Abstract
Extracellular components released from mycelia of the α and β races of the bean pathogen, Colletotrichum lindemuthianum, inhibited proton uptake in sealed vesicles prepared from bean hypocotyls. Differential sensitivity of ATP-driven proton transport to nitrate, vanadate, N,N′-dicyclohexylcarbodiimide, diethylstilbestrol, and oligomycin suggested the vesicles were enriched for tonoplast. Anion stimulation of proton transport, by enhancement of ATPase activity and dissipation of the membrane potential, was consistent with this conclusion. Although fungal components inhibited the formation of a pH gradient, the membrane potential was unaffected and the ATPase activity slightly stimulated. These data suggest that the fungal components produce an electroneutral proton exchange. Proton transport in Dark Red Kidney bean tonoplast vesicles was inhibited by mycelial preparations from the incompatible α race and compatible β race. Elicitor activity, however, was greater in the α race fractions. Elicitor purified from α race culture filtrate did not inhibit proton transport in vesicles isolated from Dark Red Kidney bean. Consequently, elicitor activity need not be associated with an ability to impair tonoplast function.
Full text
PDF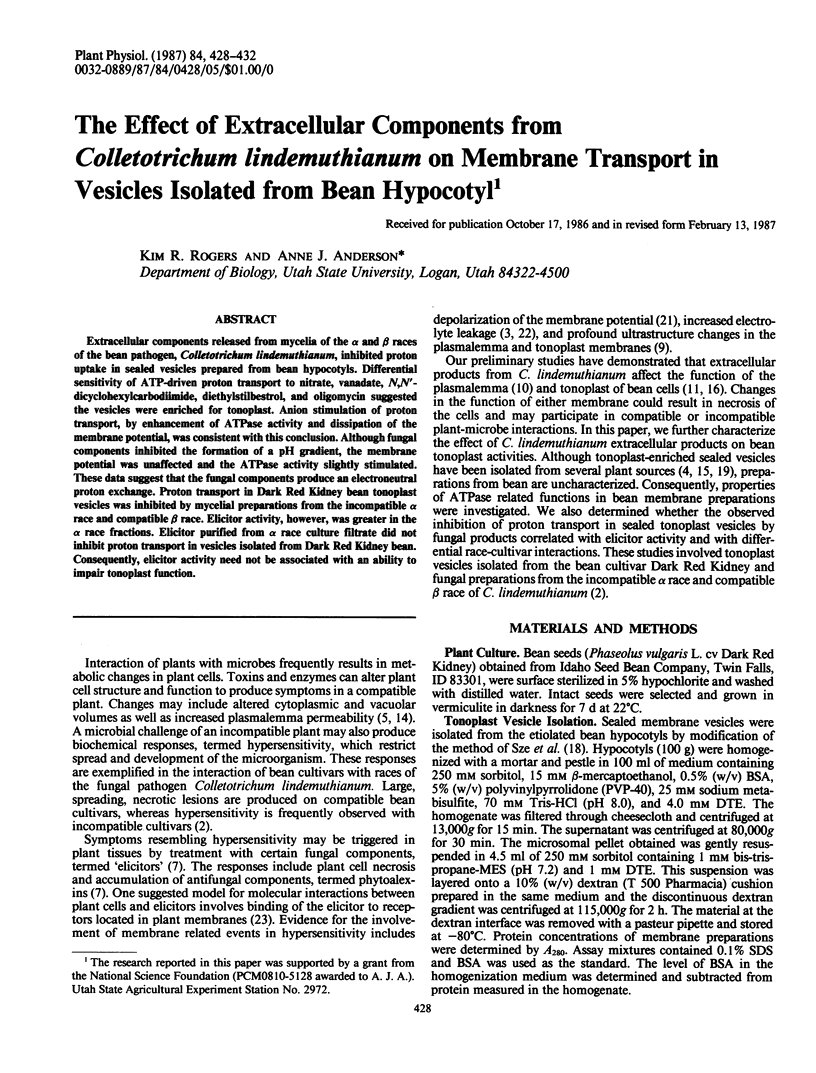



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J. Differences in the biochemical compositions and elicitor activity of extracellular components produced by three races of a fungal plant pathogen, Colletotrichum lindemuthianum. Can J Microbiol. 1980 Dec;26(12):1473–1479. doi: 10.1139/m80-244. [DOI] [PubMed] [Google Scholar]
- Briskin D. P., Thornley W. R., Wyse R. E. Membrane transport in isolated vesicles from sugarbeet taproot : I. Isolation and characterization of energy-dependent, h-transporting vesicles. Plant Physiol. 1985 Aug;78(4):865–870. doi: 10.1104/pp.78.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Hallidy L., Topp M. R. The behavior of the fluorescence lifetime and polarization of oxonol potential-sensitive extrinsic probes in solution and in beef heart submitochondrial particles. J Membr Biol. 1981;60(3):173–185. doi: 10.1007/BF01992556. [DOI] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


