Abstract
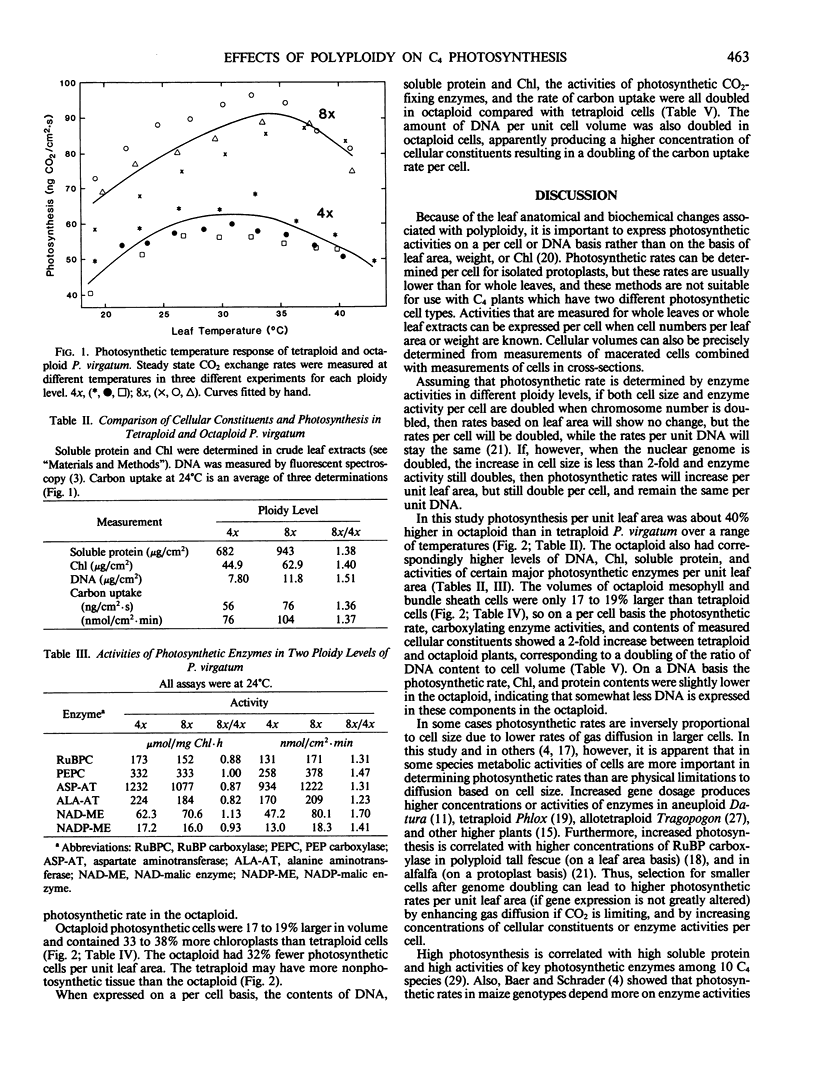
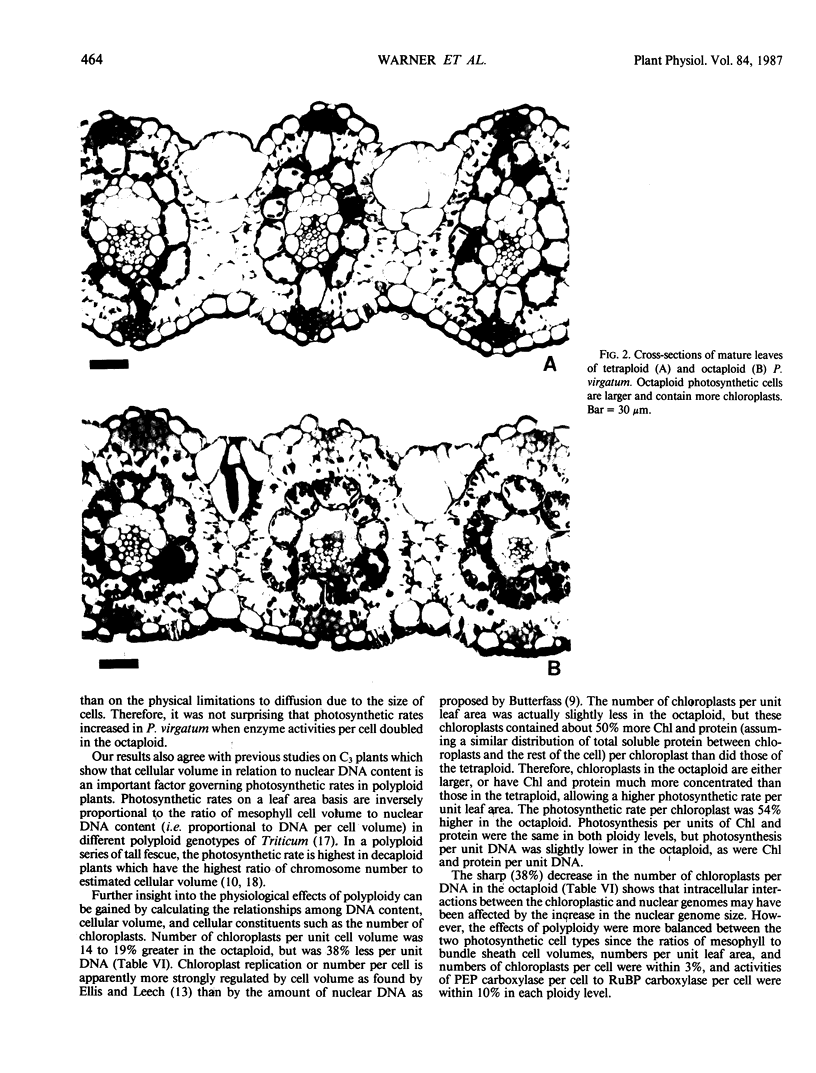
Photosynthetic gas exchange, activities of six key C4 cycle enzymes, amounts of soluble protein, chlorophyll, and DNA, and various leaf anatomical and structural features were measured in naturally occurring tetraploid and octaploid plants of the NAD-malic enzyme type C4 grass Panicum virgatum L. On a leaf area basis, the photosynthetic rate and concentrations of DNA, soluble protein, and chlorophyll were 40 to 50% higher, and enzyme activities 20 to 70% higher in the octaploid than in the tetraploid. Photosynthetic cells in the octaploid were only 17 to 19% larger in volume, yet contained 33 to 38% more chloroplasts than cells in the tetraploid. On a per cell basis the contents of DNA, soluble protein, and chlorophyll, activities of carboxylating photosynthetic enzymes, and carbon assimilation rate were all doubled in octaploid compared with tetraploid cells. Since cellular volume did not double with genome doubling, cellular constituents were more concentrated in the cells of the octaploid. The influences of polyploidy were balanced between mesophyll and bundle sheath cells since the changes in physical and biochemical parameters with ploidy level were similar in both cell types. We conclude that photosynthetic activity in these two polyploid genotypes of P. virgatum is determined by enzyme activities and concentrations of biochemical constituents, and that selection for smaller cell volume has led to higher photosynthetic rates per unit leaf area in the octaploid. The ratio of DNA content to cellular volume is a major factor determining the concentrations of gene products in cells. The number of chloroplasts, however, is controlled more by cellular volume than by the number of nuclear chromosomes.
Full text
PDF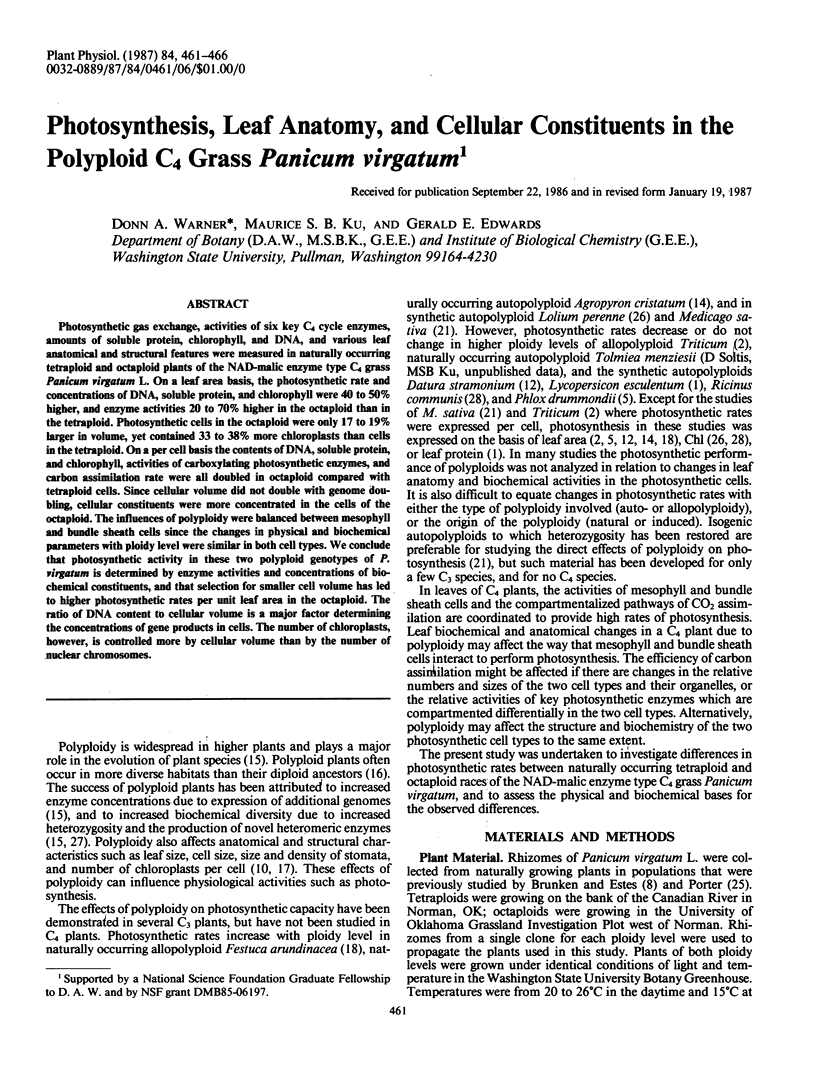


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer G. R., Meyers S. P., Molin W. T., Schrader L. E. A simple and sensitive DNA assay for plant extracts. Plant Physiol. 1982 Oct;70(4):999–1003. doi: 10.1104/pp.70.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer G. R., Schrader L. E. Relationships between CO(2) Exchange Rates and Activities of Pyruvate,Pi Dikinase and Ribulose Bisphosphate Carboxylase, Chlorophyll Concentration, and Cell Volume in Maize Leaves. Plant Physiol. 1985 Mar;77(3):612–616. doi: 10.1104/pp.77.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byrne M. C., Nelson C. J., Randall D. D. Ploidy effects on anatomy and gas exchange of tall fescue leaves. Plant Physiol. 1981 Oct;68(4):891–893. doi: 10.1104/pp.68.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. O., Palmer J. M. The oxidation of malate by isolated plant mitochondria. Eur J Biochem. 1972 Apr 24;26(4):499–509. doi: 10.1111/j.1432-1033.1972.tb01792.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Neuburger M., Douce R., Wiskich J. T. Exogenous NAD Effects on Plant Mitochondria: A Reinvestigation of the Transhydrogenase Hypothesis. Plant Physiol. 1983 Dec;73(4):1024–1027. doi: 10.1104/pp.73.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Rayner J. R., Wiskich J. T. Characteristics of External NADH Oxidation by Beetroot Mitochondria. Plant Physiol. 1976 Jul;58(1):38–42. doi: 10.1104/pp.58.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Isolation and properties of the outer membrane of plant mitochondria. Arch Biochem Biophys. 1975 Nov;171(1):117–123. doi: 10.1016/0003-9861(75)90014-4. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The Effect of Exogenous Nicotinamide Adenine Dinucleotide on the Oxidation of Nicotinamide Adenine Dinucleotide-linked Substrates by Isolated Plant Mitochondria. Plant Physiol. 1974 Sep;54(3):360–363. doi: 10.1104/pp.54.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. The oxidation of malate and exogenous reduced nicotinamide adenine dinucleotide by isolated plant mitochondria. Plant Physiol. 1974 Jan;53(1):104–109. doi: 10.1104/pp.53.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- GREENSPAN M., PURVIS J. L. ENERGY-LINKED INCORPORATION OF DIPHOSPHOPYRIDINE NUCLEOTIDE INTO RAT-LIVER MITOCHONDRIA. Biochim Biophys Acta. 1965 Apr 26;99:191–194. doi: 10.1016/s0926-6593(65)80026-1. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, MALISON R., BRIDGERS W. F., SCHUTZ B., ATCHISON A. Reincorporation of diphosphopyridine nucleotide into mitochondrial enzyme systems. J Biol Chem. 1959 Mar;234(3):693–699. [PubMed] [Google Scholar]
- Joseph M. C., Randall D. D. Photosynthesis in Polyploid Tall Fescue : II. PHOTOSYNTHESIS AND RIBULOSE-1, 5-BISPHOSPHATE CARBOXYLASE OF POLYPLOID TALL FESCUE. Plant Physiol. 1981 Oct;68(4):894–898. doi: 10.1104/pp.68.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Levin D. A., Torres A. M., Levy M. Alcohol dehydrogenase activity in diploid and autotetraploid Phlox. Biochem Genet. 1979 Feb;17(1-2):35–42. doi: 10.1007/BF00484472. [DOI] [PubMed] [Google Scholar]
- Meyers S. P., Nichols S. L., Baer G. R., Molin W. T., Schrader L. E. Ploidy effects in isogenic populations of alfalfa : I. Ribulose-1,5-bisphosphate carboxylase, soluble protein, chlorophyll, and DNA in leaves. Plant Physiol. 1982 Dec;70(6):1704–1709. doi: 10.1104/pp.70.6.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard D. L., Wiskich J. T., Robertson R. N. Ion Uptake and Phosphorylation in Mitochondria: Effect of Monovalent Ions. Plant Physiol. 1965 Nov;40(6):1129–1135. doi: 10.1104/pp.40.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin W. T., Meyers S. P., Baer G. R., Schrader L. E. Ploidy Effects in Isogenic Populations of Alfalfa : II. Photosynthesis, Chloroplast Number, Ribulose-1,5-Bisphosphate Carboxylase, Chlorophyll, and DNA in Protoplasts. Plant Physiol. 1982 Dec;70(6):1710–1714. doi: 10.1104/pp.70.6.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Day D. A., Douce R. Transport of NAD in Percoll-Purified Potato Tuber Mitochondria: Inhibition of NAD Influx and Efflux by N-4-Azido-2-nitrophenyl-4-aminobutyryl-3'-NAD. Plant Physiol. 1985 Jun;78(2):405–410. doi: 10.1104/pp.78.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Day D. A., Douce R. Transport of coenzyme A in plant mitochondria. Arch Biochem Biophys. 1984 Feb 15;229(1):253–258. doi: 10.1016/0003-9861(84)90151-6. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Douce R. Slow passive diffusion of NAD+ between intact isolated plant mitochondria and suspending medium. Biochem J. 1983 Nov 15;216(2):443–450. doi: 10.1042/bj2160443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Perchorowicz J. T., Raynes D. A., Jensen R. G. Measurement and preservation of the in vivo activation of ribulose 1,5-bisphosphate carboxylase in leaf extracts. Plant Physiol. 1982 May;69(5):1165–1168. doi: 10.1104/pp.69.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett D. M., Estes M. K., Pagano J. S. Structural proteins of simian virus 40. I. Histone characteristics of low-molecular-weight polypeptides. J Virol. 1975 Feb;15(2):379–385. doi: 10.1128/jvi.15.2.379-385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Photosynthetic and Photorespiratory Carbon Metabolism in Mesophyll Protoplasts and Chloroplasts Isolated from Isogenic Diploid and Tetraploid Cultivars of Ryegrass (Lolium perenne L.). Plant Physiol. 1980 Mar;65(3):489–494. doi: 10.1104/pp.65.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R. J., Tuskes S. E., Ozelkök S. Survival of plant mitochondria in vitro. Form and function after 4 days at 25 degrees C. Arch Biochem Biophys. 1974 Oct;164(2):743–751. doi: 10.1016/0003-9861(74)90088-5. [DOI] [PubMed] [Google Scholar]
- Roose M. L., Gottlieb L. D. Biochemical properties and level of expression of alcohol dehydrogenases in the allotetraploid plant Tragopogon miscellus and its diploid progenitors. Biochem Genet. 1980 Dec;18(11-12):1065–1085. doi: 10.1007/BF00484339. [DOI] [PubMed] [Google Scholar]
- Timko M. P., Vasconcelos A. C. Euploidy in Ricinus: EUPLOIDY EFFECTS ON PHOTOSYNTHETIC ACTIVITY AND CONTENT OF CHLOROPHYLL-PROTEINS. Plant Physiol. 1981 Jun;67(6):1084–1089. doi: 10.1104/pp.67.6.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]