmacist prepares a list of possible drugs to be stopped, which will then be discussed with the hospital physician in charge and will be submitted for consent to the patients’ General Practitioner. The active involvement of patients allows for transparency of the decision‐making process and will increase the chance for a sustainable medication optimization Patients of the control group receive care as usual” |
| Outcomes | Quote: “The independent two main primary outcomes are (1) health‐related quality of life (EQ‐5D) and (2) the difference in the number of prescribed long‐term pharmaceutical agents between intervention and control group. The secondary outcomes are appropriateness of prescribed medication (PRISCUS list, Beers Criteria, MAI), patient satisfaction (TSQM), patient empowerment (PEF‐FB‐9), patient autonomy (IADL), falls, re‐hospitalization, and death” |
| Starting date | November 2013 |
| Contact information | Christin Löffler Institute of General Practice, Rostock University Medical Center, Rostock, German christin.loeffler@med.uni‐rostock.de |
| Notes | ISRCTN42003273 Intervention phase complete, no results currently published |
McCarthy 2017.
| Study name | Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE) |
| Methods | Cluster‐randomised trial |
| Participants | Quote: “Patients will be considered eligible if they are aged ≥65 years and they are being prescribed 15 or more repeat medicines, which is a measure of both significant polypharmacy and complex multimorbidity” |
| Interventions | Quote: “Intervention arm: GPs will receive log in details to access online academic detailing and will be asked to arrange a medication review with their recruited patients. This will be supported by a website which will provide a basic structure for the review and a patient outcome form which will collect information about any changes made to the medication regime and reasons for process evaluation. Follow up data will be collected 6 months after the medication review is completed. Control arm: Usual care will be delivered for the duration of the study” |
| Outcomes | Primary outcome measures pertain to the individual patient level and are the proportion of patients with any PIP and the number of repeat medicines. Secondary outcomes: quality of life, patient’s attitudes to deprescribing and treatment burden |
| Starting date | August 2016 |
| Contact information | Professor Susan Smith susansmith@rcsi.ie |
| Notes | ISRCTN12752680 Intervention phase ongoing |
Mestres 2017.
| Study name | Supporting clinical rules engine in the adjustment of medication (SCREAM) |
| Methods | Multicentre, prospective, randomised study with a cluster group design |
| Participants | Quote: “Residents living in a nursing home in the Netherlands” |
| Interventions | Quote: “Intervention group: The datasets will be screened through the CRR on a weekly basis. The messages delivered by the CRR will be sent via mail to the specific physicians. Each remark will be sent on a separate mail in a standardised way. In response to the report, the physician will send a feedback message within 36 h indicating, in a standardised way, whether: the advice was not followed, the advice was followed or the advice was changed. After receiving this feedback, the investigators will process it in the CRR, in order to create the database for the study. Additionally, regular care will be also applied. That is according to the Dutch Healthcare Inspectorate, a yearly medication review with a physician and a pharmacist, even though there is a substantial variation [25], For the centres included in this study there are no dedicated clinical pharmacist working in the nursing home" |
| Outcomes | MAI The proportion of patients with at least one of the events, including hospital referrals (i.e. referral to a specialist, emergency department visit and hospital admission) The quality of life will be measured using the EQ‐5D |
| Starting date | June 2013 |
| Contact information | Carlota Mestres Gonzalvo c.mestresgonzalvo@zuyderland.nl |
| Notes | NTR5165 Intervention phase ongoing |
NCT00844025.
| Study name | Pharmaceutical care and clinical outcomes for the elderly taking potentially inappropriate medication: a randomized‐controlled trial |
| Methods | Randomised trial |
| Participants | Elderly with chronic disease, 65 to 90 years old, hospitalised |
| Interventions | Quote: “Behavioural: pharmacist intervention. Participants in the intervention group will receive pharmaceutical care delivered by a clinical pharmacist, including medication review, medication reconciliation, participant education and recommended actions” |
| Outcomes | Primary outcome measures: Number of unsolved drug‐related problems (time frame: 14 days after randomisation) Secondary outcome measures: Rate of ADE during hospitalisation (time frame: 14 days after randomisation) Number of potentially inappropriate medications (time frame: 14 days after randomisation) |
| Starting date | February 2009 |
| Contact information | Liu Jen Wei, MS, Principal Investigator Shin Kong Wo Ho‐Su Memorial Hospital, Department of Pharmacy, Taipei 111, Taiwan |
| Notes | Intervention phase complete, no results currently published |
NCT01034761.
| Study name | Using clinical alerts in a computerized provider order entry system to decrease inappropriate medication prescribing among hospitalized elders |
| Methods | Randomised trial |
| Participants | Patient participants: hospitalised patients over 65 years of age |
| Interventions | Quote: “A series of clinical alerts will be developed in the hospital's computerised provider order entry system to reduce the use of potentially inappropriate medications among hospitalised older patients. A synchronous alert (i.e. a 'pop‐up') will appear whenever a physician attempts to place an order for a high‐risk medication on the Beers list and the intended recipient is over 65 years of age. The alert will inform the physician about the risks associated with the medication and will propose safer alternatives” |
| Outcomes | Primary: percentage of older participants who received a specified high‐risk medication from the Beer's list (time frame: earlier hospital stay or end of study) Secondary: average number of specified high‐risk medications prescribed per participant (time frame: earlier hospital stay or end of study), restraint use (time frame: earlier hospital stay or end of study), falls (time frame: earlier hospital stay or end of study), length of stay (time frame: earlier hospital stay or end of study), total cost (time frame: earlier hospital stay or end of study), discharge status (time frame: 6 months) |
| Starting date | April 2013 |
| Contact information | Linda Canty, MD, Assistant Clinical Professor of Medicine Baystate Medical Centre, Springfield, Massachusetts, USA |
| Notes | Intervention phase complete, no results currently published |
NCT02942927.
| Study name | Team Approach to Polypharmacy Evaluation and Reduction (TAPER‐RCT) |
| Methods | Randomised trial |
| Participants | Quote: “Aged 70 years of age or older, currently taking more than 5 long term medications” |
| Interventions | Quote: “The patient will then attend an appointment with a pharmacist to review medications appropriate for discontinuation/dose reduction, after which the patient will meet with his/her family physician to discuss patient preferences for discontinuation/dose reduction. Both health care providers will have access to TAPERMD, a web based program linked to evidence and tools to support reduction in polypharmacy. Intervention: TAPER ‐ The intervention is medication reduction. This arm is comprised of: 1. Medication reconciliation 2. Identification of patient priorities for care 3. Identification of medications that are potentially appropriate for discontinuation/dose reduction 4. Linked pharmacist/family physician consultations with patient to discuss medication with intention to reduce 5. Identification of medications for trial of discontinuation/dose reduction (shared decision making) 6. Pause of medication and clinical monitoring Control: Standard of Care as wait list control. Control group will be offered intervention as part of usual clinical care at 6 months” |
| Outcomes | Beers, STOPP (personal communication) Quality of life (EQ5D‐5L and SF36v2) Healthcare resource utilisation (hospital admissions) Changes in medication side effects and symptoms (adverse) Serious adverse events |
| Starting date | April 2018 |
| Contact information | Prof. Dee Mangin mangind@mcmaster.ca |
| Notes | Recruitment and intervention phases ongoing |
NCT03156348.
| Study name | Impact of clinical pharmacist on adverse drug events in older adults |
| Methods | Randomised trial |
| Participants | 60 years and older Patients who are on pharmacological therapy |
| Interventions | Quote: “The intervention group will receive in addition to the usual care, it will receive the Clinical Pharmacist Care during hospitalization, discharge and during 2 months post‐discharge, through a home visit at 30 ± 5 days post‐discharge and a telephone call at 60 ± 5 days. During hospitalization and at discharge a clinical pharmacist (CP) will monitor daily pharmacological safety and efficacy of the medication to asses and make appropriate recommendations. CP will explain the use reasons of each of the drugs. At 30 days post‐discharge, the CP will review the updated clinical record of patient and conduct a home visit to enhance and ask about adherence, self‐medication, medication use at that time and possible results of laboratory tests performed and clarify doubts regarding the use of current medications. The same activities will be made at 60 days by telephonic way, to reinforce the recommendations” |
| Outcomes | Incidence of potentially inappropriate medication (Beers criteria and STOPP/START criteria) Incidence of adverse drug events Adherence measured with Morisky & Green Presence of clinically relevant drug interactions |
| Starting date | May 2015 |
| Contact information | Dr. Jorge Hasbun comiteetica@hcuch.cl |
| Notes | Intervention phase ongoing |
NCT03298386.
| Study name | Elderly Appropriate Treatment in Primary Care (EAT) (TAPAGE) |
| Methods | Randomised trial |
| Participants | Quote: “Patient 75 years of age or older, with polypharmacy (≥ 5 medications), not institutionalized” |
| Interventions | Quote: "Intervention Group "STOPP/START": Training of General Practitioners with the tool STOPP/START Systematic medication review by GP with STOPP/START Control group: Patient's usual care by the general practitioner (who will not be trained in the STOPP/START tool)" |
| Outcomes | STOPP/START used in the intervention Percentage of unplanned hospitalisation Decrease in the number of drugs on prescription |
| Starting date | August 2017 |
| Contact information | Dr. Akim Souag akim.souag@aphp.fr |
| Notes | Intervention phase ongoing |
NCT03909035.
| Study name | BIMEDOC |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: aged ≥ 65 years suffering from long‐term illness or aged ≥ 75 years, living at home, taking ≥ 6 medications for > 6 months Exclusion (main): no allocated GP, receiving medication review in the past 12 months |
| Interventions | Intervention: medication therapy management, which consists of a pharmacist‐led medication review aimed at detecting PIP. It includes: a pharmacist‐led interview with the patient; an evaluation of the prescriptions; detailed feedback to the GP; an appointment with the patient to explain the modifications made by the GP Control: usual pharmaceutical care |
| Outcomes | Primary outcome: hospitalisations (12 months) Main secondary outcomes (between baseline and 12 months; measured at different time points): number of PIP; number of medications per patient; compliance; quality of life; differential cost ratio |
| Starting date | June 2019 |
| Contact information | Cécile McCambridge mccambridge.c@chu‐toulouse.fr |
| Notes | — |
NCT04004936.
| Study name | EQUIPPED |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: providers at eligible healthcare facilities |
| Interventions | Intervention: "Enhancing Quality of Prescribing Practices for Older Adults Discharged from the Emergency Department (EQUIPPED is a multi‐component program to reduce the prescribing of potentially inappropriate medications (PIMs) to older adults upon discharge from the Emergency Department (ED). It has three core components: 1) provider education, 2) Electronic Health Record (EHR)‐based clinical decision support (CDS) including pharmacy quick order sets to facilitate provider order entry, and 3) provider audit and feedback with peer benchmarking. The active feedback group will receive one‐to‐one (1:1) in‐person academic detailing from a professional colleague that includes in‐person audit, feedback, and peer benchmarking and provide on‐site expertise." Active control: "Enhancing Quality of Prescribing Practices for Older Adults Discharged from the Emergency Department (EQUIPPED is a multi‐component program to reduce the prescribing of potentially inappropriate medications (PIMs) to older adults upon discharge from the Emergency Department (ED). It has three core components: 1) provider education, 2) Electronic Health Record (EHR)‐based clinical decision support (CDS) including pharmacy quick order sets to facilitate provider order entry, and 3) provider audit and feedback with peer benchmarking. The passive feedback group will receive monthly provider feedback via an electronic dashboard with audit, feedback and peer benchmarking." |
| Outcomes | Primary outcome: percentage of PIMs prescribed (12 months post‐intervention) Secondary outcomes: behavioural change; intervention micro‐costing |
| Starting date | October 2019 |
| Contact information | Elizabeth C Vaughan Elizabeth.Vaughan2@va.gov |
| Notes | — |
NCT04028583.
| Study name | TaIPE Study (TaIPE) |
| Methods | Randomised, parallel assignment |
| Participants | Aged ≥ 65 years, who met the admission criteria of the acute care for elders (ACE) unit |
| Interventions | Intervention (Quote): "In the PIM‐Check group, a medication review will be conducted using PIM‐Check within 72 hours of patient's admittance to the unit. The physician will decide whether to accept these recommendations or not and implement prescribing changes if agreed." Active comparator (Quote): "In the STOPP/START group, medication lists will be analyzed within 72 hours of patient's admittance and optimized according to STOPP/START criteria. The second physician will decide whether to accept these recommendations or not and implement prescribing changes if agreed." |
| Outcomes | Primary outcome: rate of PIPs reduction in the PIM‐Check group compared to STOPP/START (18 months) Secondary outcomes (all 18 months unless otherwise indicated): number and type of PIPs detected by each tool; rate of acceptability; number of treatment (mean and median) modification by clinicians; number of drugs at discharge; incidence rate of falls; activities of daily living (ADL) score; Confusion Assessment Method (CAM); length of stay; number of unplanned readmissions (up to 3 months after discharge); association between the number and type of PIPs at discharge with rate of re‐admission (up to 3 months after discharge) |
| Starting date | 26 February 2018 |
| Contact information | Chantal Csajka chantal.csajka@chuv.ch |
| Notes | — |
NCT04087109.
| Study name | E‐CARE Study |
| Methods | Stepped wedge randomised controlled trial |
| Participants | Inclusion criteria: residents of eligible long‐term care facilities, aged ≥ 65 years, taking a potentially inappropriate medication Exclusion criteria: language barrier or cognitive impairment |
| Interventions | Intervention: healthcare professionals (HCP) will have access to MedSafer, an application programming interface with individualised and personalised deprescribing opportunities. Patients will receive an educational brochure (EMPOWER). Control: HCP will not have access to MedSafer and will provide care as per usual protocol in each facility. |
| Outcomes | Primary outcome: proportion of patients with one or more PIM (potentially inappropriate medication) reduced or stopped (30 days) Secondary outcome: sustainability; quality of life; sleep quality; falls; transfer to acute hospital; hip fractures; delirium (30 days after each intervention cycle) |
| Starting date | 1 January 2021 |
| Contact information | Emily McDonald emily.mcdonald@mcgill.ca |
| Notes | — |
NCT04147130.
| Study name | MultiPAP Plus |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion: aged 65 to 74 years with multimorbidity (≥ 3 chronic diseases) and polypharmacy (≥ 5 drugs taken for at least 3 months) Exclusion: institutionalised, life expectancy < 12 months |
| Interventions | Intervention: 3 main components: training of GPs; patient‐centred clinical interview; clinical‐decision support system to help structured treatment plan review Control: usual clinical care based on current clinical practice guidelines |
| Outcomes | Primary outcome: hospitalisation and/or mortality (18 months) Main secondary outcomes: hospitalisation and/or mortality (12 months); therapeutic adherence questionnaire (6, 12, 18 months); medication appropriateness (6, 12, 18 months); use of health services (12, 18 months); disability (12, 18 months), quality of life (12, 18 months) |
| Starting date | February 2020 |
| Contact information | Alexandra Prados Torres |
| Notes | — |
NCT04181879.
| Study name | PolyPrime |
| Methods | Randomised, parallel assignment (pilot) |
| Participants | Inclusion: aged ≥ 70 years, receiving ≥ 4 regular medications Exclusion: cognitively impaired, terminal illness, institutionalised |
| Interventions | Intervention: the intervention consists of an online video demonstration of how GPs can improve polypharmacy with their patients, after which the GPs will conduct medication reviews with their patients with the goal of optimising their medication Control: care as usual |
| Outcomes | Primary outcomes: participation and retention; medication appropriateness Main secondary outcomes: video counts; appointments scheduled; number of medication reviews attended; length of medication reviews; healthcare resource use; costs; health‐related quality of life |
| Starting date | September 2019 |
| Contact information | Camel Hughes c.hughes@qub.ac.uk |
| Notes | — |
NTR6644.
| Study name | IMPETUS |
| Methods | Randomised, parallel assignment |
| Participants | Residents of eligible care homes, aged ≥ 65 years, with a permanent residence indication (> 6 months until the end of life), receiving or wishing to receive geriatric‐palliative based care |
| Interventions | Intervention (quote): "The Advance Care Planning (ACP+) intervention is a working method, aimed to stimulate prescribing practice based on the multidisciplinary guideline “Polypharmacy in the elderly”. Physicians, pharmacists and nursing staff will be trained in the intervention.
The ACP+ working method consists of a combination of a structured multidisciplinary medication review (SMMR) and an ACP discussion. Firstly, experiences questions and wishes of the patient in regards to his/her medication will be explored. Secondly, the physician and pharmacist conduct an SMMR. During this SMMR, the appropriateness of a patient’s medication is reviewed on the basis of key elements from the guideline “polypharmacy in the elderly”, medication appropriateness indicators, and the geriatric‐palliative algoritm.
The recommendations from this SMMR are then discussed with the patient and/or representatives in an ACP discussion.
This SMMR and ACP working method will be repeated every six months ( between T0 and T1, between T1 and T2, and between T2 and T3), three times for each patient during the study." Control: care as usual |
| Outcomes | Primary outcome: change in prescription of preventive/chronic medication (3 months) Secondary outcomes (all 3 months unless otherwise indicated): quality of life; social wellbeing; pain; frequency of falls; hospitalisations; deaths (any cause; any time point); appropriateness of medication prescription |
| Starting date | Not reported |
| Contact information | C.A.M. Pouw c.pouw@vumc.nl |
| Notes | — |
Prados‐Torres 2017.
| Study name | Improving prescription in primary care patients with multimorbidity and polypharmacy (Multi‐PAP) |
| Methods | Randomised clinical trial (cluster) |
| Participants | Age 65 to 74 years, multimorbidity, defined as ≥ 3 chronic diseases, polypharmacy, defined as ≥ 5 drugs prescribed over at least the 3 months prior to inclusion in the study |
| Interventions | Quote: “Intervention group: A complex intervention with two phases is conducted: First phase: FP training. This will consist of a previously designed training activity, delivered using the massive online open courses (MOOC) format, including basic concepts relating to multimorbidity, appropriateness of prescribing, treatment adherence, the Ariadne principles, and physician‐patient shared decision making. Second phase: Physician‐patient interview based on the Ariadne principles. Control group: Patients in the control group will receive usual clinical care based on the provision of advice and information and will undergo examinations as recommended in the CPGs corresponding to each of the patient’s chronic diseases” |
| Outcomes | Medication appropriateness index (MAI) Use of health services: unplanned and/or avoidable hospitalisations, use of emergency services and PC (FP and nurse) Quality of life: measured using the EuroQol 5D‐5L questionnaire Medication safety: measured as the incidence of adverse drug reactions and potentially hazardous interactions Treatment adherence: measured using the Morisky‐Green test and the Haynes‐Sackett questionnaire |
| Starting date | November 2016 |
| Contact information | Alexandra Prados‐Torres sprados.iacs@aragon.es |
| Notes |
NCT02866799 Intervention phase ongoing |
Selic 2016.
| Study name | Use of web‐based application to improve prescribing in home‐living elderly |
| Methods | Randomised trial |
| Participants | Patient participants: chronically ill elderly people, older than 65 years who live at home and regularly receive at least one drug |
| Interventions | Quote: “Participants' data will be entered into a web‐based application and screened for potentially inappropriate prescribing using STOPP and START criteria. Identified potentially inappropriate prescriptions will be presented to participants' physicians for consideration and change. Physicians of participants in the control group will not be informed about potentially inappropriate prescriptions” |
| Outcomes | Quote: “Quality of life index (EQ‐5D); quality of prescribing–the presence of inappropriate prescribing according to the START/STOPP criteria (at least one criterion from both lists was violated) or the presence of polypharmacy (more than 5 concomitant medications); the number of active ingredients regularly taken by the patient; adherence according to the Morisky 4‐item questionnaire; non‐planned hospitalizations and non‐planned/urgent visits to a clinical specialist; number of visits to the emergency room or the emergency physician’s home visits in the previous year; number of visits to the GP in the year concerned; number of inappropriate prescriptions according to the START/STOPP criteria; and number of interactions between the prescribed medications marked 'major'” |
| Starting date | 2014 |
| Contact information | Polona Selic Department of Family Medicine, Faculty of Medicine, University of Ljubljana, Poljanski nasip 58, Ljubljana, Slovenia polona.selic@siol.net |
| Notes | Protocol: Selic et al. (2016). The Effects of a Web Application and Medical Monitoring on the Quality of Medication, Adverse Drug Events and Adherence in the Elderly Living at Home: a Protocol of the Study. Materia Socio‐Medica, 28(6), 432‐436 Intervention phase complete, no results currently published |
ADEs: adverse drug events ADR: adverse drug reactions CRR: Clinical Rule Reporter CQC: Care Quality Commission ED: emergency department GP: general practitioner FP: family physician HCP: healthcare professionals HRQoL: health‐related quality of life IADL: Instrumental Activities of Daily Living MAI: Medication Appropriateness Index MMAS‐4: Morisky Medication Adherence Scale PC: primary care PIMs: Potentially inappropriate medications PIP: potentially inappropriate prescribing PPOs: potential prescribing omissions START: Screening Tool to Alert doctors to the Right Treatment STOPP: Screening Tool of Older Person’s Prescriptions TRIM: Tool to Reduce Inappropriate Medication TSQM: Treatment Satisfaction Questionnaire for Medication
Differences between protocol and review
As only two studies (Bucci 2003; Crotty 2004a) reported the primary outcome measure of change in medication appropriateness used in the first iteration of this review, we used postintervention results of potentially inappropriate medications (PIMs) and potential prescribing omissions (PPOs) in the meta‐analyses to compare the effect sizes of the interventions.
Furthermore, we modified our approach to pooling outcome data for potentially inappropriate prescribing (PIP), to instead classify the outcomes under the broad categorisation of PIMs or PPOs. For example, rather than looking at explicit tools or implicit tools individually (i.e. the Screening Tool of Older Person’s Prescriptions (STOPP) versus the Medication Appropriateness Index (MAI)), the current review has focused on PIMs (i.e. the number of PIMs), while the meta‐analysis previously entitled “change in MAI score” has been refocused to include studies including data on “medication appropriateness (as measured by an implicit tool)” to align with the original primary outcomes of interest.
The search strategy was modified slightly from that used in the original review to avoid limiting the search unnecessarily. Based on a recommendation made following the search development process for the previous review, the term 'polypharmacy' was searched alone (e.g. not combined with the concept of “age” using the Boolean operator “AND”) because most of the literature on polypharmacy focuses on older populations. The search strategy was also modified to include relevant new index terms in MEDLINE since the last search (such as: potentially inappropriate medication list/) and additional search terms were included (such as deprescribing and drug discontinuation).
EBM Reviews, ACP Journal Club, The Joanna Briggs Institute EBP Database and PsycINFO were not searched for this update because they ceased updates, are currently indexed in other databases (MEDLINE, Embase and CINAHL) and they were deemed unlikely to yield anything unique for the topic respectively.
To comply with Cochrane and EPOC requirements, we have now included the most important outcomes in the summary of findings table, which were: medication appropriateness (as measured by an implicit tool), the number of PIMs, the proportion of patients with one or more PIMs, the number of PPOs, the proportion of patients with one or more PPOs, quality of life and hospital admissions.
For the current update of the review we only considered randomised controlled trials for inclusion.
Contributions of authors
J Cole (JC) selected trials, extracted data, assessed risk of bias, was responsible for data management and data analysis, was involved in the interpretation of the results, drafted the manuscript, contributed to additional manuscript writing and approved the final version of the manuscript.
D Goncalves Bradley (DCB) selected trials, was involved in interpretation of the results and approved the final version of the manuscript.
M Alqahtani (MA) selected trials, extracted data, assessed risk of bias, was involved in interpretation of the results and approved the final version of the manuscript.
H Barry (HB) selected trials, extracted data, assessed risk of bias and approved the final version of the manuscript.
CA Cadogan (CAC) designed, conducted, analysed and reported previous versions of this review. For this update, CAC selected trials, extracted data, assessed risk of bias, conducted data analysis and interpretation of results, contributed to writing the manuscript and approved the final version of the manuscript.
A Rankin (AR) conducted, analysed and reported a previous version of this review, and for this update approved the final version of the manuscript.
S Patterson (SP) prepared the protocol, conducted, analysed and reported previous versions of this review.
Ngaire Kerse (NK) conducted, analysed and reported previous versions of this review. For this update, NK approved the final version of the manuscript.
CR Cardwell (CRC) advised on analysis in previous versions of this review and on this updated version.
C Ryan (CR) conducted, analysed and reported previous versions of this review. For this update, CR approved the final version of the manuscript.
C Hughes (CH) directed the design of the protocol for this review and designed, conducted, analysed and reported previous versions. For this update, CH selected trials, extracted data, assessed risk of bias, conducted data analysis and interpretation of results, contributed to writing the manuscript and approved the final version of the manuscript.
Sources of support
Internal sources
-
Queen's University Belfast, UK
School of Pharmacy
External sources
-
Research and Development Office, Northern Ireland, UK
Fellowship awarded to Dr. Susan Patterson to undertake the original review for 2 years, 2 days per week
-
The Dunhill Medical Trust, London, UK
A grant from the Dunhill Medical Trust supported Dr. Cathal Cadogan to undertake an update of the original review [grant number: R298/0513]
-
The Health Research Board (HRB) Centre for Primary Care Research, Royal College of Surgeons in Ireland (RCSI), Dublin, Ireland
A grant from the HRB Centre for Primary Care Research supported Dr. Audrey Rankin to undertake an update of the original review [grant number: HRC/2014/1]
Declarations of interest
JC is currently employed as a systematic reviewer at the Clinical Trial Service Unit, University of Oxford and is a co‐author on another Cochrane Review. JC was involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme.
DCG‐B is a Cochrane editor and was not involved in the editorial process for this review.
MA: none known.
HB: involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme.
CAC is an associate editor with the Cochrane Effectiveness of Practice and Organisation of Care (EPOC) group and was involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme.
AR: involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme.
SP: none known.
NK: holds the position of the Joyce Cook Chair in Ageing Well at the University of Auckland, which was funded by a gift from the Cook family. Health Research Council of New Zealand grant held by the University of Auckland. Recipient of payment as member of the Health Research Council of New Zealand Data Safety Monitoring Board. Payment made by New Zealand Retirement Villages Association to the University of Auckland to conduct research and for expert witness services. Works as a locum general practitioner for Auckland City Mission. Affiliated to the Royal New Zealand College of General Practitioners, which has opinions on prescribing. Involved in studies funded by the Health Research Council of New Zealand on the impact of falls in residential care.
CRC: none known.
CR: involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme.
CH: is an editor with the Cochrane EPOC Group and was not involved in the editorial process for this review. CH was involved in conducting a study eligible for inclusion in this review: An external pilot cluster randomised controlled trial of a theory‐based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime), funded by the HSC R&D Division Cross‐border Healthcare Intervention Trials in Ireland Network (CHITIN) programme through the European Union’s INTERREG VA Programme. CH is a registered pharmacist and has published papers and opinion pieces in medical journals, and is a non‐executive director with the Belfast Health and Social Care Trust and a Trustee with the Dunhill Medical Trust.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Auvinen 2021 {published data only}
- Auvinen KJ, Räisänen J, Voutilainen A, Jyrkkä J, Mäntyselkä P, Lönnroos E. Interprofessional medication assessment has effects on the quality of medication among home care patients: randomised controlled intervention study. Journal of the American Medical Directors Association 2021;22:74-81. [CLINICALTRIALS.GOV: NCT02398812] [DOI] [PubMed] [Google Scholar]
Basger 2015 {published data only (unpublished sought but not used)}
- Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. International Journal of Clinical Pharmacy 2015;37(6):1194-205. [DOI] [PubMed] [Google Scholar]
Bladh 2011 {published data only}
- Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Quality & Safety 2011;20(9):738-46. [DOI] [PubMed] [Google Scholar]
Blum 2021 {published data only}
- Blum MR, Sallevelt BTGM, Spinewine A, O’Mahony D, Moutzouri E, Feller M, et al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ 2021;374:n1585:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02986425. OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid older people (OPERAM). clinicaltrials.gov/ct2/show/NCT02986425;(first received 8 December 2016).
- NTR6012. OPtimising thERapy to prevent Avoidable hospital admissions in the Multimorbid elderly. trialregister.nl/trialreg/admin/rctview.asp?TC=6012;(first received 28 July 2016).
Boersma 2019 {published data only}
- Boersma MN, Huibers CJA, Drenth‐van Maanen AC, Emmelot‐Vonk MH, Wilting I, Knol W. The effect of providing recommendations on appropriate prescribing: a cluster-randomised controlled trial in older adults in a preoperative setting. British Journal of Clinical Pharmacology 2019;85:1974-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR5750. PROPOSE: PReoperative Optimization of Pharmacotherapy in frail Older patients with use of STRIP assistant. trialregister.nl/trialreg/admin/rctview.asp?TC=5750;(first received 12 February 2016).
Bucci 2003 {published data only}
- Bucci C, Jackevicius C, McFarlane K, Liu P. Pharmacist's contribution in a heart function clinic: patient perception and medication appropriateness. Canadian Journal of Cardiology 2003;19(4):391-6. [PubMed] [Google Scholar]
Campins 2017 {published data only}
- Campins L, Serra-Prat M, Gozalo I, Lopez D, Palomera E, Agusti C, et al. Randomized controlled trial of an intervention to improve drug appropriateness in community-dwelling polymedicated elderly people. Family Practice 2017;34(1):36-42. [DOI] [PubMed] [Google Scholar]
- Campins L, Serra-Prat M, Palomera E, Bolibar I, Martinez M A, Gallo P. Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain). Gaceta Sanitaria 2017;Epub ahead of print:1-6. [DOI] [PubMed] [Google Scholar]
Clyne 2015 {published data only}
- Clyne B, Smith SM, Hughes CM, Boland F, Bradley MC, Cooper JA, et al. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster-randomized controlled trial (OPTI-SCRIPT Study). Annals of Family Medicine 2015;13(6):545-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne B, Smith SM, Hughes CM, Boland F, Cooper JA, Fahey T, et al. Sustained effectiveness of a multifaceted intervention to reduce potentially inappropriate prescribing in older patients in primary care (OPTI-SCRIPT study). Implementation Science 2016;11(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coronado‐Vazquez 2019 {published data only}
- Coronado-Vázquez V, Gómez-Salgado J, Cerezo-Espinosa de los Monteros J, Ayuso-Murillo D, Ruiz-Frutos C. Shared decision-making in chronic patients with polypharmacy: an interventional study for assessing medication appropriateness. Journal of Clinical Medicine 2019;8(6):904. [DOI: 10.3390/jcm8060904] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crotty 2004a {published data only}
- Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age & Ageing 2004;33(6):612-7. [DOI] [PubMed] [Google Scholar]
Crotty 2004b {published and unpublished data}
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. American Journal Geriatric Pharmacotherapy 2004;2(4):257-64. [DOI] [PubMed] [Google Scholar]
Curtin 2020 {published data only}
- Curtin D, Jennings E, Daunt R, Curtin S, Randles M, Gallagher P, et al. Deprescribing in older people approaching end of life: a randomized controlled trial using STOPPFrail criteria. Journal of the American Geriatrics Society 2020;68:762-9. [DOI] [PubMed] [Google Scholar]
Dalleur 2014 {published data only}
- Boland B, Dalleur O, Losseau C, Spinewine A. Reduction of inappropriate medications in frail older inpatients through recommendations by a geriatrician within the internal liaison team. European Geriatric Medicine 2012;3(S1):120. [Google Scholar]
- Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs & Aging 2014;31(4):291-8. [DOI] [PubMed] [Google Scholar]
Franchi 2016 {published and unpublished data}
- Franchi C, Tettamanti M, Djade CD, Pasina L, Mannucci PM, Onder G, et al, ELICADHE Invesigators. E-learning in order to improve drug prescription for hospitalized older patients: a cluster-randomized controlled study. British Journal of Clinical Pharmacology 2016;82(1):53-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frankenthal 2014 {published and unpublished data}
- Frankenthal D, Israeli A, Caraco Y, Lerman Y, Kalendaryev E, Zandman-Goddard G. Long-term outcomes of medication intervention using the screening tool of older persons potentially inappropriate prescriptions screening tool to alert doctors to right treatment criteria. Journal of the American Geriatrics Society 2017;65(2):e33-8. [DOI] [PubMed] [Google Scholar]
- Frankenthal D, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. Journal of the American Geriatrics Society 2014;62(9):1658-65. [DOI] [PubMed] [Google Scholar]
Fried 2017 {published data only}
- Fried TR, Niehoff KM, Street RL, Charpentier PA, Rajeevan N, Miller PL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. Journal of the American Geriatrics Society 2017;65(10):2265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 2011 {published data only}
- Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clinical Pharmacology and Therapeutics 2011;89(6):845-54. [DOI] [PubMed] [Google Scholar]
Garcia‐Gollarte 2014 {published data only (unpublished sought but not used)}
- Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cuenllas-Diaz A, Cruz-Jentoft A. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. Journal of the American Medical Directors Association 2014;15(12):885–91. [DOI] [PubMed] [Google Scholar]
Haag 2016 {published data only}
- Haag JD, Davis AZ, Hoel RW, Armon JJ, Odell LJ, Dierkhising RA, et al. Impact of pharmacist-provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. American Health & Drug Benefits 2016;9(5):259-68. [PMC free article] [PubMed] [Google Scholar]
Hanlon 1996 {published data only (unpublished sought but not used)}
- Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. American Journal of Medicine 1996;100(4):428-37. [DOI] [PubMed] [Google Scholar]
Koberlein‐Neu 2016 {published data only}
- Koberlein-Neu J, Mennemann H, Hamacher S, Waltering I, Jaehde U, Schaffert C, et al. Interprofessional medication management in patients with multiple morbidities. Deutsches Ärzteblatt international 2016;113(44):741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose O, Mennemann H, John C, Lautenschlager M, Mertens-Keller D, Richling K, et al. Priority setting and influential factors on acceptance of pharmaceutical recommendations in collaborative medication reviews in an ambulatory care setting - analysis of a cluster randomized controlled trial (WestGem-Study). PLOS One 2016;11(6):e0156304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose O, Mennemann HS, Hamacher S, Waltering I, Jaehde U, Koberlein-Neu J. Whom to serve first with a Medication Review? International Journal of Clinical Pharmacy 2016;38(4):1009. [Google Scholar]
- Rose O, Schaffert C, Czarnecki K, Mennemann HS, Waltering I, Hamacher S, et al. PMC4508809; Effect evaluation of an interprofessional medication therapy management approach for multimorbid patients in primary care: a cluster-randomized controlled trial in community care (WestGem study protocol). BMC Family Practice 2015;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michalek 2014 {published data only (unpublished sought but not used)}
- Michalek C, Wehling M, Schlitzer J, Frohnhofen H. Effects of "fit fOR the aged" (FORTA) on pharmacotherapy and clinical endpoints: a pilot randomized controlled study. European Journal of Clinical Pharmacology 2014;70(10):1261–7. [DOI] [PubMed] [Google Scholar]
Milos 2013 {published data only}
- Milos V, Rekman E, Bondesson A, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs & Aging 2013;30(4):235-46. [DOI] [PubMed] [Google Scholar]
Muth 2016 {published data only}
- Muth C, Harder S, Uhlmann L, Rochon J, Fullerton B, Guthlin C, et al. Pilot study to test the feasibility of a trial design and complex intervention on PRIoritising MUltimedication in Multimorbidity in general practices (PRIMUMpilot). BMJ Open 2016;6(7):e011613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muth 2018 {published data only}
- Muth C, Uhlmann L, Haefeli WE, Rochon J, den Akker M, Perera R, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ Open 2018;8(2):e017740. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Mahony 2020 {published data only}
- O’Mahony D, Gudmundsson A, Soiza RL, Petrovic M, Cruz-Jentoft AJ, Cherubini A, et al. Prevention of adverse drug reactions in hospitalised older patients with multi-morbidity and polypharmacy: the SENATOR randomised controlled trial. Age and Ageing 2020;49:605-14. [DOI] [PubMed] [Google Scholar]
Olsson 2012 {published data only}
- Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scandinavian Journal of Primary Health Care 2012;30(1):3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pitkala 2014 {published and unpublished data}
- Pitkala KH, Juola AL, Kautiainen H, Soini H, Finne-Soveri U, Bell JS, et al. Education to reduce potentially harmful medication use among residents of assisted living facilities: a randomized controlled trial. Journal of the American Medical Directors Association 2014;15(12):892–8. [DOI] [PubMed] [Google Scholar]
Romskaug 2020 {published data only}
- Romskaug R, Molden E, Straand J, Kersten H, Skovlund E, Pitkala KH, et al. Cooperation between geriatricians and general practitioners for improved pharmacotherapy in home-dwelling elderly people receiving polypharmacy - the COOP Study: study protocol for a cluster randomised controlled trial. Trials 2017;18(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romskaug R, Skovlund E, Straand J, Molden E, Kersten H, Pitkala KH, et al. Effect of clinical geriatric assessments and collaborative medication reviews by geriatrician and family physicians for improving health-related quality of life in home-dwelling older patients receiving polypharmacy. A cluster randomized clinical trial. JAMA Internal Medicine 2020;180(2):181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmader 2004 {published data only (unpublished sought but not used)}
- Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. American Journal of Medicine 2004;116(6):394-401. [DOI] [PubMed] [Google Scholar]
Shim 2018 {published data only}
- Shim YW, Chua SS, Wong HC, Alwi S. Collaborative intervention between pharmacists and physicians on elderly patients: a randomised controlled trial. Therapeutics and Clinical Risk Management 2018;14:1114-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinewine 2007 {published and unpublished data}
- Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized controlled trial. Journal of the American Geriatrics Society 2007;55(5):658-65. [DOI] [PubMed] [Google Scholar]
Strauven 2019 {published data only}
- Strauven G, Anrys P, Vandael E, Henrard S, De Lepeleire J, Spinewine A, et al. Cluster-controlled trial of an intervention to improve prescribing in nursing homes study. Journal of the American Medical Directors Association (JAMDA) 2019;20(11):1404-11. [DOI] [PubMed] [Google Scholar]
Syafhan 2021 {published data only}
- Syafhan NF, Azzam SA, Williams SD, Wilson W, Brady J, Lawrence P, et al. General practitioner practice-based pharmacist input to medicines optimisation in the UK: pragmatic, multicentre, randomised, controlled trial. Journal of Pharmaceutical Policy and Practice 2021;14:Article number 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2003 {published data only}
- Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, et al. The medical office of the 21st century (MOXXI): effectiveness of computerised decision-making support in reducing inappropriate prescribing in primary care. Canadian Medical Association Journal 2003;169(6):549-56. [PMC free article] [PubMed] [Google Scholar]
Taylor 2003 {published data only (unpublished sought but not used)}
- Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. American Journal of Health-System Pharmacy 2003;60(11):1123-9. [DOI] [PubMed] [Google Scholar]
Thyrian 2017 {published and unpublished data}
- Thyrian JR, Hertel J, Wucherer D, Eichler T, Michalowsky B, Dreier-Wolfgramm A, et al. Effectiveness and safety of dementia care management in primary care: a randomized clinical trial. JAMA Psychiatry 2017;74(10):996-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wehling 2016 {published data only (unpublished sought but not used)}
- Pazan F, Burkhardt H, Frohnhofen H, Weiss C, Throm C, Kuhn-Thiel A, et al. Changes in prescription patterns in older hospitalized patients: the impact of FORTA on disease-related over- and under-treatments. European Journal of Clinical Pharmacology 2017;74(3):339-47. [DOI] [PubMed] [Google Scholar]
- Wehling M, Burkhardt H, Kuhn-Thiel A, Pazan F, Throm C, Weiss C, et al. VALFORTA: A randomised trial to validate the FORTA (fit fOR the aged) classification. Age and Ageing 2016;45(2):262-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
DRKS00013588 {unpublished data only}
- DRKS00013588. Drug safety for nursing-home residents - findings of a pragmatic, cluster-randomized, controlled intervention trial in 44 nursing homes. Deutsches Arzteblatt International 2021;118(42):705-12. [DOI: 10.3238/arztebl.m2021.0297] [DOI] [PMC free article] [PubMed]
- Krause O, Wiese B, Doyle IM, Kirsch C, Thurmann P, Wilm S, et al, H IOPP-3-iTBX study. Multidisciplinary intervention to improve medication safety in nursing home residents: protocol of a cluster randomised controlled trial (HIOPP-3-iTBX study). BMC Geriatrics 2019;19(1):24. [DOI: 10.1177/2042098620918459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogg 2009 {published data only}
- Fletcher J, Hogg W, Farrell B, Woodend K, Dahrouge S, Lemelin J, et al. Effect of nurse practitioner and pharmacist counseling on inappropriate medication use in family practice. Canadian Family Physician 2012;58(8):862-8. [PMC free article] [PubMed] [Google Scholar]
- Hogg W, Lemelin J, Dahrouge S, Liddy C, Armstrong CD, Legault F, et al. Randomized controlled trial of anticipatory and preventive multidisciplinary team care: for complex patients in a community-based primary care setting. Canadian Family Physician (Medecin de Famille Canadien) 2009;55(12):e76-85. [PMC free article] [PubMed] [Google Scholar]
Hugtenburg 2017 {published data only}
- Hugtenburg JG. The effectiveness of optimised clinical medication reviews for geriatric patients: a cluster randomized controlled trial. Pharmacoepidemiology and Drug Safety 2017;26:194. [DOI] [PubMed] [Google Scholar]
Juola 2015 {published data only}
- Juola AL, Bjorkman MP, Pylkkanen S, Finne-Soveri H, Soini H, Kautiainen H, et al. Nurse education to reduce harmful medication use in assisted living facilities: effects of a randomized controlled trial on falls and cognition. Drugs & Aging 2015;32(11):947-55. [DOI] [PubMed] [Google Scholar]
Rieckert 2020 {published data only}
- Rieckert A, Reeves D, Altiner A, Drewelow E, Esmail A, Flamm M, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ 2020;369:m1822. [DOI: 10.1136/bmj.m1822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt‐Mende 2017 {published data only}
- Schmidt-Mende K, Andersen M, Wettermark B, Hasselstrom J. An educational intervention to reduce acute health care consumption in elderly patients with inappropriate drugs - a cluster randomised trial in primary care. Pharmacoepidemiology and Drug Safety 2016;25:378-9. [DOI] [PubMed] [Google Scholar]
- Schmidt-Mende K, Andersen M, Wettermark B, Hasselstrom J. Educational intervention on medication reviews aiming to reduce acute healthcare consumption in elderly patients with potentially inappropriate medicines-A pragmatic open-label cluster-randomized controlled trial in primary care. Pharmacoepidemiology and Drug Safety 2017;26(11):1347-56. [DOI] [PubMed] [Google Scholar]
Simon 2006 {published data only}
- Simon SR, Smith DH, Feldstein AC, Perrin N, Yang X, Zhou Y, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. Journal of the American Geriatrics Society 2006;54(6):963-8. [DOI] [PubMed] [Google Scholar]
Wouters 2017 {published data only}
- Taxis K, Scheper J, Koning H, Brouwer C, Twisk J, Meer H, et al. Discontinuing inappropriate medication in nursing home residents (DIM-NHR study)-a cluster randomized controlled trial. Pharmacoepidemiology and Drug Safety 2017;26:449-50. [Google Scholar]
- Wouters H, Scheper J, Koning H, Brouwer C, Twisk JW, Meer H, et al. Discontinuing inappropriate medication use in nursing home residents: a cluster randomized controlled trial. Annals of Internal Medicine 2017;167(9):609-17. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aharaz 2021 {published data only}
- Aharaz A, Rasmussen JH, McNulty HBO, Cyron A, Fabricius PK, Bengaard AK, et al. A collaborative deprescribing intervention in a subacute medical outpatient clinic: a pilot randomised controlled trial. Metabolites 2021;11:1-14. [DOI: 10.3390/metabo11040204] [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Cura‐Gonzalez 2022 {published data only}
- Del Cura-Gonzalez I, Lopez-Rodriguez JA, Leiva-Fernandez F, Gimeno-Miguel A, Poblador-Plou B, Lopez-Verde F, et al. How to improve healthcare for patients with multimorbidity and polypharmacy in primary care: a pragmatic cluster-randomized clinical trial of the multipap intervention. Journal of Personalized Medicine 2022;12(5):1-16. [DOI: 10.3390/jpm12050752] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grischott 2022 {published data only}
- Grischott T, Rachamin Y, Senn O, Hug P, Rosemann T, Neuner-Jehle S. Medication review and enhanced information transfer at discharge of older patients with polypharmacy: a cluster-randomized controlled trial in Swiss hospitals. Journal of General Internal Medicine 2022 Aug 31 [Epub ahead of print]. [DOI: 10.1007/s11606-022-07728-6] [DOI] [PMC free article] [PubMed]
Kirwan 2022 {published data only}
- Kirwan C, Hynes L, Hart N, Mulligan S, Leathem C, McQuillan L, et al. The multimorbidity collaborative medication review and decision making (MyComrade) study: a pilot cluster randomised trial in two healthcare systems. Pilot and Feasibility Studies 2022;8(1):1-29. [DOI: 10.1186/s40814-022-01107-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kornholt 2022 {published data only}
- Kornholt J, Feizi ST, Hansen AS, Laursen JT, Reuther LO, Petersen TS, et al. Effects of a comprehensive medication review intervention on health-related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: A pragmatic randomized clinical trial. British Journal of Clinical Pharmacology 2022;88(7):3360-9. [DOI: 10.1111/bcp.15287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahlknecht 2021 {published data only}
- Mahlknecht A, Wiedermann CJ, Sandri M, Engl A, Valentini M, Vogele A, et al. Expert-based medication reviews to reduce polypharmacy in older patients in primary care: a northern-Italian cluster-randomised controlled trial. BMC Geriatrics 2021;21(1):1-19. [DOI: 10.1186/s12877-021-02612-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2022 {published data only}
- McCarthy C, Clyne B, Boland F, Moriarty F, Flood M, Wallace E, et al. GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE Study): a cluster randomised controlled trial. PLOS Medicine 2022;19(1):1-19. [DOI: 10.1371/ journal.pmed.1003862] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2022 {published data only}
- McDonald EG, Wu PE, Rashidi B, Wilson MG, Bortolussi-Courval E, Atique A, et al. The MedSafer Study-Electronic decision support for deprescribing in hospitalized older adults: a cluster randomized clinical trial. JAMA Internal Medicine 2022;182(3):265-73. [DOI: 10.1001/jamainternmed.2021.7429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rankin 2022 {published data only}
- Rankin A, Gorman A, Cole J, Cadogan CA, Barry HE, Agus A, et al. An external pilot cluster randomised controlled trial of a theory-based intervention to improve appropriate polypharmacy in older people in primary care (PolyPrime). Pilot and Feasibility Studies 2022;8(1):1-19. [DOI: 10.1186/s40814-022-01161-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rudolf 2021 {published data only}
- Rudolf H, Thiem U, Aust K, Krause D, Klaassen-Mielke R, Greiner W, et al. Reduction of potentially inappropriate medication in the elderly — results of a cluster-randomized, controlled trial in German primary care practices (RIME). Deutsches Ärzteblatt International 2021;118:875–82. [DOI: 10.3238/arztebl.m2021.0372] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617000665336 {unpublished data only}
- ACTRN12617000665336. Impact of clinical pharmacist medication review on appropriate prescribing in elderly patients: a randomized, controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372670 (first received 30 March 2017).
Correard 2020 {published data only}
- Correard F, Montaleytang M, Costa M, Astolfi M, Baumstarck K, Loubière S, et al. Impact of medication review via tele-expertise on unplanned hospitalizations at 3 months of nursing homes patients (TEM-EHPAD): study protocol for a randomized controlled trial. BMC Geriatrics 2020;20:147. [DOI: 10.1186/s12877-020-01546-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dauphinot 2017 {published data only}
- Dauphinot V, Jean-Bart E, Krolak-Salmon P, Mouchoux C. A multi-center, randomized, controlled trial to assess the efficacy of optimization of drug prescribing in an elderly population, at 18 months of follow-up, in the evolution of functional autonomy: the OPTIM study protocol. BMC Geriatrics 2017;17(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00003610 {unpublished data only}
- Thiem U, Wilm S, Greiner W, Rudolf H, Trampisch HJ, Müller C, et al. Reduction of potentially inappropriate medication in the elderly: design of a cluster-randomised controlled trial in German primary care practices (RIME). Therapeutic Advances in Drug Safety 2020;11:1-13. [DOI: 10.1177/2042098620918459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greiver 2019 {published data only}
- Greiver M, Dahrouge S, O’Brien P, Manca D, Lussier MT, Wang J, et al. Improving care for elderly patients living with polypharmacy: protocol for a pragmatic cluster randomized trial in community-based primary care practices in Canada. Implementation Science 2019;14:55. [DOI: 10.1186/s13012-019-0904-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husebo 2015 {published data only}
- Husebo BS, Flo E, Aarsland D, Selbaek G, Testad I, Gulla C, et al. COSMOS - improving the quality of life in nursing home patients: protocol for an effectiveness-implementation cluster randomized clinical hybrid trial. Implementation Science 2015;10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ie 2020 {published data only}
- Ie K, Hirose M, Sakai T, Motohashi I, Aihara M, Otsuki T, et al. Protocol of a randomised controlled trial on the efficacy of medication optimisation in elderly inpatients: medication optimisation protocol efficacy for geriatric inpatients (MPEG) trial. BMJ Open 2020;10(10):e041125. [DOI: 10.1136/bmjopen-2020-041125] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN18427377 {unpublished data only}
- Grischott T, Zechmann S, Rachamin Y, Markun S, Chmiel C, Senn O, et al. Improving inappropriate medication and information transfer at hospital discharge: study protocol for a cluster RCT. Implementation Science 2018;13:155. [DOI: 10.1186/s13012-018-0839-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN18427377. Hospital discharge study. https://www.isrctn.com/ISRCTN18427377 2018;(first received 3 January 2018).
ISRCTN18752158 {published data only}
- ISRCTN18752158. The general practice-based pharmacist: supporting medicines management in older adults. www.isrctn.com/ISRCTN18752158 (first received 3 March 2020).
ISRCTN41009897 {published data only}
- ISRCTN41009897. Pilot testing of a new approach to improving the prescribing of many drugs for older people who live in their own home and are cared for by general practitioners. www.isrctn.com/ISRCTN41009897 (first received 18 November 2019).
ISRCTN90146150 {published data only}
- ISRCTN90146150. Improving medicines use in people who take multiple medicines. https://www.isrctn.com/ISRCTN90146150 (first received 19 March 2019).
Johansen 2018 {published data only}
- Johansen JS, Havnes K, Halvorsen KH, Haustreis S, Skaue LW, Kamycheva E, et al. Interdisciplinary collaboration across secondary and primary care to improve medication safety in the elderly (IMMENSE study): study protocol for a randomised controlled trial. BMJ Open 2018;8(1):e020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02816086. A new interdisciplinary collaboration structure to improve medication safety in the elderly. clinicaltrials.gov/ct2/show/NCT02816086 (first received 28 June 2016).
Jungo 2019 {published data only}
- Jungo KT, Rozsnyai Z, Mantelli S, Floriani C, Lowe AL, Lindemann F, et al. 'Optimising PharmacoTherapy In the multimorbid elderly in primary CAre' (OPTICA) to improve medication appropriateness: study protocol of a cluster randomised controlled trial. BMJ Open 2019;9(9):e031080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komagamine 2018 {published data only}
- Komagamine J, Sugawara K, Kaminaga M, Tatsumi S. Study protocol for a single-centre, prospective, non-blinded, randomised, 12-month, parallel-group superiority study to compare the efficacy of pharmacist intervention versus usual care for elderly patients hospitalised in orthopaedic wards. BMJ Open 2018;8:e021924. [DOI: 10.1136/bmjopen-2018-021924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kua 2017 {published and unpublished data}
- Kua CH, Yeo C YY, Char CWT, Tan CWY, Tan PC, Mak VS, et al. Nursing home team-care deprescribing study: a stepped-wedge randomised controlled trial protocol. BMJ Open 2017;7(5):e015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02863341. Nursing home team-care deprescribing study. clinicaltrials.gov/ct2/show/NCT02863341 2018;(first received 11 August 2016).
Loffler 2014 {published data only}
- Loffler C, Drewelow E, Paschka SD, Frankenstein M, Eger J, Jatsch L, et al. Optimizing polypharmacy among elderly hospital patients with chronic diseases: study protocol of the cluster randomized controlled POLITE-RCT trial. Implementation Science 2014;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2017 {published and unpublished data}
- ISRCTN12752680. Supporting medicines management in older adults with multiple medical conditions. isrctn.com/ISRCTN12752680 (first received 26 September 2016).
- McCarthy C, Clyne B, Corrigan D, Boland F, Wallace E, Moriarty F, et al. Supporting prescribing in older people with multimorbidity and significant polypharmacy in primary care (SPPiRE): a cluster randomised controlled trial protocol and pilot. Implementation Science 2017;12(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mestres 2017 {published data only}
- Mestres Gonzalvo C, Wit HA, Oijen BP, Hurkens KP, Janknegt R, Schols JM, et al. Supporting clinical rules engine in the adjustment of medication (SCREAM): protocol of a multicentre, prospective, randomised study. BMC Geriatrics 2017;17(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00844025 {unpublished data only}
- NCT00844025. Pharmaceutical care and clinical outcomes for the elderly taking potentially inappropriate medication. clinicaltrials.gov/ct2/show/NCT00844025 (first received 13 February 2009).
NCT01034761 {unpublished data only}
- NCT01034761. Using clinical alerts to decrease inappropriate medication prescribing. clinicaltrials.gov/ct2/show/NCT01034761 (first received 17 December 2009).
NCT02942927 {published and unpublished data}
- NCT02942927. Team approach to polypharmacy evaluation and reduction. clinicaltrials.gov/ct2/show/NCT02942927 (first received 24 October 2016).
NCT03156348 {unpublished data only}
- NCT03156348. Impact of clinical pharmacist on adverse drug events in older adults. clinicaltrials.gov/ct2/show/NCT03156348;(first received 17 May 2017).
NCT03298386 {unpublished data only}
- NCT03298386. Elderly Appropriate Treatment in primary care (EAT). clinicaltrials.gov/ct2/show/NCT03298386;(first received 2 October 2017).
NCT03909035 {published data only}
- NCT03909035. A collaborative approach to medication reviews for older patients with polypharmacy (BIMEDOC). clinicaltrials.gov/ct2/show/NCT03909035 (first received 9 April 2019).
NCT04004936 {published data only}
- NCT04004936. Reducing potentially inappropriate medication prescribing for older patients in the ED (EQUIPPED). clinicaltrials.gov/ct2/show/NCT04004936 (first received 2 July 2019).
NCT04028583 {published data only}
- NCT04028583. Tool for Inappropriate Prescription Evaluation: The TaIPE Study (TaIPE). clinicaltrials.gov/ct2/show/NCT04028583 (first received 22 July 2019).
NCT04087109 {published data only}
- NCT04087109. MedSafer E-care: an automated deprescribing solution (E-CARE Study) (E-CARE) [MedSafer E-care: an automated deprescribing solution for community-dwelling older adults lving with polypharmacy (E-CARE Study)]. clinicaltrials.gov/ct2/show/NCT04087109 (first received 12 September 2019).
NCT04147130 {published data only}
- NCT04147130. MultiPAP Plus: improving prescription in primary care patients with multimorbidity and polypharmacy (MultiPAP Plus). clinicaltrials.gov/ct2/show/NCT04147130 (first received 31 October 2019).
NCT04181879 {published data only}
- NCT04181879. Appropriate polypharmacy in older people in primary care (PolyPrime). clinicaltrials.gov/ct2/show/NCT04181879 (first received 2 December 2019).
NTR6644 {published data only}
- NTR6644. Improving medication prescription in the context of advanced care planning for patients receiving nursing home care. trialsearch.who.int/Trial2.aspx?TrialID=NTR6644 (first received 23 August 2017).
- Pouw CAM, Smalbrugge MS, Hugtenburg JG, Van Marum RJ, Hertogh CMPM. Improving medication prescription in the context of advanced care planning for patients receiving nursing home care (IMPETUS): study protocol of a cluster randomized controlled trial. European Geriatric Medicine 2017;8:S218. [Google Scholar]
Prados‐Torres 2017 {published and unpublished data}
- NCT02866799. Multi-PAP RCT: Improving prescription in primary care patients with multimorbidity and polypharmacy. clinicaltrials.gov/ct2/show/NCT02866799 (first received 15 August 2016).
- Prados-Torres A, Del Cura-Gonzalez I, Prados-Torres D, Lopez-Rodriguez JA, Leiva-Fernandez F, Calderon-Larranaga A, et al. Effectiveness of an intervention for improving drug prescription in primary care patients with multimorbidity and polypharmacy: study protocol of a cluster randomized clinical trial (Multi-PAP project). Implementation Science 2017;12(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Selic 2016 {published data only}
- Cedilnik Gorup E, Petek-Ster M. Use of web-based application to improve prescribing in home-living elderly: a randomised controlled study protocol. In: 9th Congress of the European Union Geriatric Medicine Society, Venice, Italy. 2013.
- Selic P, Gorup EC, Gorup S, Ster MP, Rifel J, Ketis ZK. The effects of a web application and medical monitoring on the quality of medication, adverse drug events and adherence in the elderly living at home: a protocol of the study. Materia Socio Medica 2016;28(6):432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agbabiaka 2017
- Agbabiaka TB, Wider B, Watson LK, Goodman C. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review protocol. Drugs & Aging 2017;34(12):891-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
AGS 2012
- American Geriatrics Society 2012. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. Journal of the American Geriatrics Society 2012;60(4):616-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
AGS 2019
- 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society 2019;67(4):674-94. [DOI: 10.1111/jgs.15767] [DOI] [PubMed] [Google Scholar]
Ali 2022
- Ali MU, Sherifali D, Fitzpatrick-Lewis D, Kenny M, Lamarche L, Raina P, et al. Interventions to address polypharmacy in older adults living with multimorbidity. Canadian Family Physician 2022;68:e215-26. [DOI: 10.46747/cfp.6807e215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anrys 2016
- Anrys P, Strauven G, Boland B, Dalleur O, Declercq A, Degryse JM, et al. Collaborative approach to Optimise MEdication use for Older people in Nursing homes (COME-ON): Study protocol of a cluster controlled trial. Implementation Science 2016;11(1):1-11. [DOI: 10.1186/s13012-016-0394-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Appleton 2014
- Appleton SC, Abel GA, Payne RA. Cardiovascular polypharmacy is not associated with unplanned hospitalisation: Evidence from a retrospective cohort study. BMC Family Practice 2014;15(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aronson 2006
- Aronson JK. Polypharmacy, appropriate and inappropriate. British Journal of General Practice 2006;56(528):484-5. [PMC free article] [PubMed] [Google Scholar]
Barnett 2012
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012;380(9836):37-43. [DOI] [PubMed] [Google Scholar]
Basger 2012
- Basger B, Chen TF, Moles RJ. Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method. BMJ Open 2012;2(5):e001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beers 1991
- Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC. Explicit criteria for determining inappropriate medication use in nursing home residents. Archives of Internal Medicine 1991;151(9):1825-32. [PubMed] [Google Scholar]
Beers 1997
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Archives of Internal Medicine 1997;157(14):1531-6. [PubMed] [Google Scholar]
Bradley 2012
- Bradley MC, Fahey T, Cahir C, Bennett K, O'Reilly D, Parsons C, et al. Potentially inappropriate prescribing and cost outcomes for older people: a cross-sectional study using the Northern Ireland Enhanced Prescribing Database. European Journal of Clinical Pharmacology 2012;68(10):1425-33. [DOI] [PubMed] [Google Scholar]
Cadogan 2016
- Cadogan CA, Ryan C, Francis JJ, Gormley GJ, Passmore P, Kerse N, et al. Development of an intervention to improve appropriate polypharmacy in older people in primary care using a theory-based method. BMC Health Services Research 2016;16(1):661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadogan 2016a
- Cadogan CA, Ryan C, Hughes CM. Appropriate polypharmacy and medicine safety: when many is not too many. Drug Safety 2016;39(2):109-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahir 2010
- Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. British Journal of Clinical Pharmacology 2010;69(5):543-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahir 2014
- Cahir C, Moriarty F, Teljeur C, Fahey T, Bennett K. Potentially inappropriate prescribing and vulnerability and hospitalization in older community-dwelling patients. Annals of Pharmacotherapy 2014;48(12):1546-54. [DOI] [PubMed] [Google Scholar]
Coast 2008
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Social Science and Medicine 2008;67(5):874-82. [DOI: 10.1016/j.socscimed.2008.05.015] [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337(7676):979-83. [DOI: 10.1136/bmj.a1655] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2022
- Davies LE, Kingston A, Todd A, Hanratty B. Is polypharmacy associated with mortality in the very old: findings from the Newcastle 85+ Study. British Journal of Clinical Pharmacology 2022;88(6):2988-95. [DOI: 10.1111/bcp.15211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Del Cura‐Gonzalez 2022
- Del Cura-Gonzalez I, Lopez-Rodriguez JA, Leiva-Fernandez F, Gimeno-Feliu LA, Pico-Soler V, Bujalance-Zafra MJ, et al, MULTIPAP PLUSGroup. Effectiveness of the MULTIPAP Plus intervention in youngest-old patients with multimorbidity and polypharmacy aimed at improving prescribing practices in primary care: study protocol of a cluster randomized trial. Trials 2022;23(479):1-13. [DOI: 10.1186/s13063-022-06293-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Department of Health and Social Care 2021
- Good for you, good for us, good for everybody: a plan to reduce overprescribing to make patient care better and safer, support the NHS, and reduce carbon emissions. Department of Health and Social Care 2021. [CROWN COPYRIGHT: 2021]
Elwyn 2012
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. Journal of General Internal Medicine 2012;27(10):1361–7. [DOI: 10.1007/s11606-012-2077-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2015
- Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy, 2015. Available at: epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/epoc_taxonomy_13.12.16.pdf.
EPOC 2016
- Effective Practice and Organisation of Care (EPOC). The EPOC taxonomy of health systems interventions. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2016. Available at: epoc.cochrane.org/epoc-specific-resources-review-authors.
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). What study designs should be included in an EPOC review? EPOC Resources for review authors, 2017. Available at: epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/what_study_designs_should_be_included_in_an_epoc_review.pdf.
EPOC 2017b
- Effective Practice and Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors, 2017. Available at: epoc.cochrane.org/epoc-specific-resources-review-authors.
Ettema 2007
- Ettema TP, Droes RM, Lange J, Mellenbergh GJ, Ribbe MW. QUALIDEM: development and evaluation of a dementia specific quality of life instrument. Scalability, reliability and internal structure. International Journal of Geriatric Psychiatry 2007;22(6):549-56. [DOI: 10.1002/gps.1713] [DOI] [PubMed] [Google Scholar]
Fastbom 2015
- Fastbom J, Johnell K. National indicators for quality of drug therapy in older persons: the Swedish experience from the first 10 years. Drugs & Aging 2015;32(3):189-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fick 2003
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults. Archives of Internal Medicine 2003;163:2716-24. [DOI] [PubMed] [Google Scholar]
Fick 2008
- Fick DM, Mion LC, Beers MH, Waller JL. Health outcomes associated with potentially inappropriate medication use in older adults. Research in Nursing and Health 2008;31(1):42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fulton 2005
- Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. Journal of the American Academy of Nurse Practitioners 2005;17(4):123-32. [DOI] [PubMed] [Google Scholar]
Furniss 2000
- Furniss LBA, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. British Journal of Psychiatry 2000;176:563-7. [DOI] [PubMed] [Google Scholar]
Gallagher 2001
- Gallagher LP. The potential for adverse drug reactions in elderly patients. Applied Nursing Research 2001;14(4):220-4. [DOI] [PubMed] [Google Scholar]
Gallagher 2008
- Gallagher P, Ryan C, Byrne S, Kennedy J, O'Mahony D. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. International Journal of Clinical Pharmacology and Therapeutics 2008;46(2):72-83. [DOI] [PubMed] [Google Scholar]
Galvin 2014
- Galvin R, Moriarty F, Cousins G, Cahir C, Motterlini N, Bradley M, et al. Prevalence of potentially inappropriate prescribing and prescribing omissions in older Irish adults: findings from The Irish LongituDinal Study on Ageing study (TILDA). European Journal of Clinical Pharmacology 2014;70(5):599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADEpro GDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Guthrie 2015
- Guthrie B, Makubate B, Hernandez-Santiago V, Dreischulte T. The rising tide of polypharmacy and drug-drug interactions: population database analysis 1995-2010. BMC Medicine 2015;13(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 1992
- Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK, et al. A method for assessing drug therapy appropriateness. Journal of Clinical Epidemiology 1992;45(10):1045-51. [DOI] [PubMed] [Google Scholar]
Hepler 1990
- Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. American Journal of Hospital Pharmacy 1990;47(3):533-43. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne, JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook. [WEBSITE: www.training.cochrane.org/handbook]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Holt 2010
- Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Deutsches Ärzteblatt International 2010;107(31-32):543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2012
- Hughes LD, McMurdo MET, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age and Ageing 2012;42(1):62-9. [DOI] [PubMed] [Google Scholar]
Hughes 2014
- Hughes C, Cooper JA, Ryan C. Going beyond the numbers: a call to redefine polypharmacy. British Journal of Clinical Pharmacology 2014;77(6):915-6. [DOI: 10.1111/bcp.12284] [DOI] [PMC free article] [PubMed] [Google Scholar]
Information Centre 2017
- Prescribing and Medicines Team, NHS Digital. Prescriptions dispensed in the community. Statistics for 2006-16. www.digital.nhs.uk/publication/s/o/pres-disp-com-eng-2006-16-rep.pdf (accessed 16 April 2018).
Jeffery 1999
- Jeffery S, Ruby CM, Hanlon JT, Twersky JI. The impact of an interdisciplinary team on suboptimal prescribing in a long term care facility. Consultant Pharmacist 1999;14:1386–9. [Google Scholar]
Jennings 2022
- Jennings ELM, O'Mahony D, Gallgher PF. Medication-related quality of life (MRQoL) in ambulatory older adults with multi-morbidity and polypharmacy. European Geriatric Medicine 2022;13(3):579-83. [DOI: 10.1007/s41999-021-00573-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kantor 2015
- Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015;314(17):1818-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufmann 2014
- Kaufmann CP, Tremp R, Hersberger KE, Lampert ML. Inappropriate prescribing: a systematic overview of published assessment tools. European Journal of Clinical Pharmacology 2014;70(1):1-11. [DOI] [PubMed] [Google Scholar]
Kaur 2009
- Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly. A systematic review. Drugs & Aging 2009;26(12):1013-28. [DOI] [PubMed] [Google Scholar]
King's Fund 2013
- The King's Fund. Polypharmacy and medicines optimisation: Making it safe and sound. www.kingsfund.org.uk/sites/default/files/field/field_publication_file/polypharmacy-and-medicines-optimisation-kingsfund-nov13.pdf (accessed 26 April 2016).
Kirkham 2016
- Kirkham JJ, Gorst S, Altman DG, Blazeby JM, Clarke M, Devane D, et al. Core outcome set STAndards for reporting: The COS-STAR statement. PLOS Medicine 2016;13(10):e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2004
- Ko DT, Mamdani M, Alter DA. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA 2004;291(15):1864–70. [DOI] [PubMed] [Google Scholar]
Kuhn‐Thiel 2014
- Kuhn-Thiel AM, Weiss C, Wehling M. Consensus Validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs & Aging 2014;31(2):131-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuijpers 2007
- Kuijpers MA, Marum RJ, Egberts AC, Jansen PA, OLDY (OLd people Drugs & dYsregulations) Study Group. Relationship between polypharmacy and underprescribing. British Journal of Clinical Pharmacology 2007;65(1):130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2005
- Lee DS, Tu JV, Juurlink DN, Alter DA, Ko DT, Austin PC, et al. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA 2005;294(24):1240-7. [DOI] [PubMed] [Google Scholar]
Mangoni 2003
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British Journal of Clinical Pharmacology 2003;57(1):6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masnoon 2017
- Masnoon N, Shakib S, Kalisch-Ellett L, Caughey G. What is polypharmacy? A systematic review of definitions. BMC Geriatrics 2017;17:1-10. [DOI: 10.1186/s12877-017-0621-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2022
- McCarthy C, Clyne B, Boland F, Moriarty F, Flood M, Wallace E, et al, SPPiRE Study team. GP-delivered medication review of polypharmacy, deprescribing, and patient priorities in older people with multimorbidity in Irish primary care (SPPiRE Study): A cluster randomised controlled trial. PLOS Medicine/Public Library of Science 2022;19(1):1-19. [DOI: 10.1371/journal.pmed.1003862] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGarrigle 2017
- McGarrigle C, Donoghue O, Scarlett S, Kenny RA. Health and Wellbeing: Active Ageing for Older Adults in Ireland. Dublin: The Irish Longitudinal Study on Ageing (TILDA), 2017. [Google Scholar]
McLeod 1997
- McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. Canadian Medical Association Journal 1997;156(3):385-91. [PMC free article] [PubMed] [Google Scholar]
Meds75+ [Computer program]
- Meds75+ database of medication. Meds75+ - fimea englanti, accessed 5 January 2022.
Mekonnen 2021
- Mekonnen AB, Redley B, Courten B. Potentially inappropriate prescribing and its associations with health-related and system-related outcomes in hospitalised older adults: a systematic review and meta-analysis. British Journal of Clinical Pharmacology 2021;87:4150-72. [DOI: 10.1111/bcp.14870] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2011
- Michie S, Abraham C, Eccles MP, Francis JJ, Hardeman W, Johnston M. Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implementation Science 2011;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
- Moore G, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:1-7. [DOI: 10.1136/bmj.h1258] [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC 2008
- Medical Research Council. A framework for the development and evaluation of RCTs for complex interventions to improve health. Medical Research Council, UK 2008.
Mucherino 2022
- Mucherino S, Casula M, Galimberti F, Guarino I, Olmastroni E, Tragni E, et al, on behalf of the EDUREDRUG Group. The effectiveness of interventions to evaluate and reduce healthcare costs of potentially inappropriate prescriptions among the older adults: a systematic review. International Journal of Environmental Research and Public Health 2022;19(6724):1-19. [DOI: 10.3390/ijerph19116724] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2022
- Murphy M, Bennett K, Ryan S, Hughes CM, Lavan AH, Cadogan CA. A systematic scoping review of interventions to optimise medication prescribing and adherence in older adults with cancer. Research in Social and Administrative Pharmacy 2022;18:2392–402. [DOI: 10.1016/j.sapharm.2021.04.011] [DOI] [PubMed] [Google Scholar]
O'Connor 2012
- O'Connor MN, Gallagher P, O'Mahony D. Inappropriate prescribing. Drugs & Aging 2012;29(6):437-52. [DOI] [PubMed] [Google Scholar]
O'Mahony 2015
- O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age & Ageing 2015;44(2):213-8. [DOI: 10.1093/ageing/afu145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2013
- Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation Science 2013;8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rankin 2018
RENBASE [Computer program]
- RENBASE - use of drugs in renal failure. Lääkkeet ja munuaiset (terveysportti.fi), accessed 5 January 2022.
RevMan 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.13. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rogers 2003
- Rogers EM. Diffusion of Innovations. 5th edition. New York: Free Press, 2003. [Google Scholar]
Rollason 2003
- Rollason V, Vogt N. Reduction of polypharmacy in the elderly. A systematic review of the role of the pharmacist. Drugs & Aging 2003;20(11):817-32. [DOI] [PubMed] [Google Scholar]
Romskaug 2017
- Romskaug R, Molden E, Straand J, Kersten H, Skovlund E, Pitkala KH, et al. Cooperation between geriatricians and general practitioners for improved pharmacotherapy in home-dwelling elderly people receiving polypharmacy - the COOP Study: study protocol for a cluster randomised controlled trial. Trials 2017;18:1-9. [DOI: 10.1186/s13063-017-1900-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saeed 2021
- Saeed D, Carter G, Parsons C. Interventions to improve medicines optimisation in frail older patients in secondary and acute care settings: a systematic review of randomised controlled trials and non‑randomised studies. International Journal of Clinical Pharmacy 2021;44:15-26. [DOI: 10.1007/s11096-021-01354-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salm 2022
- Salm C, Sauer J, Binder N, Pfefferle A, Sofroniou M, Metzner G, et al. Over- and under-prescribing, and their association with functional disability in older patients at risk of further decline in Germany – a cross-sectional survey conducted as part of a randomised comparative effectiveness trial. BMC Geriatrics 2022;22:1-10. [DOI: 10.1186/s12877-022-03242-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuttner 2022
- Schuttner L, Hockett Sherlock S, Simons C, Ralson JD, Rosland AM, Nelson K, et al. Factors affecting primary care physician decision-making for patients with complex multimorbidity: a qualitative interview study. BMC Primary Care 2022;23:1-10. [DOI: 10.1186/s12875-022-01633-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 2010
- Scott IA, Guyatt GH. Cautionary tales in the interpretation of clinical studies involving older persons. Archives of Internal Medicine 2010;170(7):587-9. [DOI] [PubMed] [Google Scholar]
SFINX database [Computer program]
- SFINX database for drug-drug interactions. Lääkeinteraktiot ja -haitat (terveysportti.fi), accessed 5 January 2022.
Simonson 2005
- Simonson W, Feinberg JL. Medication-related problems in the elderly. Drugs and Aging 2005;22(7):559-69. [DOI] [PubMed] [Google Scholar]
Skivington 2021
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. [DOI: 10.1136/bmj.n2061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinewine 2007a
- Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007;370(9582):173-84. [DOI] [PubMed] [Google Scholar]
Steinman 2007
- Steinman MA. Polypharmacy and the balance of medication benefits and risks. American Journal of Geriatric Pharmacotherapy 2007;5(4):314-5. [DOI] [PubMed] [Google Scholar]
Stewart 1990
- Stewart RB. Polypharmacy in the elderly: a fait accompli? Drug Intelligence and Clinical Pharmacy 1990;24(3):321-3. [DOI] [PubMed] [Google Scholar]
Stewart 2017
- Stewart D, Mair A, Wilson M, Kardas P, Lewek P, Alonso A, et al. Guidance to manage inappropriate polypharmacy in older people: systematic review and future developments. Expert Opinion on Drug Safety 2017;16(2):203-13. [DOI] [PubMed] [Google Scholar]
The Priscus List
- Holt S, Schmiedl S, Thürmann PA. Potentially Inappropriate Medications in the Elderly: The PRISCUS List. Deutsches Ärzteblatt International 2010;107:543-51. [DOI: 10.3238/arztebl.2010.0543] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2012
- Wells M, Williams B, Treweek S, Coyle J, Taylor J. Intervention description is not enough: Evidence from an in-depth multiple case study on the untold role and impact of context in randomised controlled trials of seven complex interventions. Trials 2012;13:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wenger 2001
- Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Annals of Internal Medicine 2001;135(8 Pt 2):642-6. [DOI] [PubMed] [Google Scholar]
Werder 2003
- Werder SF, Preskorn SH. Managing polypharmacy. Walking the fine line between help and harm. Current Psychiatry 2003;2(2):24-36. [Google Scholar]
Williamson 2017
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18(3):280. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2022
- The World Bank in Malaysia. www.worldbank.org/en/country/malaysia/overview#1 (accessed 18 March 2022).
Xu 2021
- Xu Z, Liang X, Zhu Y, Lu Y, Ye Y, Fang L, et al. Factors associated with potentially inappropriate prescriptions and barriers to medicines optimisation among older adults in primary care settings: a systematic review. Family Medicine and Community Health 2021;9(e001325):1-16. [DOI: 10.1136/fmch-2021-001325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yourman 2008
- Yourman L, Concato J, Agostini JV. Use of computer decision support interventions to improve medication prescribing in older adults: a systematic review. American Journal of Geriatric Pharmacotherapy 2008;6(2):119-29. [DOI: 10.1016/j.amjopharm.2008.06.001] [DOI] [PubMed] [Google Scholar]
Zermansky 2006
- Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes — randomised controlled trial. Age and Ageing 2006;35:586-91. [DOI: 10.1093/ageing/afl075] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Patterson 2009
- Patterson SM, Hughes C, Kerse N, Cardwell CR. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2009, Issue 4. Art. No: CD008165. [DOI: 10.1002/14651858.CD008165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 2012
- Patterson SM, Hughes C, Kerse N, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD008165. [DOI: 10.1002/14651858.CD008165.pub2] [DOI] [PubMed] [Google Scholar]
Patterson 2014
- Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD008165. [DOI: 10.1002/14651858.CD008165.pub3] [DOI] [PubMed] [Google Scholar]
Rankin 2018a
- Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD008165. [DOI: 10.1002/14651858.CD008165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


